Microwave-Assisted Solvent-Free Acetylation of Cellulose with Acetic Anhydride in the Presence of Iodine as a Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the microwave irradiation
2.2. Effects of iodine quantities
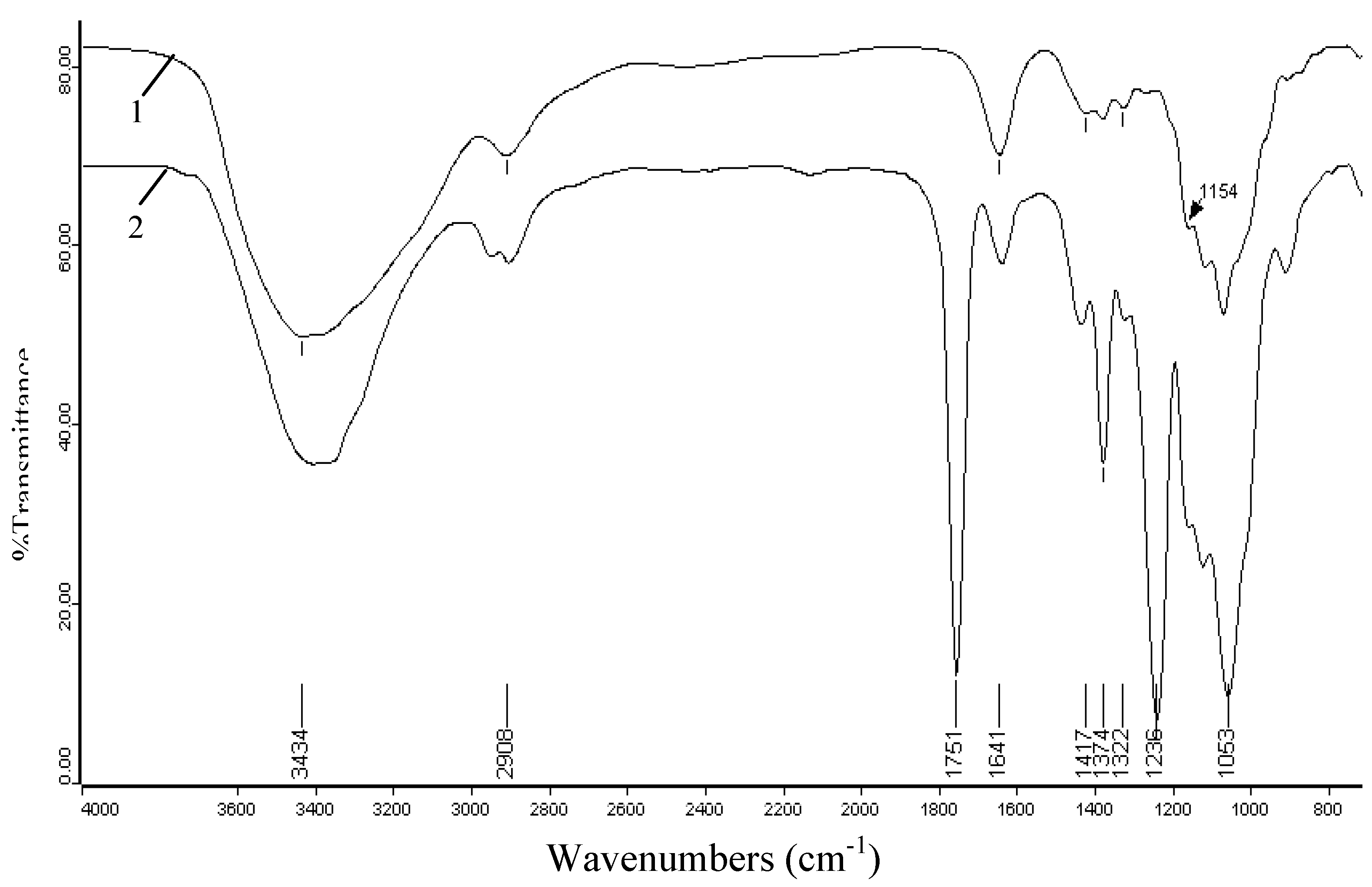
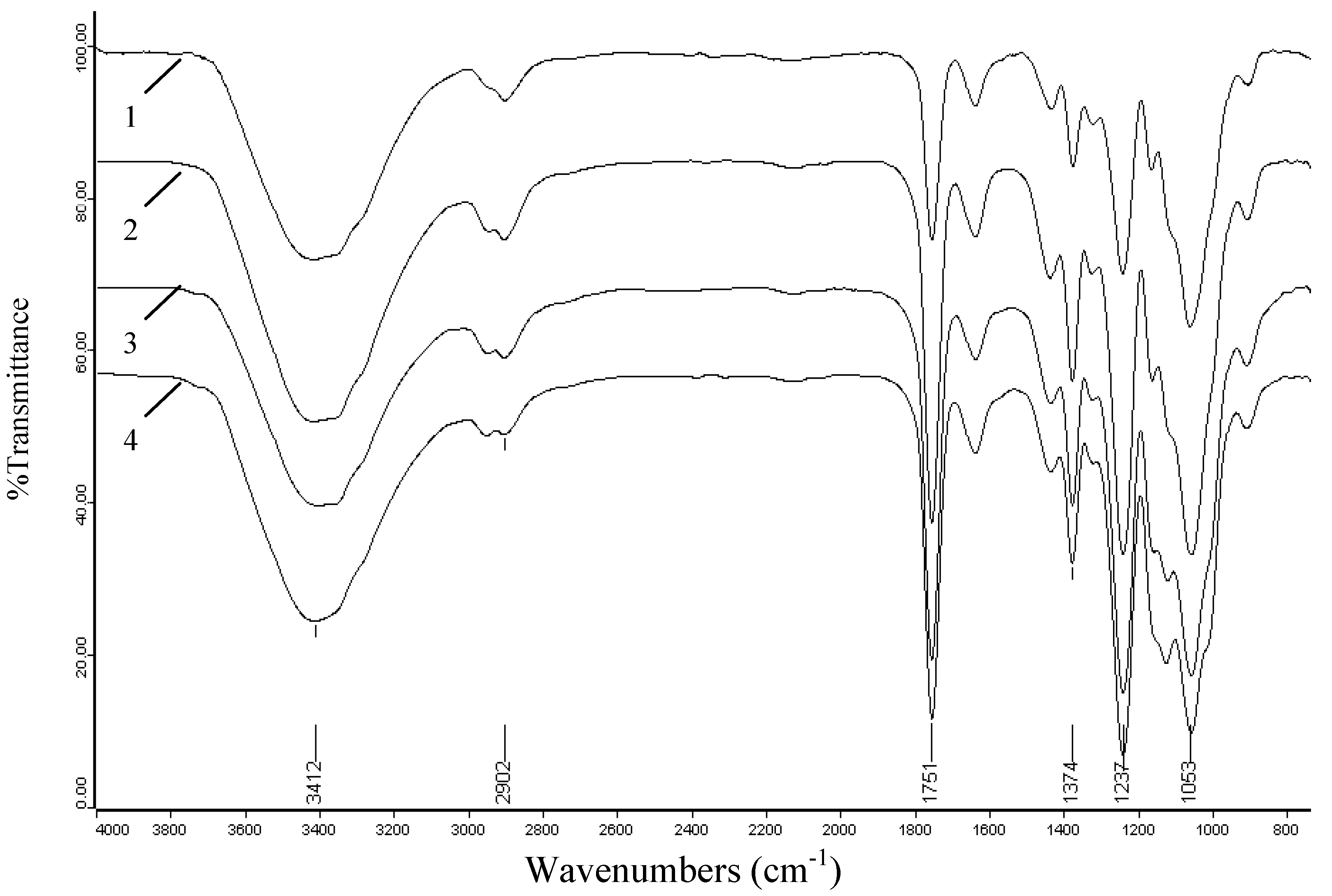
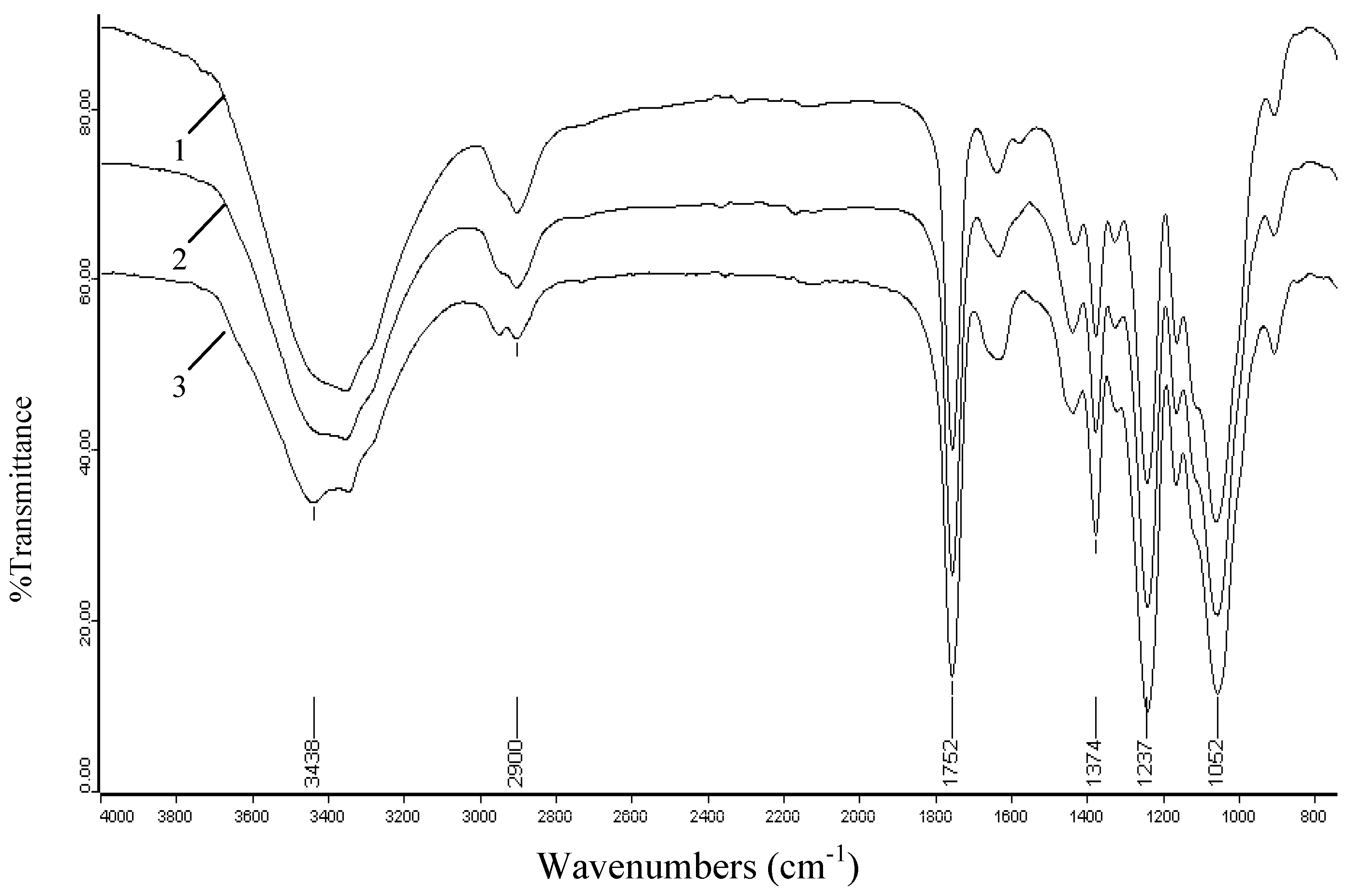
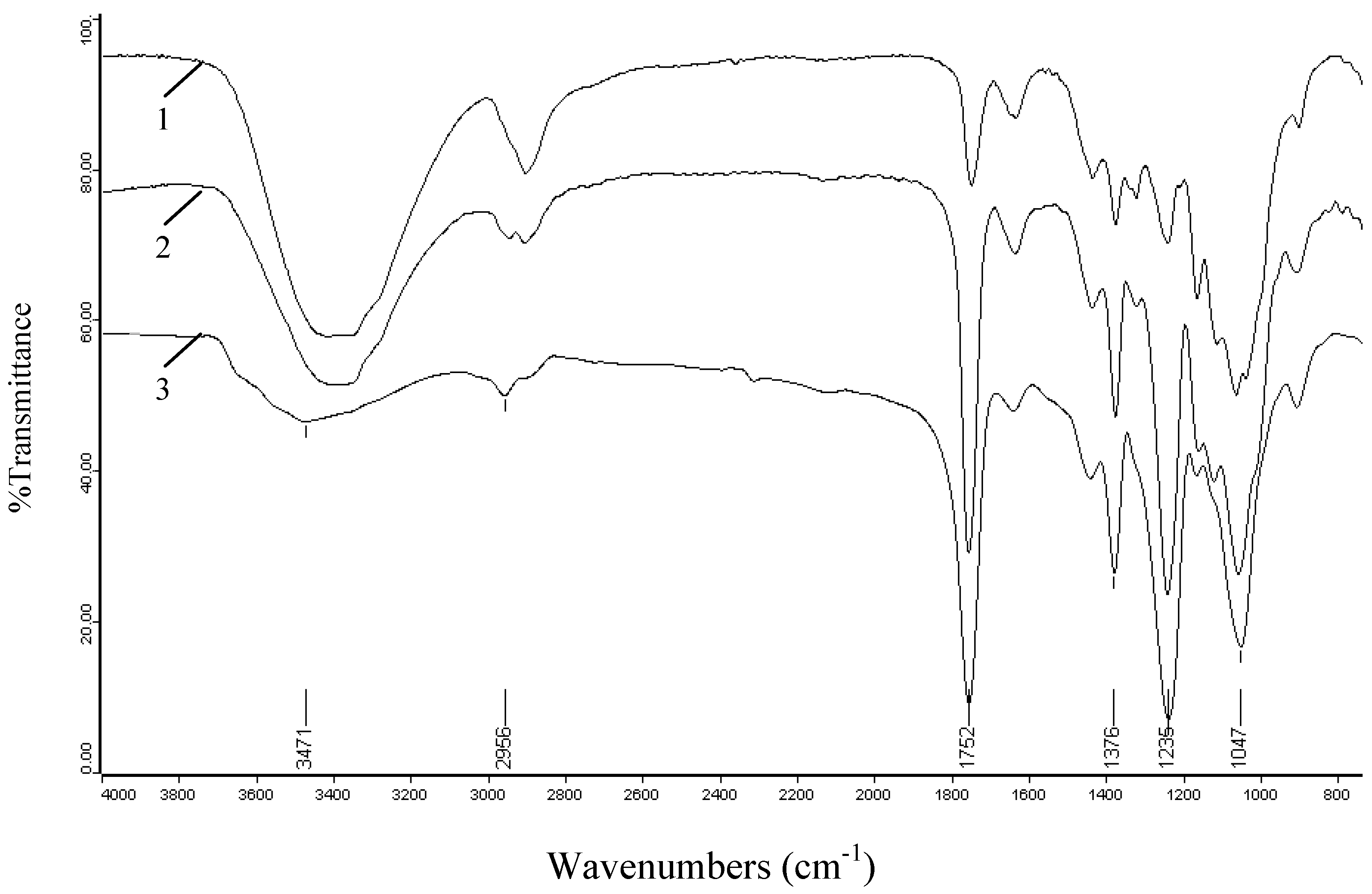
2.3. FT-IR spectra
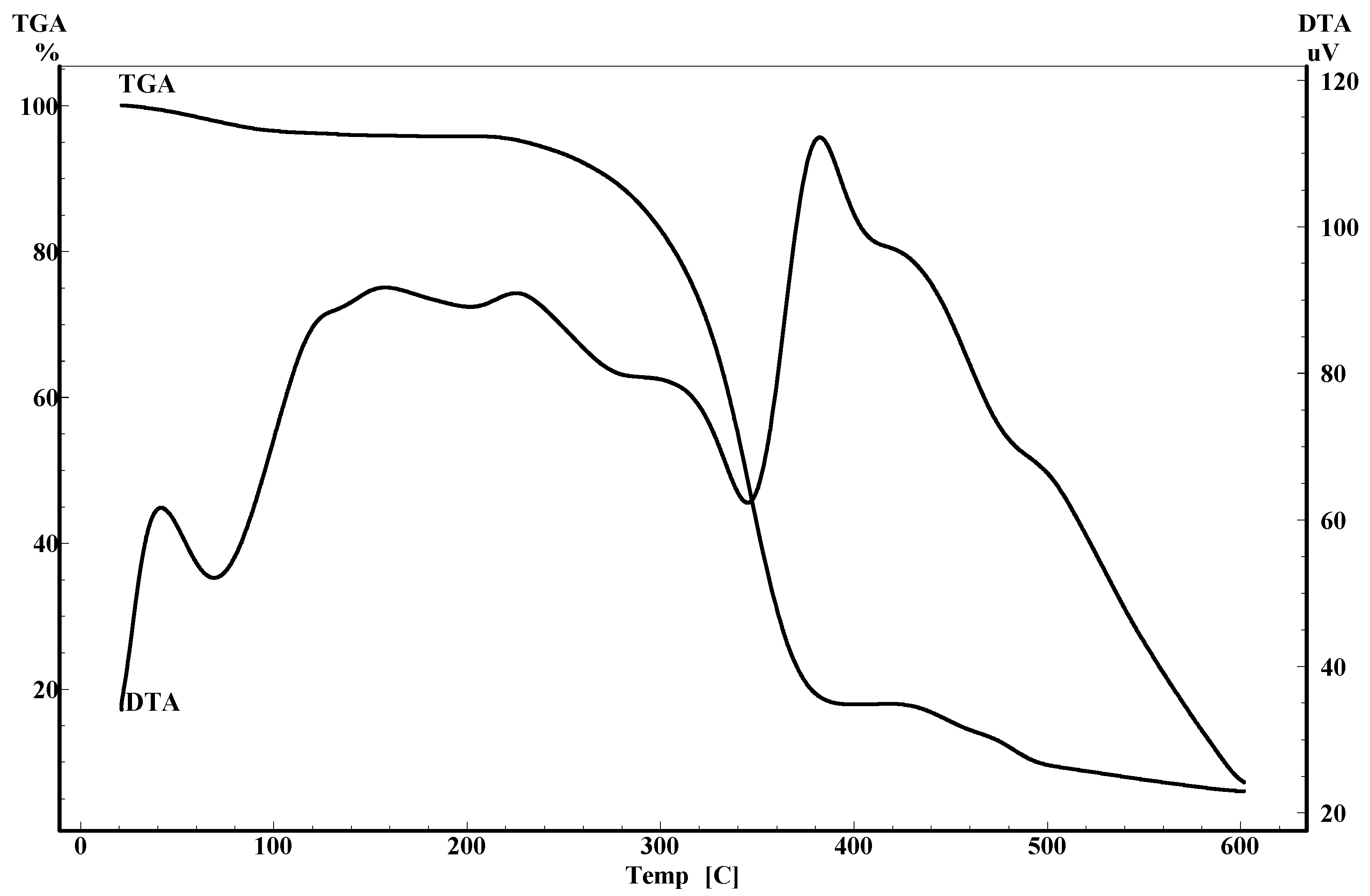
2.4. Thermal analysis
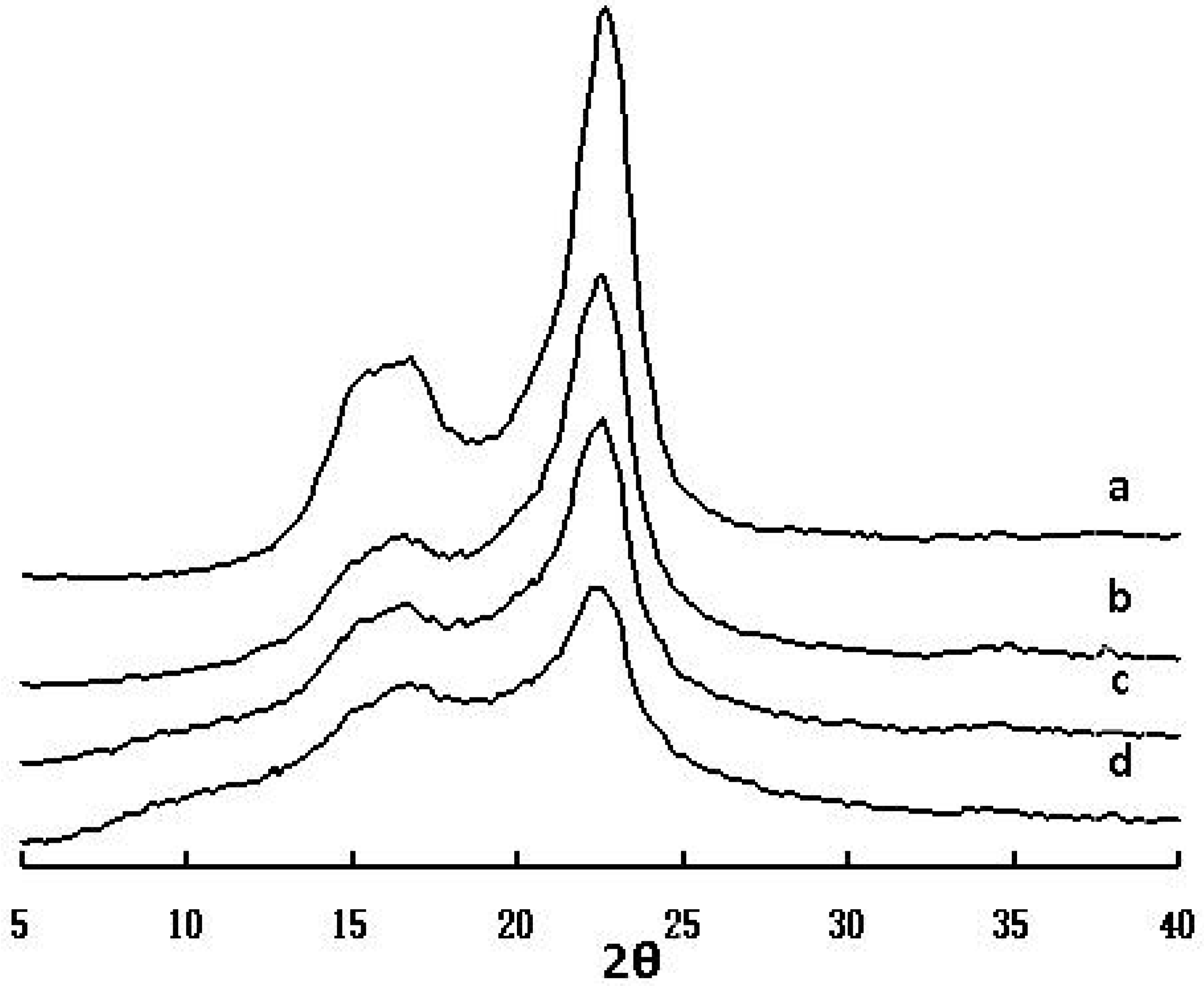
2.5. WAXD spectra
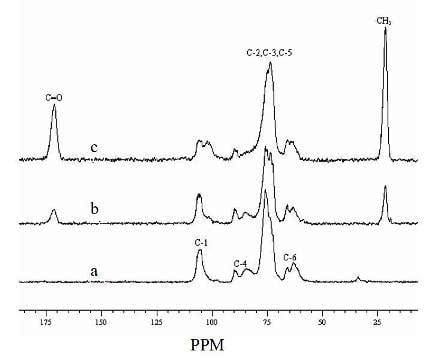
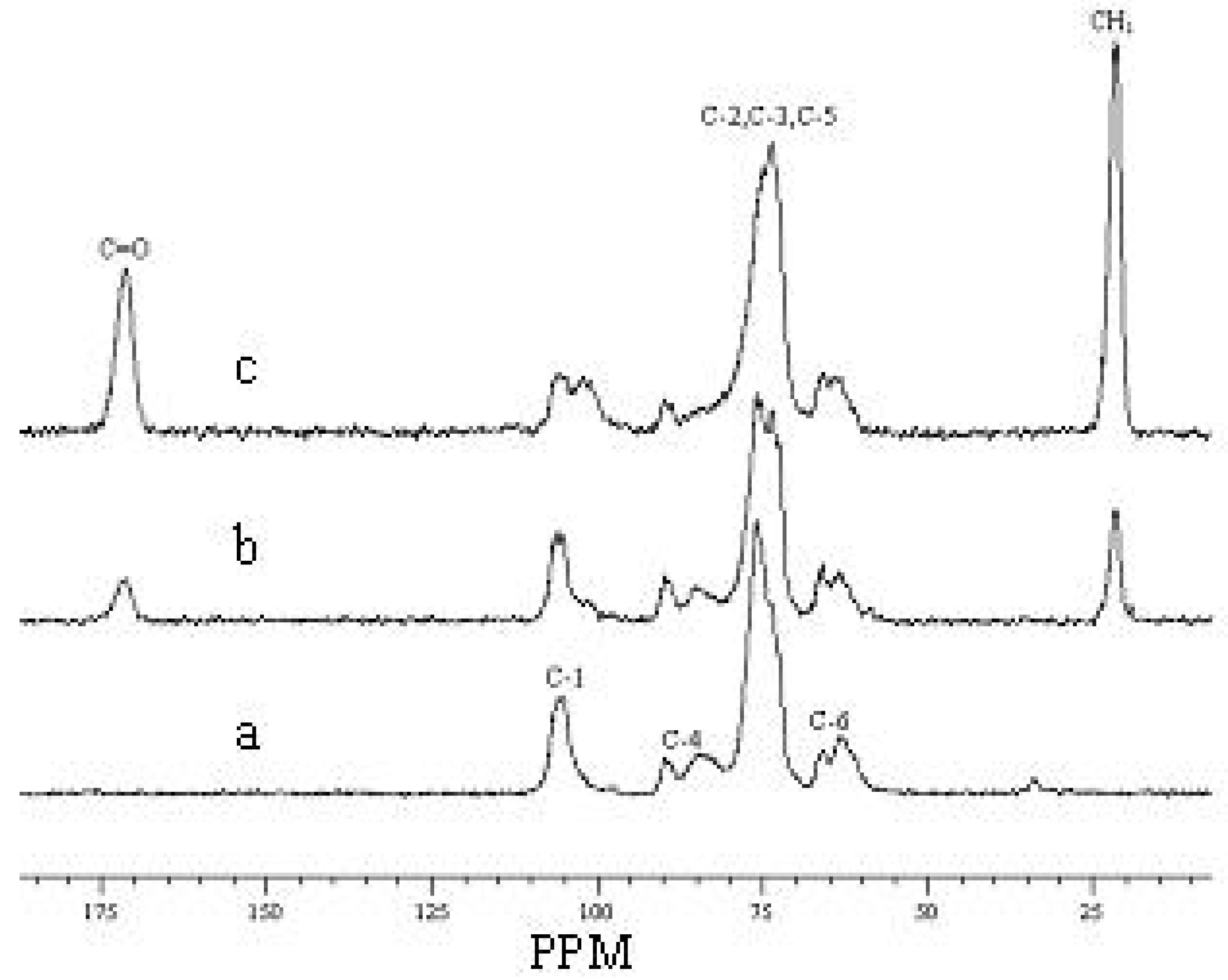
2.6. CP/MAS 13C-NMR spectra
3. Conclusions
4. Experimental
4.1. Materials and reagents
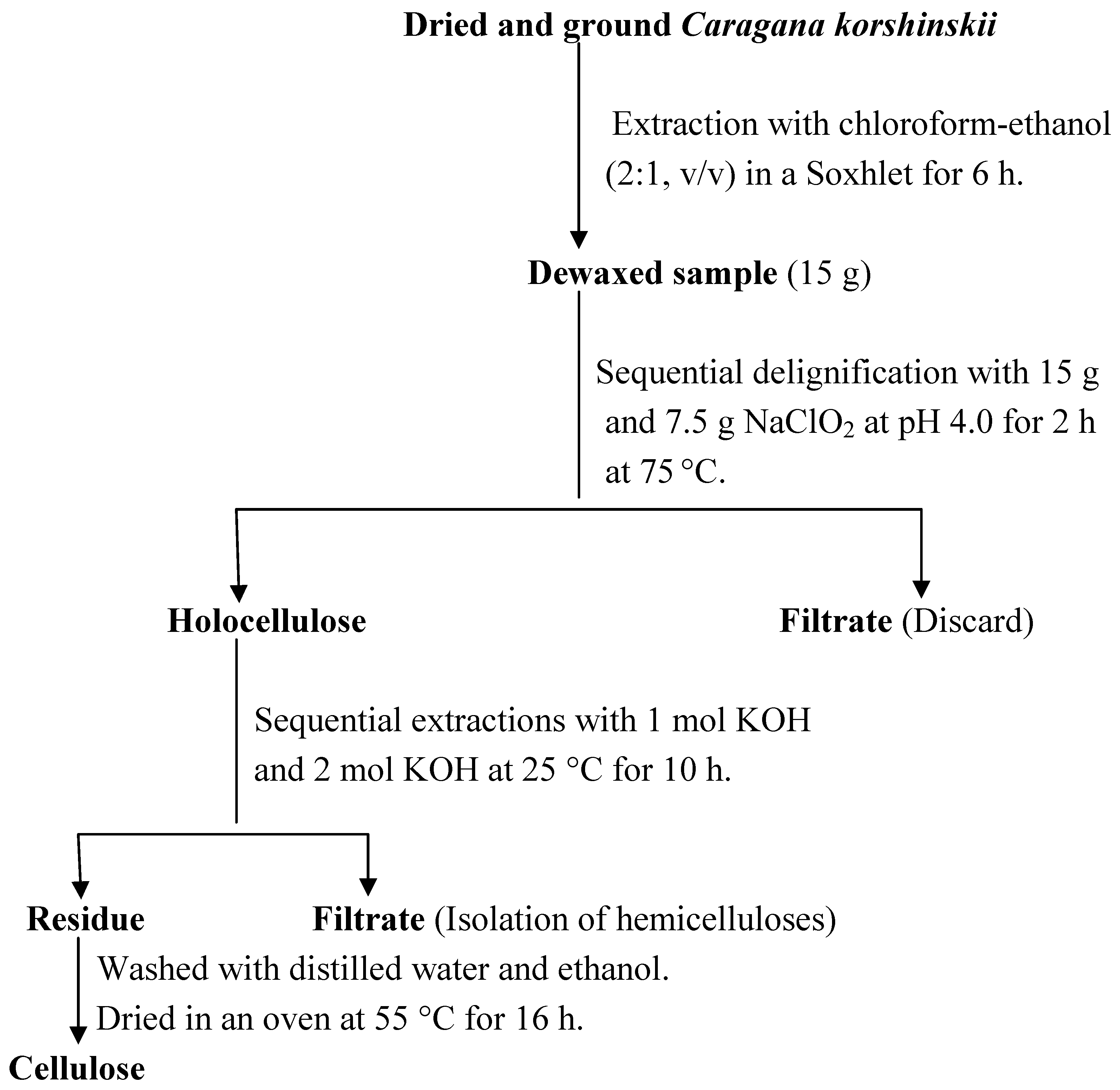
4.2. The isolation of cellulose
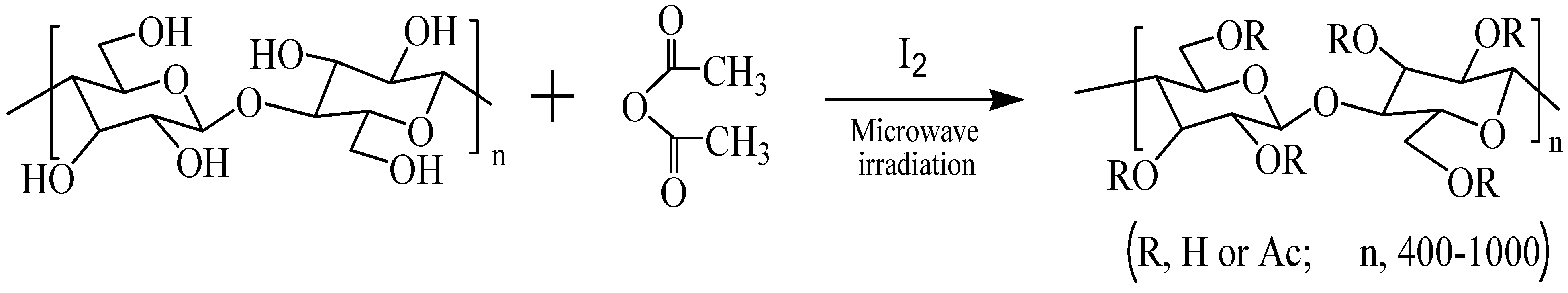
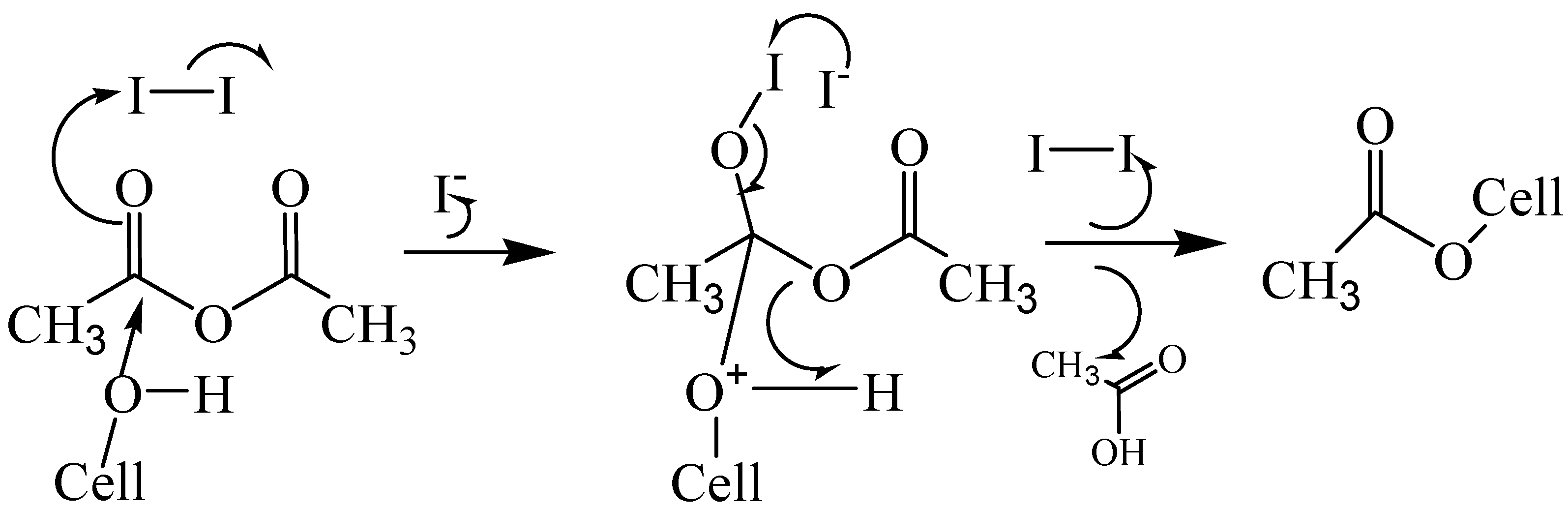
4.3. Acetylation of cellulose
4.4. Structural characterization
Acknowledgements
References
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T. Biotechnological potential of agro-industrial residues. I: Sugarcane bagasse. Bioresour. Technol. 2000, 74, 69–80. [Google Scholar] [CrossRef]
