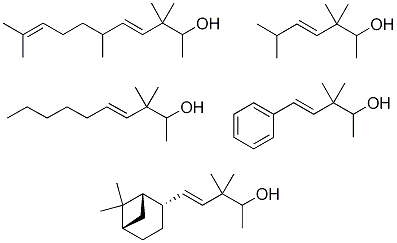
Synthesis and Olfactory Evaluation of Bulky Moiety-Modified Analogues to the Sandalwood Odorant Polysantol®
Abstract
:1. Introduction
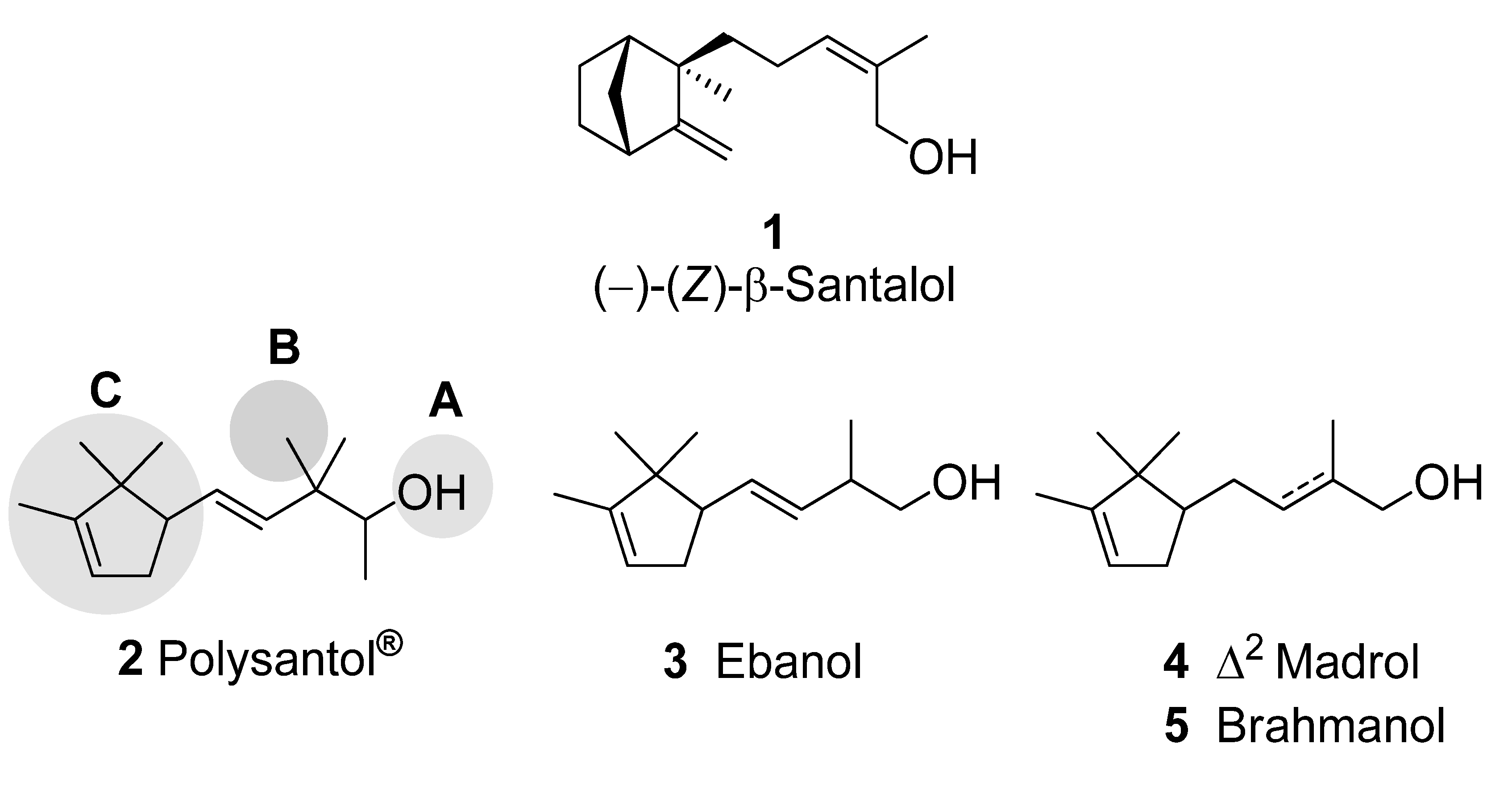
2. Results and Discussion
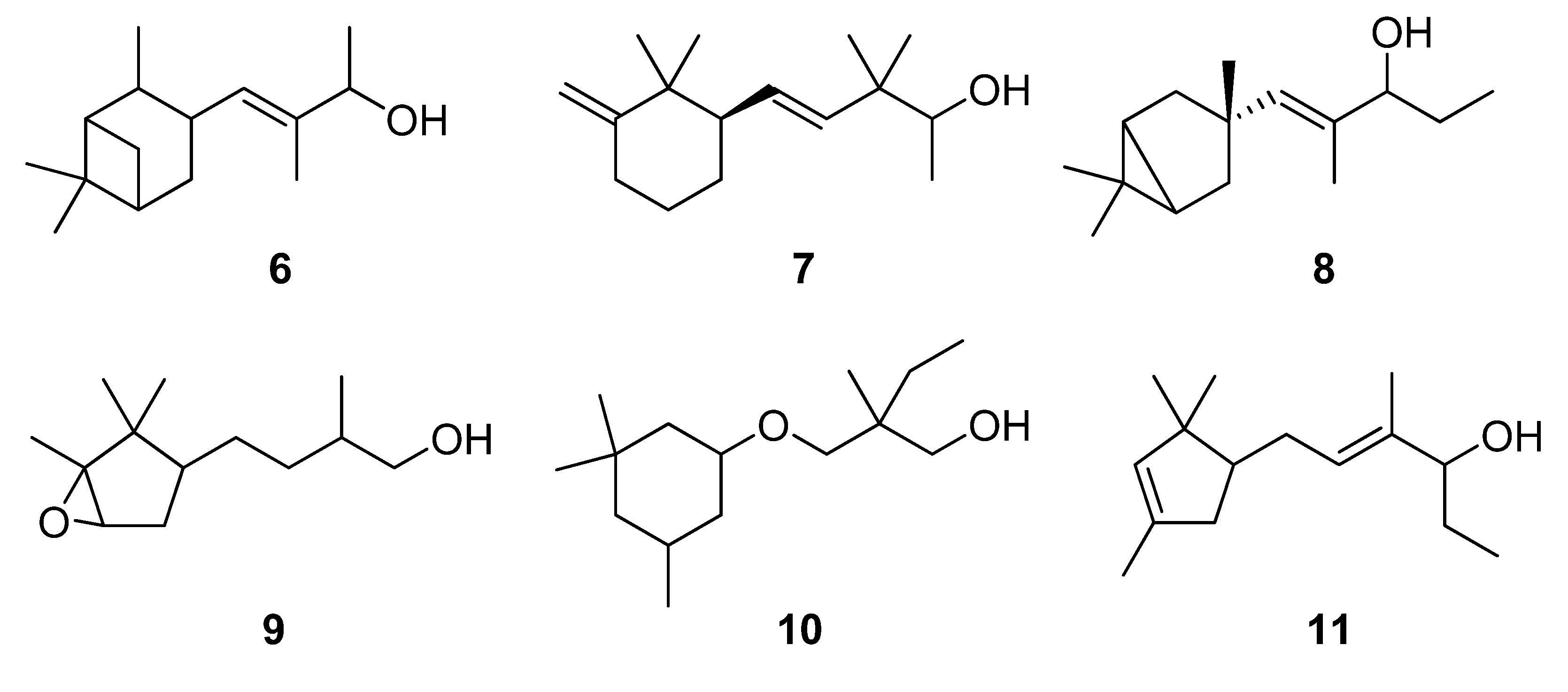
2.1. Synthesis
2.1.1. Conversion of nopol (16) into dihydronopal (18)
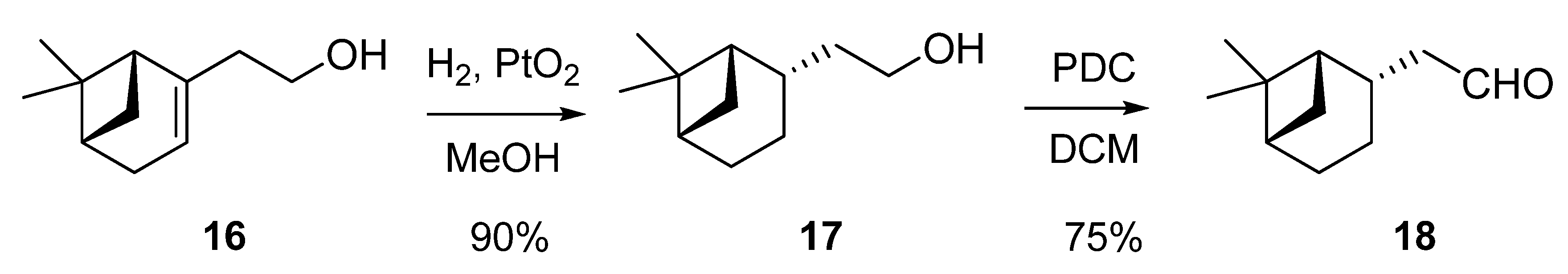
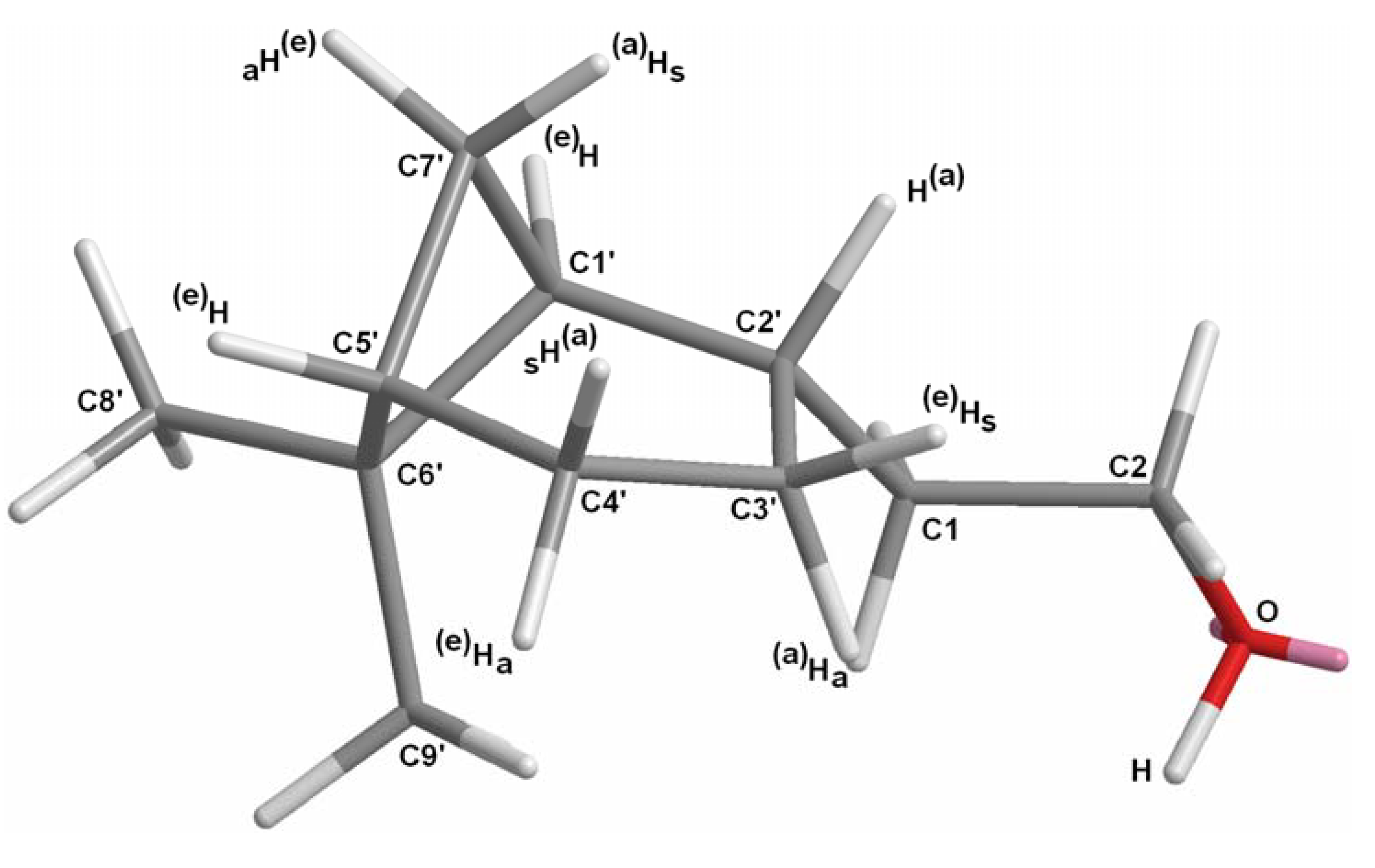
2.1.2. Aldol condensation of the aldehydes 12−15 and 18 with butanone to give the α,β-unsaturated ketones 19, 20, 22, 24 and 27
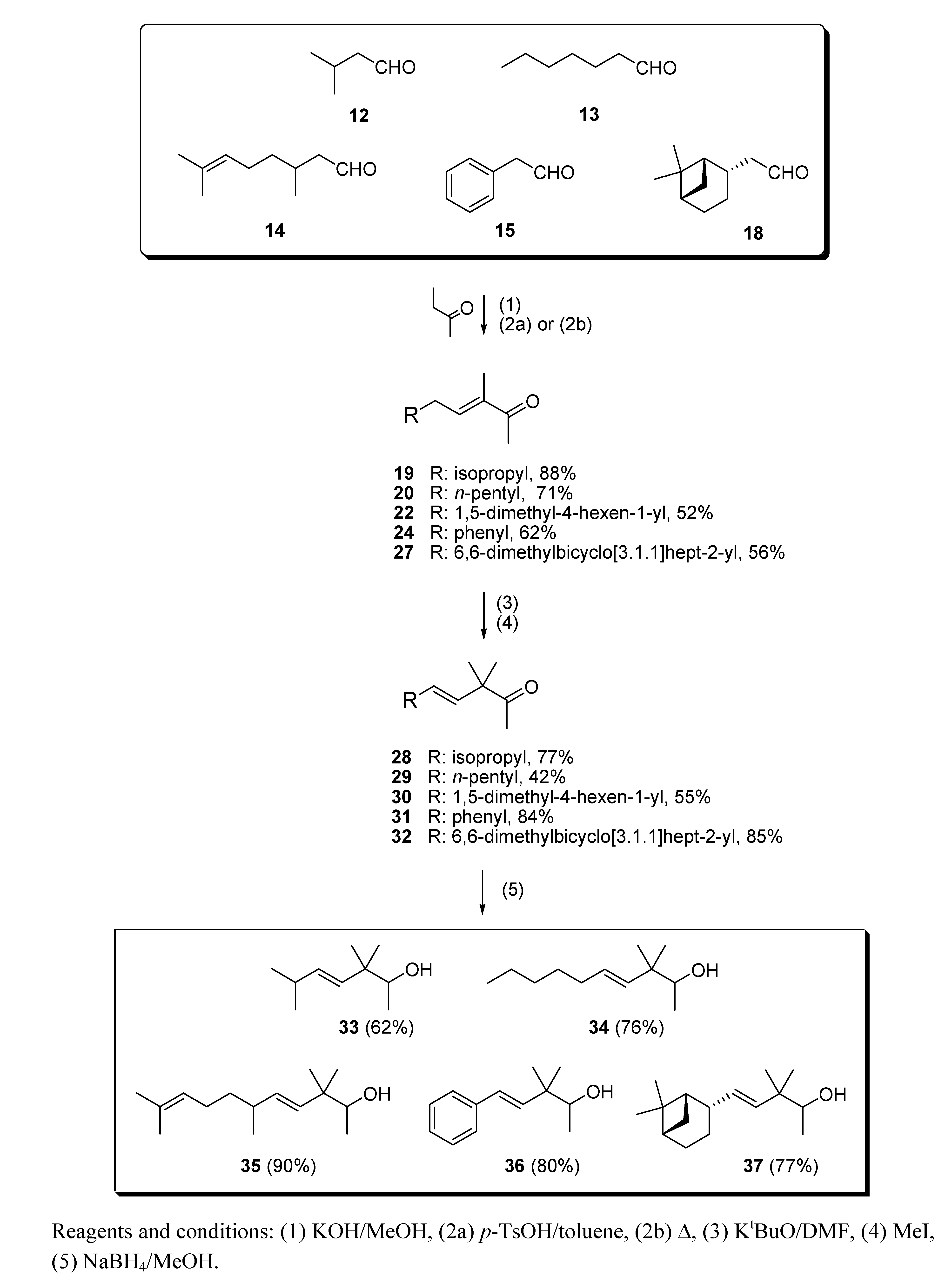
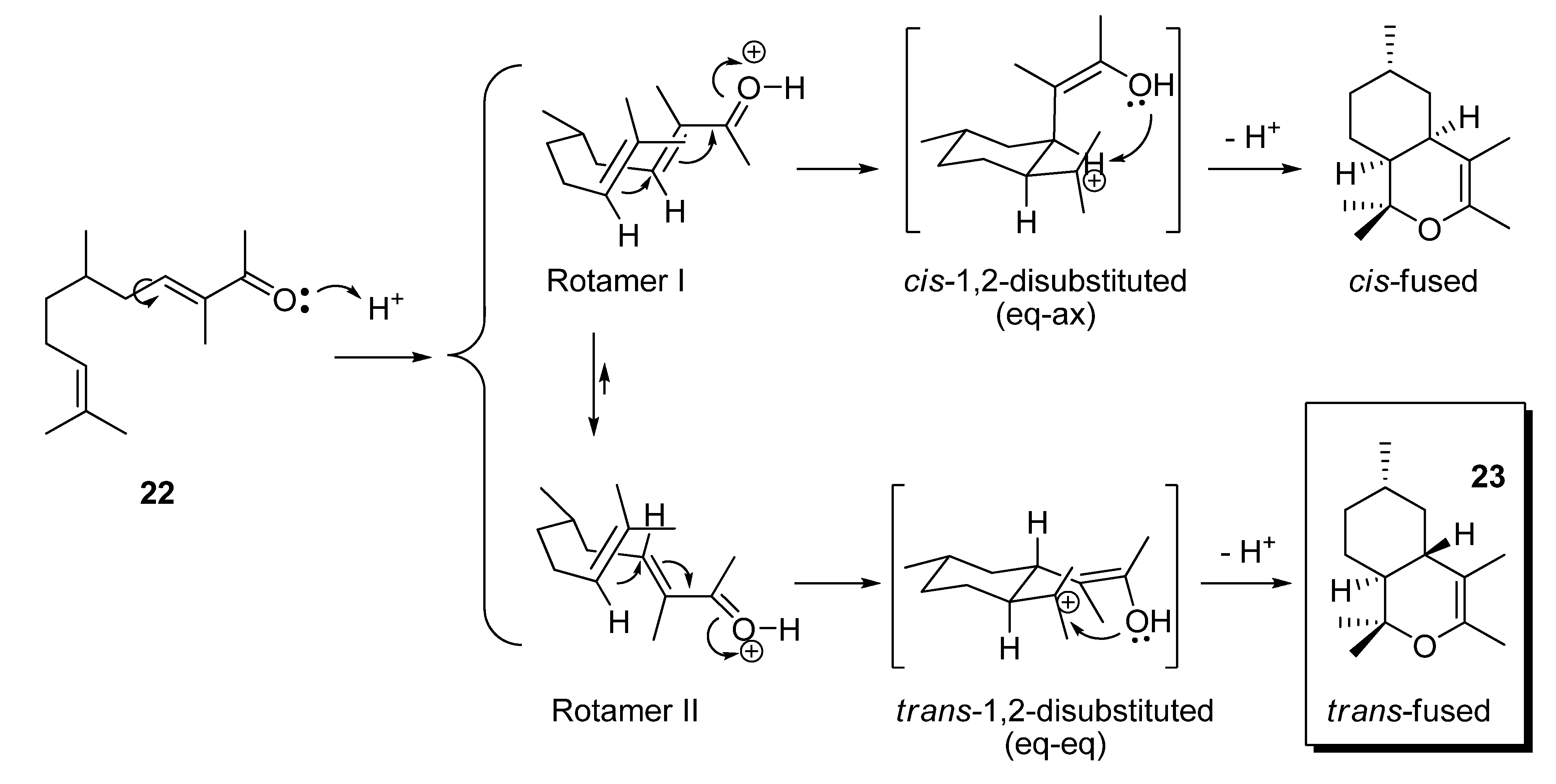
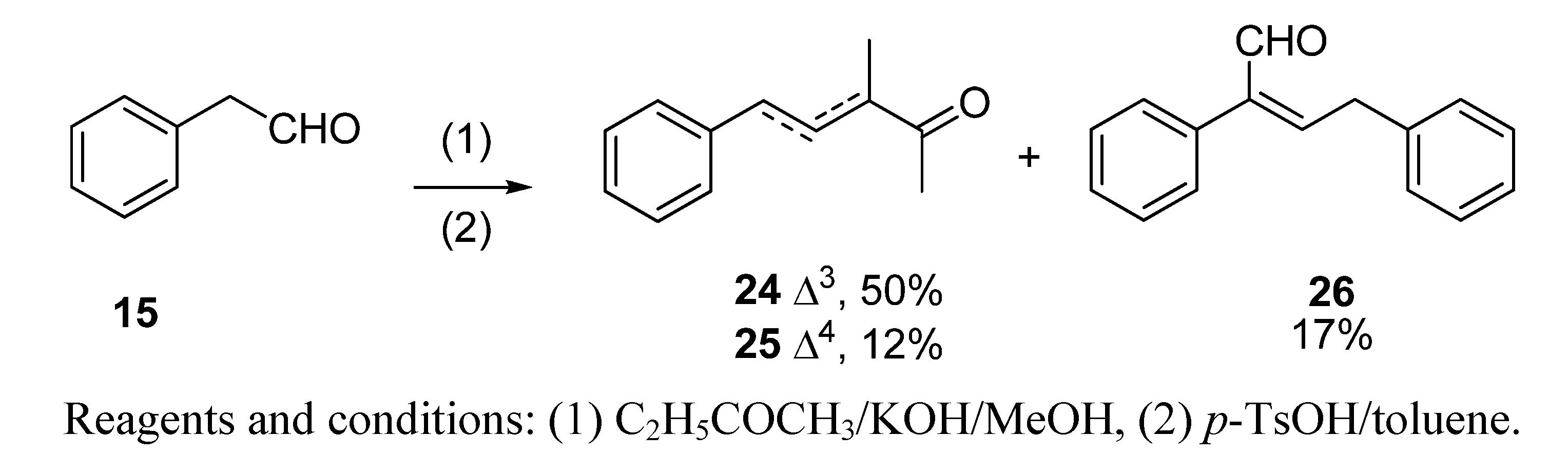
2.1.3. Deconjugative α-methylation of the α, β-unsaturated ketones 19, 20, 22, 24 and 27 to give the β, γ-unsaturated ketones 28–32
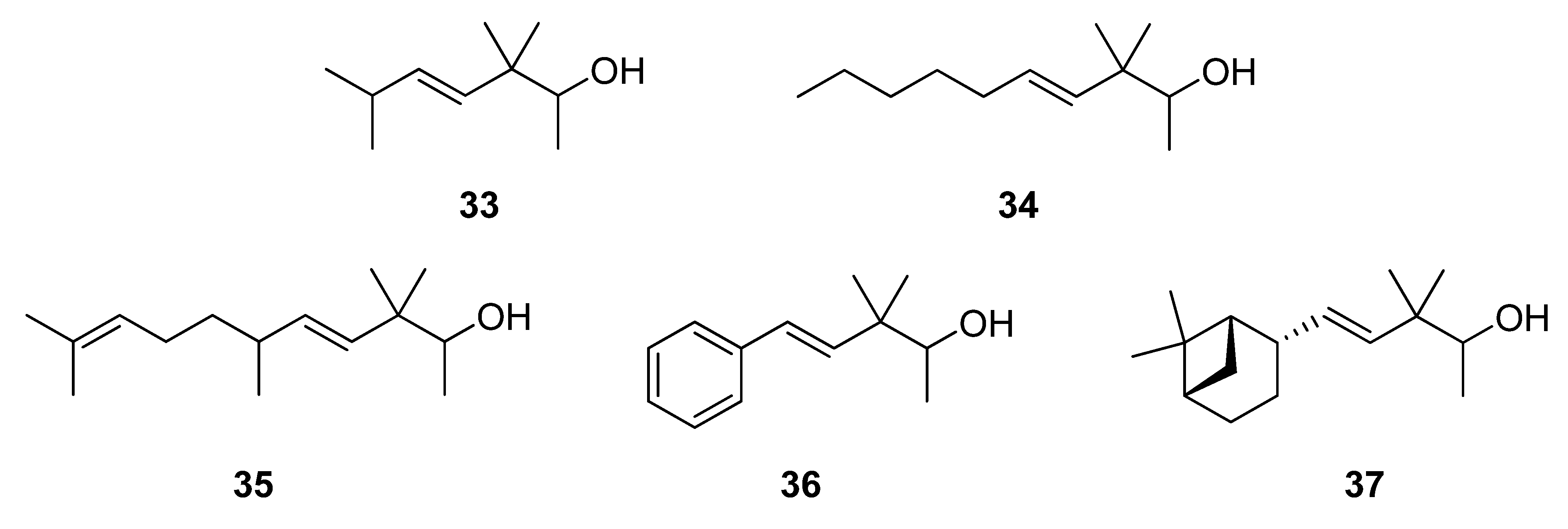
2.1.4. Reduction of the β,γ-unsaturated ketones 28–32 with NaBH4 to give the alcohols 33–37
2.2. Odour evaluation
