Abstract
A number of 5-aryl-1-methyl-4-nitroimidazoles 5a-f have been synthesized in good yields by the Suzuki coupling reaction between 5-chloro-1-methyl-4-nitroimidazole (3) and arylboronic acids 4a-f, aided by dichlorobis-(triphenylphosphine)palladium(II), K2CO3, and tetrabutylammonium bromide in water at 70-80 °C. Compounds 5a-f were characterized by elemental analysis, NMR and MS spectral data. On the basis of in vitro screening data, 5-(3-chlorophenyl)-1-methyl-4-nitro-1H-imidazole (5f) exhibited potent lethal activity against Entamoeba histolytica and Giardia intestinalis with IC50 = 1.47 µM/mL, a value lower by a factor of two than that of the standard drug, metronidazole. The boosted activity of 5f was not accompanied by any increased cytotoxicity. The rest of the series also exhibited potent antiparasitic activity with IC50 values in the 1.72-4.43 µM/mL range. The cytotoxicity of the derivatives 5c and 5e was increased compared to the precursor compound, metronidazole, although they remain non-cytotoxic at concentrations much higher than the antiparasitic concentration of the two derivatives.
Introduction
The imidazole nucleus occurs naturally in L-histidine, histamine (a vasodelator hormone), thiamine (vitamin B1), and in several other biomolecules [1,2,3,4]. An example of an imidazole-containing synthetic drugs is cimetidine (1, Figure 1), widely used for the treatment of peptic ulcers [5,6,7,8]. Nitroimidazoles have wide applications in the drug synthesis due to their biological activity. Examples include natural azomycin (antibiotic, 2-nitroimidazole) [9,10,11,12] and synthetic metronidazole (2, anti-amoebic dysentry) [13,14,15,16] (Figure 1).
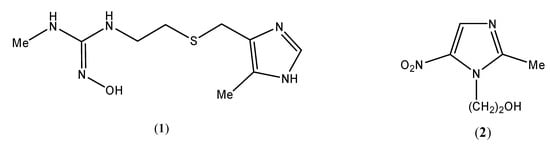
Figure 1.
Model imidazole-based synthetic drugs.
(Substituted)-4-nitroimidazoles also showed activity against various microbes and found applications in chemotherapy [17,18,19]. Thus, a series of 1-methyl-4-nitro-5-substituted imidazoles were reported to exhibit antileishmanial, antiamebic and anthelmintic activities [17]. Also, a number of synthetic 5-(substituted azolyl)-1-methyl-4-nitroimidazoles were reported to exhibit anti-parasitic activity [20,21,22].
Herein, we wish to report a one-pot synthesis of 5-aryl-1-methyl-4-nitroimidazoles 5a-f by reaction of 5-chloro-1-methyl-4-nitroimidazole (3) with arylboronic acids 4 under Suzuki reaction conditions [25,26,27,28,29,30] (Scheme 1), and the evaluation of their antiparasitic activity.
Results and Discussion
Chemistry
Previously, the synthesis of 5-aryl-1-methyl-4-nitroimidazoles (exemplified by 5a/Scheme 2) was achieved via N(1)-methylation of the respective 5(4)-aryl-4(5)-nitroimidazole (6A  6B) [23]. This step led to a mixture of 5a and its isomeric 1-methyl-4-aryl-5-nitroimidazole 7A, the isolation of which required extra separation and purification efforts. Additionally, the required synthon (6A
6B) [23]. This step led to a mixture of 5a and its isomeric 1-methyl-4-aryl-5-nitroimidazole 7A, the isolation of which required extra separation and purification efforts. Additionally, the required synthon (6A  6B) was prepared by condensation of (substituted)phenacyl chloride with formamide, followed by acidification and then neutralization with aqueous ammonium hydroxide [24].
6B) was prepared by condensation of (substituted)phenacyl chloride with formamide, followed by acidification and then neutralization with aqueous ammonium hydroxide [24].
In the present work, reaction of the commercially available 5-chloro-1-methyl-4-nitroimidazole (3) with arylboronic acids 4 under Suzuki reaction conditions provided an efficient route towards the formation of 5-aryl-1-methyl-4-nitroimidazoles 5a-f, as outlined in Scheme 1.
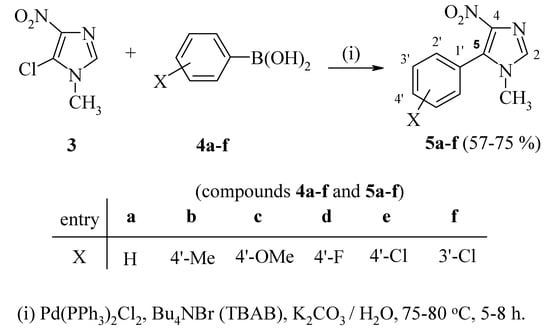
Scheme 1.