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in cellulose ester performance and application. Prog. Polym. Sci. 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Mahendran, R.; Malaisamy, R.; Mohan, D. Cellulose acetate and epoxy resin blend ultrafiltration membranes: reparation, characterization, and application. J. Macromol. Sci. A. 2002, 39, 1025–1035. [Google Scholar] [CrossRef]
- Paula, M.P.; Lacerda, T.M.; Frollini, E. Sisal cellulose acetates obtained from heterogeneous reactions. Express Polym. Lett. 2008, 2, 423–428. [Google Scholar] [CrossRef]
- Sassi, J.F.; Chanzy, H. Ultrastructural aspects of the acetylation of cellulose. Cellulose 1995, 2, 111–127. [Google Scholar] [CrossRef]
- Li, J.Z.; Furuno, T.; Katoh, S.; Uehara, T. Chemical modification of wood by anhydredes without solvents or catalyst. J. Wood Sci. 2000, 46, 215–221. [Google Scholar] [CrossRef]
- Hou, A.Q.; Wang, X.J.; Wu, L.H. Effect of microwave irradiation on the physical properties and morphological structures of cotton cellulose. Carbohyd. Polym. 2008, 74, 934–937. [Google Scholar] [CrossRef]
- Idris, A.; Ahmed, I.; Jye, H.W. Performance of cellulose acetate-polyethersulphone blend membrane prepared using microwave heating for palm oil mill effluent treatment. Water Sci. Technol. 