| Compounds | Odour | ||
|---|---|---|---|
| Top notes | Heart notes | Base notes | |
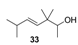 | Turpentine, varnish and woody with oriental bottom, patchouli, humid tar, smoky and earthy | Nuance of woody, slightly damp in a mixture between the scent of fresh wood and antique furniture | Slightly eastern woody, not very intense |
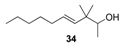 | Greasy, citrus and earthy-green notes suitable with a moist mushroom scent | Citronellic-type of citrus odour with weak woody | Green and grassy resembling to freshly cut stalk of palms |
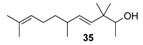 | Phenolic, dump, cresolic, milky and sandela flavour | weak citrus odour | almost odourless |
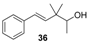 | Cresolic, citrus on citronella-type odours, green, phenolic and slightly exotic oriental odour | Slight woody note with flowery touch of roses, but less intense | Imperceptible odour (nearly odourless) |
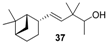 | Solvent and woody note, sandalwood-type alike to the essential oil | Sandal and sandela scent | Reminiscence of sandalwood odour with saffron touch |
| Compounds | Odour |
|---|---|
 | Borneol, balsamic, camphoraceus woody notes, but not sandalwood. Also fencholic, slightly valerianic with a note which remembers to wet mossy forest soil at the end. |
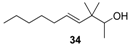 | Woody notes with dryness and amber nuances Iso E Super-type. Also fatty, green, floral and soapy notes, with an animalic and valerianic tone at the end. |
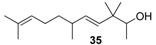 | Very clean and natural sandalwood note, Polysantol-type and as intense as this. The woody bouquet is harmonized with amber, balsamic, animalic, sweet, green and a slightly cresolic background. |
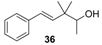 | Woody and mild sandalwood scent. It is also slightly greasy with burn, moist and green nuances, and a reminiscent of citrus fruits at the end. |