Synthesis of 5-aryl-1-methyl-4-nitroimidazoles 5a-f via Suzuki coupling.
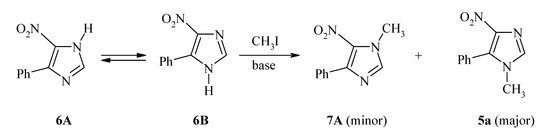
Scheme 2.
N-Methylation of 5(4)-aryl-4(5)-nitroimidazole [23].
Compared to the multistep synthetic route noted above, the direct Suzuki coupling reaction provides a more convenient and efficient route for the preparation of 5-aryl-1-methyl-4-nitroimidazoles 5a-f. Thus, treatment of 5-chloro-1-methyl-4-nitroimidazole (3) with arylboronic acids 4a-f using 3 mole % of dichloro bis-(triphenylphosphin)palladium(II), K2CO3, and tetrabutylammonium bromide in water at 70-80 °C, gave the corresponding 5-arylimidazole derivatives 5a-f [31]. These Pd-catalyzed cross-coupling reactions proceeded nicely in the presence of the ‘additive’ (TBAB). The latter is known to stabilize colloidal palladium nanoparticles that act as catalysts in the Suzuki coupling of aryl halides [32], and thus enhances the rate of the coupling reaction. The presence of the strongly electron-withdrawing nitro group on the imidazole ring facilitates the coupling process which results in good yields of 5a-f.
The microanalytical and spectral (HRMS and NMR) data, given in the Experimental part, are in accordance with the assigned structures for compounds 5a-f. Thus, the observed high resolution MS data for M+ are in good agreement with the values calculated for the respective molecular formulas. The NMR spectra displayed 1H- and 13C- signals characteristic of the respective aryl moieties introduced at the C-5. Assignments of the 1H- and 13C- signals to the different respective protons and carbons are based on DEPT and 2D (COSY, HMQC, HMBC) experiments which showed correlations consistent with these assignments. The 1H-NMR spectra for compounds 5a-f show aromatic protons for the aryl group introduced by Suzuki coupling besides the imidazole C-2 proton at ~ 7.45 ppm. The CH3 and OCH3 aryl substituents for compounds 5b and 5c appear at 2.41 and 3.85 ppm, respectively. Compound 5d containing 4'- fluorine substituent showed additional coupling caused by the 19F nucleus with a vicinal coupling 1H-19F (3J= 8.5 Hz). The 13C-NMR spectra of the prepared compounds 5a-f showed carbon signals expected for aryl groups introduced by Suzuki coupling besides the imidazole C-2, C-4 and C-5 carbons. In compound 5d, the skeletal carbons of the benzo ring at C-5 are readily recognizable by their signal splitting 'as doublets' arising from spin-spin coupling with the nearby fluorine atom at C-4' with varying JC-F values for the four different carbons C1'-C4' (1J = 250 Hz ; 2J = 22 Hz; 3J = 8.6 Hz ; 4J = 3.6 Hz). DEPT experiments were employed to differentiate primary and tertiary carbons from quaternary carbons.
Antiamoebic and antigiardial activity
The antiamoebic and antigiardial activities of the compounds 5a-f were investigated using in vitro bioassays that included the standard antiamoebic and antigiardial drug metronidazole as a control. The cytotoxicity of the compounds on the two cell lines, Hep-2 and Vero cells, was also compared with that of metronidazole. The IC50 values of the compounds against Entamoeba histolytica, Giardia intestinalis, and the two cell lines are given in Table 1. As indicated in the Table, all the tested compounds showed biological activities against Entamoeba and Giardia. Compounds 5c, 5e, and 5f showed the highest activity against the parasites, with IC50 values ranging from around one to two micromolar, compared to around four micromolar for the standard drug, metronidazole. When the cytotoxicity of the prepared molecules is considered, compound 5f appears to be the best among the prepared derivatives of metronidazole, as indicated in Table 2. This Table presents the IC50 ratio of metronidazole over that of the derivative compounds 5a-f against the parasites and the two investigated cell lines. Compound 5f was around two to three times more active than metronidazole, against Entamoeba and Giardia, respectively. Fortunately, the boosted activity of this compound was not accompanied with any increased cytotoxicity. In contrast, the cytotoxicity of the derivatives 5c and 5e has increased compared to the precursor compound, metronidazole, although they remain non-cytotoxic at concentrations much higher than the antiparasitic concentration of the two derivatives. As can be concluded from Table 1, the IC50 values of compounds 5c and 5e against the two cell lines were ≥ 230 times higher than that against the parasites under investigation.