2007, 56, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Moharram, M.A.; Mahmoud, O.M. X-ray diffraction methods in the study of the effect of microwave heating on the transformation of cellulose I into cellulose II during mercerization. J. Appl. Polym. Sci. 2007, 105, 2978–2983. [Google Scholar] [CrossRef]
- Biswas, A.; Shogren, R.L.; Selling, G.; Salch, J.; Willett, J.L.; Buchanan, C.M. Rapid and environmentally friendly preparation of starch esters. Carbohyd. Polym. 2008, 74, 137–141. [Google Scholar] [CrossRef]
- Biswas, A.; Sharma, B.K.; Willet, J.L.; Vermillion, K.; Erhan, S.Z.; Cheng, H.N. Novel modified soybean oil containing hydrazine-ester: Synthesis and characterization. Green Chem. 2007, 9, 85–89. [Google Scholar] [CrossRef]
- Biswas, A.; Shogren, R.L.; Kim, S.; Willett, J.L. Rapid preparation of starch maleate half-esters. Carbohyd. Polym. 2006, 64, 484–487. [Google Scholar] [CrossRef]
- Tao, Y.Z.; Xu, W.L. Microwave-assisted solubilization and solution properties of hyperbranched polysaccharide. Carbohyd. Res. 2008, 343, 3071–3078. [Google Scholar] [CrossRef] [PubMed]
- Koroskenyi, B.; Mccarthy, S.P. Microwave-assisted solvent-free or aqueous-based synthesis of biodegradable polymers. J. Polym. Environ. 2002, 10, 93–104. [Google Scholar] [CrossRef]