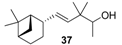 | Very clean, intense and rounded sandalwood note, more natural scent than Polysantol-type. It is also woody, vetiver, green in mossy-type, with animal and vanilla notes at the end. |
3. Experimental
3.1. General
3.2. Starting materials
3.3. Aldol condensation of 12−15 and 18 with butanone to give 19, 20, 22, 24 and 27
3.4. Deconjugative α-methylation of 19, 20, 22,24 and 27 to give 28−32
3.5. Reduction of 28–32 with NaBH4 to give 33–37
3.6. Sensory evaluation
4. Conclusions
Acknowledgements
- Samples Availability: Samples of the compounds are available from the authors.
References and Notes
- Brunke, E.J.; Klein, E. Fragrance Chemistry, The Science of the Sense of Smell; Academic Press: New York, NY, USA, 1982; p. 397. [Google Scholar]
- Ohloff, G.; Winter, B.; Fehr, C. Perfumes. Art, Science and Technology; Elsevier Applied Science: London, UK, 1991; p. 287. [Google Scholar]
- Fráter, G.; Bajgrowicz, J.A.; Kraft, P. Fragrance chemistry. Tetrahedron 1998, 54, 7633–7703. [Google Scholar]
- Naipawer, R.E. Cyclopentene derivatives and their use as odorants. US4696766.
- Chapuis, C.; Gautier, A.; Blanc, P.A. Use of optically active isomers of (E)-3,3-dimethyl-5-(2,2,3-trimethyl-3-cyclopenten-1-yl-)-4-penten-2-ol in perfumery. US5512543.
- Buchbauer, G.; Spreitzer, H.; Zechmeister-Machhart, F.; Klinsky, A.; Weiss-Greiler, P.; Wolschann, P. Synthesis and olfactoric activity of keto-β-santalol and methoxy-β-santalol. Eur. J. Med. Chem. 1998, 33, 463–470. [Google Scholar]
- Buchbauer, G.; Stappen, I.; Pretterklieber, C.; Wolschann, P. Structure–activity relationships of sandalwood odorants: synthesis and odor of tricyclo β-santalol. Eur. J. Med. Chem. 2004, 39, 1039–1046. [Google Scholar]
- Hölscher, B.; Braun, N.A.; Weber, B.; Kappey, C.H.; Meier, M.; Pickenhagen, W. Enantioselectivity in odor perception synthesis and olfactory properties of the new tricyclic sandalwood odorant Fleursandol. Helv. Chim. Acta 2004, 87, 1666–1680. [Google Scholar]
- Axel, R. Scents and sensibility: A molecular logic of olfactory perception (Nobel Lecture). Angew. Chem. Int. Ed. 2005, 44, 6111–6127. [Google Scholar]
- Buck, L. Unraveling the sense of smell (Nobel Lecture). Angew. Chem. Int. Ed. 2005, 44, 6128–6140. [Google Scholar]
- Buchbauer, G.; Hillisch, A.; Mraz, K.; Wolschann, P. Conformational parameters of the sandalwood-odor activity: conformational calculations on sandalwood odor Part X. Helv. Chim. Acta 1994, 77, 2286–2296. [Google Scholar]
- Bajgrowicz, J.A.; Frank, I.; Fráter, G. Synthesis and structure elucidation of a new potent sandalwood-oil substitute. Helv. Chim. Acta 1998, 81, 1349–1358. [Google Scholar]
- Auger, B.; Bajgrowicz, J.A.; Giraudi, E. Preparation of formylpinanes for fragrances. WO9311094.
- Chapuis, C. Preparation of 3,3-dimethyl-5-(2,2,3-trimethyl-3-cyclohexenyl)-4-penten-2-ol enantiomers and analogs as perfume fragrances. EP572797.