- Satgé, C.; Verneuil, B.; Branland, P.; Granet, R.; Krausz, P.; Rozier, J.; Petit, C. Rapid homogeneous esterification of cellulose induced by microwave irradiation. Carbohyd. Polym. 2002, 49, 373–376. [Google Scholar] [CrossRef]
- Biswas, A.; Selling, G.; Appell, M.; Woods, K.K.; Willett, J.L.; Buchanan, C.M. Iodine catalyzed esterification of cellulose using reduced levels of solvent. Carbohyd. Polym. 2007, 68, 555–560. [Google Scholar] [CrossRef]
- Behmadi, H.; Roshani, M.; Saadati, S.M. Synthesis of phenanthrimidazole from 9, 10-phenanthraquinone and aldehydes by molecular iodine as catalyst. Chinese Chem. Lett. 2009, 20, 5–8. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Sengupta, S.; Guptal, M.K.; Baishya1, G.; Harshavardhanal, S.J.; Dash1, U. Iodine as a mild, efficient, and cost-effective catalyst for the synthesis of thiiranes from oxiranes. Monatsh. Chem. 2008, 139, 1363–1367. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Rao, C.V.; Reddy, M.S. Molecular iodine-catalyzed highly stereoselective synthesis of sugar acetylenes. Synthesis Stuttgart 2003, 2, 247–250. [Google Scholar] [CrossRef]
- Ren, J.L.; Sun, R.C.; Liu, C.F.; Cao, Z.N.; Luo, W. Acetylation of wheat straw hemicelluloses in ionic liquid using iodine as a catalyst. Carbohyd. Polym. 2007, 70, 406–414. [Google Scholar] [CrossRef]
- Ahmed, N.; van Lier, J.E. Molecular iodine in isopropenyl acetate (IPA): a highly efficient catalyst for the acetylation of alcohols, amines and phenols under solvent free conditions. Tetrahedron Lett. 2006, 47, 5345–5349. [Google Scholar] [CrossRef]
- Phukan, P. Iodine as an extremely powerful catalyst for the acetylation of alcohols under solvent-free conditions. Tetrahedron Lett. 2004, 45, 4785–4787. [Google Scholar] [CrossRef]
- Biswas, A.; Shogren, R.L.; Willett, J.L. Solvent-free process to esterify polysaccharides. Biomacromolecules 2005, 6, 1843–1845. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Shogren, R.L.; Selling, G.; Salch, J.; Willett, J.L.; Buchanan, C.M. Rapid and environmentally friendly preparation of starch esters. Carbohyd. Polym. 2008, 74, 137–141. [Google Scholar] [CrossRef]
- Satchell, D.P.N. An outline of acylation. Q. Rev. (London) 1963, 17, 160–163. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Jones, D.; Strickland, G.; Centin, N.S. Kinetic and mechanisticaspects of the acetylation of wood with acetic anhydride. Holzforschung 1998, 52, 623–629. [Google Scholar] [CrossRef]