- Dimoglo, A.S.; Beda, A.A.; Shvets, N.M.; Gorvachov, M.Y.; Kheifits, L.A.; Aulchenko, I.S. Investigation of the relationship between sandalwood odor and chemical structure: Electron-topological approach. New J. Chem. 1995, 19, 149–154. [Google Scholar]
- Wahren, U.; Sprung, I.; Schulze, K.; Findensen, M.; Buchbauer, G. Synthesis of syn- and anti-epoxides of α-campholenic and fencholenic derivatives. Tetrahedron Lett. 1999, 40, 5991–5992. [Google Scholar]
- Ono, S.; Etsuno, J.; Fukuda, K.; Toi, S.; Fujikura, Y. 3-(3,3,5-Trimethylcyclohexyloxy)-1-propanols and perfume compositions containing them. JP 7165655.
- Schulze, K.; Uhlig, H. Aroma chemical syntheses with fencholenic aldehyde. Monatsh. Chem. 1989, 120, 547–559. [Google Scholar]
- Linares-Palomino, P.J.; Salido, S.; Altarejos, J.; Nogueras, M.; Sánchez, A. Synthesis and odor evaluation of stereoisomers of octahydrobenzopyran derivatives. Flavour Fragr. J. 2006, 21, 659–666. [Google Scholar]
- Linares-Palomino, P.J.; Salido, S.; Altarejos, J.; Sánchez, A. Chlorosulfonic acid as a convenient electrophilic olefin cyclization agent. Tetrahedron Lett. 2003, 44, 6651–6655. [Google Scholar]
- Castro, J.M.; Salido, S.; Altarejos, J.; Nogueras, M.; Sánchez, A. Synthesis of Ambrox from labdanolic acid. Tetrahedron 2002, 58, 5941–5949. [Google Scholar]
- Castro, J.M.; Linares-Palomino, P.J.; Salido, S.; Altarejos, J.; Nogueras, M.; Sánchez, A. Synthesis of Polysantol and related sandalwood-type odorants using magnesium α-bromoketone enolates. Tetrahedron Lett. 2004, 45, 2619–2622. [Google Scholar]
- Castro, J.M.; Linares-Palomino, P.J.; Salido, S.; Altarejos, J.; Nogueras, M.; Sánchez, A. Enantiospecific synthesis, separation and olfactory evaluation of all diastereomers of a homologue of the sandalwood odorant Polysantol. Tetrahedron 2005, 61, 11192–11203. [Google Scholar]
- Chapado, L.; Linares-Palomino, P.J.; Salido, S.; Nogueras, M.; Sánchez, A.; Altarejos, J. Preparation of a novel odorant with sandalwood fragrance. WO 2008092981.
- Höfinghoff, J.; Buchbauer, G.; Holzer, W.; Wolschann, P. Syntheses and odor of “bulky group”-modified sandalwood odorants: isophorono-β-santalol analogues. Eur. J. Med. Chem. 2006, 41, 905–913, and references cited therein. [Google Scholar]
- (1R)-(–)-Nopol is a commercially available compound, but it can be prepared by reaction of β-pinene with formaldehyde [28].
- Heitmann, W.; Mätzel, U.; Blanc, P.A. Preparation of cis-dihydronopol. EP406742.
- Eigenmann, G.W.; Arnold, R.T. Stereospecific hydrogenation of α-pinene derivatives. J. Am. Chem. Soc. 1959, 81, 3440–3442. [Google Scholar]
- Corey, E.J.; Schmidt, G. Useful procedures for the oxidation of alcohols involving pyridinium dichromate in aprotic media. Tetrahedron Lett. 1979, 20, 399–402. [Google Scholar]
- We also obtained cis-dihydronopol (17) by hydrogenation of nopol (1 g) in absolute MeOH (50 mL) over Raney-Ni (1.5 g) under pressure of H2 gas on a hydrogenation apparatus for 48 h. The GC analysis indicated a conversion of ca. 82% [24].
- Kim, K.Y.; Lee, S.G. Complete assignment of 1H and 13C NMR spectra of trans- and cis-myrtanol. Magn. Reson. Chem. 1997, 35, 451–454. [Google Scholar]
- Badjah-Hadj-Ahmed, A.Y.; Meklati, B.Y.; Waton, H.; Pham, Q.T. Structural studies in the bicyclo[3.1.1]heptane series by proton and carbon-13 NMR. Magn. Reson. Chem. 1992, 30, 807–816. [Google Scholar]
- Azzaroni, F.; Biscarini, P.; Bordoni, S.; Longoni, G.; Venturini, E. Catalytic hydroformylation of (1S,5S)-(–)- and (1R,5R)-(+)-β-pinene: stereoselective synthesis and spectroscopic characterization of (1S,2R,5S)-, (1S,2S,5S)-, (1R,2R,5R)- and (1R,2S,5R)-10-formylpinan. J. Organomet. Chem. 1996, 508, 59–67. [Google Scholar]
- Tishchenko, I.G.; Bubel, O.N.; Kovaleva, A.F. Use of optically active isomers of (E)-3,3-dimethyl-5-(2,2,3-trimethyl-3-cyclopenten-1-yl-)-4-penten-2-ol in perfumery. US5512543.
- Naipawer, R.E.; Easter, W.M. 3-Methyl-5-(2,2,3-trimethylcyclopent-3-en-1-yl)pentan-2-ol compound and perfume compositions. US4052341.