- Pandey, K.K. A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Xu, F.; Sun, R.C.; Lu, Q. Characteristics of cellulose isolated by a totally chlorine-free method from caragana korshinskii. J. Appl. Polym. Sci. 2006, 101, 3251–3263. [Google Scholar] [CrossRef]
- Cerqueira, D.A.; Filho, G.R.; Assunção, R.M.N. A new value for the heat of fusion of a perfect crystal of cellulose acetate. Polym. Bull. 2006, 56, 475–484. [Google Scholar] [CrossRef]
- Vanderhart, D.L.; Hyatt, J.A.; Atalla, R.H.; Tirumalai, V.C. Solid-state 13C NMR and Raman studies of cellulose triacetate: oligomers, polymorphism, and inference about chain polarity. Macromolecules 1996, 29, 730–739. [Google Scholar] [CrossRef]
- Kono, H.; Numata, Y.; Nagai, N.; Erata, T.; Takai, M. CPMAS 13C NMR and X-Ray studies of cellooligosaccharide acetates as a model for cellulose triacetate. J. Polym. Sci. Pol. Chem. 1999, 37, 4100–4107. [Google Scholar] [CrossRef]
- Sanios, E.; Dart, R.K.; Dawkins, J.V. Preparation, characterization and biodegradation studies on cellulose acetates with varying degrees of substitution. Polymer 1997, 38, 3045–3054. [Google Scholar] [CrossRef]
- Stojanović, Ž.; Jeremić, K.; Jovanović, S.; Lechner, M.D. A comparison of some methods for the determination of the degree of substitution of carboxymethyl starch. Starch-Strake 2005, 57, 79–83. [Google Scholar] [CrossRef]
- Luo, J.; Sun, Y.Y. Acetylation of cellulose using recyclable polymeric catalysts. J. Appl. Polym. Sci. 2006, 100, 3288–3296. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Acetylation conditions | Acetylated cellulose | ||||
|---|---|---|---|---|---|
| T ( °C )a | t (min)b | P (w)c | Sample No. | DS | Yield (% dry weight)d |
| 120 | 15 | 300 | 1 | 1.4 | 22 |
| 120 | 15 | 400 | 2 | 1.5 | 23 |
| 120 | 15 | 500 | 3 | 1.4 | 22 |
| 120 | 15 | 600 | 4 | 1.4 | 22 |
| 120 | 15 | 700 | 5 | 1.4 | 22 |
| 120 | 15 | 800 | 6 | 1.4 | 7 |
| 80 | 15 | 400 | 7 | 0.78 | 13 |
| 90 | 15 | 400 | 8 | 0.84 | 12 |
| 100 | 15 | 400 | 9 | 1.2 | 16 |
| 110 | 15 | 400 | 10 | 1.1 | 21 |
| 120 | 15 | 400 | 11 | 1.4 | 21 |
| 130 | 15 | 400 | 12 | 1.6 | 25 |
| 130 | 5 | 400 | 13 | 1.5 | 26 |
| 130 | 10 | 400 | 14 | 1.6 | 27 |
| 130 | 20 | 400 | 15 | 1.7 | 22 |
| 130 | 25 | 400 | 16 | 1.8 | 26 |
| 130 | 30 | 400 | 17 | 1.8 | 35 |
| 130 | 40 | 400 | 18 | 1.9 | 46 |
| Sample No. | 19 | 20 | 21 | 22 | 23 | 24 |
|---|---|---|---|---|---|---|
| Iodine(%)a | 1 | 3 | 5 | 8 | 10 | 15 |
| DS | 0.48 | 1.1 | 1.8 | 2.0 | 2.3 | 2.8 |