- Zhao, H.; Cai, M.Z. Stereoselective synthesis of (Z)-α,β-unsaturated ketones via hydromagnesiation of alkynylsilanes. Synth. Commun. 2003, 33, 1643–1650. [Google Scholar]
- Mahrwald, R.; Schick, H. Synthesis of α,β-unsaturated carbonyl compounds by titanium tetraalkoxide-induced aldol condensation under neutral conditions. Synthesis 1990, 7, 592–595. [Google Scholar]
- .
- Robert, F.; Héritier, J.; Quiquerez, J.; Simian, H.; Blank, I. Synthesis and sensorial properties of 2-alkylalk-2-enals and 3-(acetylthio)-2-alkyl alkanals. J. Agric. Food Chem. 2004, 52, 3525–3529. [Google Scholar]
- Sasaki, K. A modified Wittig synthesis of 6,10-dimethyl-3,9-undecadien-2-one, ethyl 5,9-dimethyl-2,8-decadienonate, and their α-alkyl homologs: stereochemistry of the reaction and conformation of the products. Bull. Chem. Soc. Jpn. 1968, 41, 1252–1254. [Google Scholar]
- Sasaki, K. Cyclization of 6,10-dimethyl-3,9-undecadien-2-one and its homologs by concentrated phosphoric acid. Nippon Kagaku Zasshi 1968. [Google Scholar]
- Dana, G.; Gharbi-Benarous, J.; Thuan, S.L.T. Dehydration of α,β-ethylenic 1,2-diols. IV. Role of the stereomutation of α-hydroxyl allylic carbocations on the orientation of observed reactions. Can. J. Chem. 1980, 58, 1451–1462. [Google Scholar]
- Sato, T.; Watanabe, M.; Watanabe, T.; Onoda, Y.; Murayama, E. Trisubstituted stannyllithium as a double electron equivalent. Reaction with α,β-enones. J. Org. Chem. 1988, 53, 1894–1899. [Google Scholar]
- Kim, J.H.; Kulawiec, R.J. A Tandem epoxide isomerization-aldol condensation process catalyzed by palladium acetate-tributylphosphine. J. Org. Chem. 1996, 61, 7656–7657, and references cited therein. [Google Scholar]
- .
- Chang, S.; Yoon, J.; Brookhart, M. Carbon-carbon bond forming reactions of η3-allyl iron tricarbonyl anions with carbon electrophiles. J. Am. Chem. Soc. 1994, 116, 1869–1879. [Google Scholar]
- Mookherjee, B.D.; Trenkle, R.W.; Wolff, R.K.; Boden, R.M.; Yoshida, T. Use of methyl-substituted pinyl oxopentenes, for augmenting, enhancing or modifying the aroma or taste of smoking tobacco and smoking tobacco articles. US4428387.
- Naipawer, R.E. Cyclopentene derivatives and their use as odorants. EP0203528.
- Bajgrowicz, J.A.; Fráter, G. Preparation of optically pure isomers of campholenic aldehyde derivatives for use as detergent fragrances. EP0841318.
- Armesto, D.; Ortiz, M.J.; Agarrabeitia, A.R.; Martín-Fontecha, M. Novel Oxa-di-π -methane and Norrish type I reactions in the S2 (π,π*) excited state of a series of β,γ-unsaturated ketones. Org. Lett. 2005, 7, 2687–2690. [Google Scholar]
- de Graauw, C.F.; Peters, J.A.; van Bekkum, H.; Huskeus, J. Meerwein-Ponndorf-Verley reductions and Oppenauer oxidations: an integrated approach. Synthesis 1994, 10, 1007–1017. [Google Scholar]
- Yoshii, F.; Yamada, Y.; Hoshy, T.; Hagiwara, H. The creation of a database of odorous compounds focused on molecular rigidity and analysis of the molecular features of the compounds in the database. Chem. Senses 2002, 27, 399–405. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chapado, L.; Linares-Palomino, P.J.; Badía, C.; Salido, S.; Nogueras, M.; Sánchez, A.; Altarejos, J. Synthesis and Olfactory Evaluation of Bulky Moiety-Modified Analogues to the Sandalwood Odorant Polysantol®. Molecules 2009, 14, 2780-2800. https://doi.org/10.3390/molecules14082780
Chapado L, Linares-Palomino PJ, Badía C, Salido S, Nogueras M, Sánchez A, Altarejos J. Synthesis and Olfactory Evaluation of Bulky Moiety-Modified Analogues to the Sandalwood Odorant Polysantol®. Molecules. 2009; 14(8):2780-2800. https://doi.org/10.3390/molecules14082780
Chicago/Turabian StyleChapado, Laura, Pablo J. Linares-Palomino, Concepción Badía, Sofía Salido, Manuel Nogueras, Adolfo Sánchez, and Joaquín Altarejos. 2009. "Synthesis and Olfactory Evaluation of Bulky Moiety-Modified Analogues to the Sandalwood Odorant Polysantol®" Molecules 14, no. 8: 2780-2800. https://doi.org/10.3390/molecules14082780
APA StyleChapado, L., Linares-Palomino, P. J., Badía, C., Salido, S., Nogueras, M., Sánchez, A., & Altarejos, J. (2009). Synthesis and Olfactory Evaluation of Bulky Moiety-Modified Analogues to the Sandalwood Odorant Polysantol®. Molecules, 14(8), 2780-2800. https://doi.org/10.3390/molecules14082780