| Yield (% dry weight)b | − | 7.2 | 35.3 | 37.4 | 43.6 | 78 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, J.; Zhang, L.-P.; Peng, F.; Bian, J.; Yuan, T.-Q.; Xu, F.; Sun, R.-C. Microwave-Assisted Solvent-Free Acetylation of Cellulose with Acetic Anhydride in the Presence of Iodine as a Catalyst. Molecules 2009, 14, 3551-3566. https://doi.org/10.3390/molecules14093551
Li J, Zhang L-P, Peng F, Bian J, Yuan T-Q, Xu F, Sun R-C. Microwave-Assisted Solvent-Free Acetylation of Cellulose with Acetic Anhydride in the Presence of Iodine as a Catalyst. Molecules. 2009; 14(9):3551-3566. https://doi.org/10.3390/molecules14093551
Chicago/Turabian StyleLi, Jing, Li-Ping Zhang, Feng Peng, Jing Bian, Tong-Qi Yuan, Feng Xu, and Run-Cang Sun. 2009. "Microwave-Assisted Solvent-Free Acetylation of Cellulose with Acetic Anhydride in the Presence of Iodine as a Catalyst" Molecules 14, no. 9: 3551-3566. https://doi.org/10.3390/molecules14093551
APA StyleLi, J., Zhang, L.-P., Peng, F., Bian, J., Yuan, T.-Q., Xu, F., & Sun, R.-C. (2009). Microwave-Assisted Solvent-Free Acetylation of Cellulose with Acetic Anhydride in the Presence of Iodine as a Catalyst. Molecules, 14(9), 3551-3566. https://doi.org/10.3390/molecules14093551