Interestingly, the tested compounds exhibited an almost similar pattern of activity against both G. intestinalis and E. histolytica (Table 1), indicating that each compound affects both parasites by a similar mechanism of action. In addition, the molecular modifications on our derivatives did not render any of the compounds inactive. The other molecules, 5a, 5b, and 5d remained as active as metronidazole (Table 1,Table 2).
The activities exhibited by the derivatives, especially compound 5f, suggest that the derivatives may be used as new lead compounds in the development of new antiparasitic drugs. Although drug resistance to Entamoeba and Giardia does not, so far, appear to be a serious problem, occasional reports of failure with metronidazole [33] and the reported variations in drug sensitivities of isolates [34] may be alarming. Therefore, the importance of such biologically active, non-cytotoxic metronidazole derivatives, especially 5f, lies in their potential contribution to overcome the problem of resistance of pathogens to the standard drugs. Additionally, because of the limited number of drugs available in the market against anaerobic protozoal parasites and bacteria there is a serious need for new active compounds. The molecular modification on the original drugs, therefore, offers alternatives that may bypass the already developed mechanisms adopted by the anaerobic pathogens against the standard drugs. Our derivatized compounds (5c, 5e, 5f) are good drug candidates to be tested against metronidazole-resistant parasites and bacteria.

Table 1.
Antiparasitic activities of compounds 5a-f.
| Mean IC50 ± SD(n) (μM) | ||||
|---|---|---|---|---|
| Compound | Giardia intestinalis | Entamoeba histolytica | Hep-2 cells | Vero cells |
| 5a | 4.43 ± 1.97 | 4.04 ± 0.28 | 1040.27 ± 19.18 | 1748.28 ± 18.38 |
| 5b | 4.01 ± 0.75 | 3.10 ± 0.41 | 1610.74 ± 22.23 | 1633.32 ± 13.61 |
| 5c | 1.72 ± 0.57 | 1.16 ± 0.19 | 568.80 ± 22.71 | 868.24 ± 22.02 |
| 5d | 3.76 ± 0.2 | 4.39 ± 0.71 | 1894.12 ± 21.13 | 1918.41 ± 13.37 |
| 5e | 1.90 ± 0.14 | 1.56 ± 0.156 | 437.19 ± 16.39 | 725.05 ± 11.79 |
| 5f | 1.47 ± 0.14 | 1.89 ± 0.14 | 1780.21 ± 15.71 | 1783.16 ± 19.66 |
| Metronidazole | 4.39 ± 0.59 | 4.10 ± 0.78 | 2044.20 ± 26.36 | 2071.35 ± 16.37 |
SD(n): Standard deviation

Table 2.
IC50 ratios.
| IC50 Ratio | ||||
|---|---|---|---|---|
| (metronidazole/compound) | ||||
| Compound | Giardia intestinalis | Entamoeba histolytica | Hep-2 cells | Vero cells |
| 5a | 1 | 1 | 2 | 1.2 |
| 5b | 1.1 | 1.3 | 1.3 | 1.3 |
| 5c | 3.5 | 2.6 | 3.6 | 2.4 |
| 5d | 1.2 | 0.93 | 1.1 | 1.1 |
| 5e | 2.6 | 2.3 | 4.7 | 2.9 |
| 5f | 2.2 | 2.9 | 1.2 | 1.2 |
Experimental
General
The following chemicals, employed in this study, were purchased and used without further purification: 5-chloro-1-methyl-4-nitroimidazole, phenylboronic acid, 4-tolylboronic acid, 4-methoxy-phenylboronic acid, 4-flourophenylboronic acid, 4-chlorophenylboronic acid, 3-chlorophenylboronic acid, tetrabutyl ammonium bromide and dichloro bis-(triphenylphosphin)palladium(II). Melting points (uncorrected) were determined on a Gallenkamp electrothermal melting temperature apparatus. 1H- and 13C-NMR spectra were recorded on a Bruker DPX-300 instrument with TMS as internal reference. High-resolution mass spectra (HRMS) were measured in positive ion mode using electrospray ion trap (ESI) technique by collision-induced dissociation on a Bruker APEX-4 (7 Tesla) instrument. The samples were dissolved in acetonitrile, diluted in spray solution (methanol/water 1:1 v/v + 0.1% formic acid) and infused using a syringe pump with a flow rate of 2 µL/min. External calibration was conducted using Arginine cluster in a mass range m/z 175-871. IR spectra were recorded as KBr discs on a Nicolet Impact-400 FT-IR spectrophotometer. Elemental analyses were preformed at the Microanalytical Laboratory of the Hashemite University, Zarqa-Jordan, and the results were found to be in good agreement (± 0.4%) with the calculated values.
General procedure for the synthesis of 5-aryl-1-methyl-4-nitroimidazoles 5a-f
A mixture of 5-chloro-1-methyl-4-nitroimidazole 3 (4 mmol), the particular arylboronic acid 4a-f (4 mmol), Pd(PPh3)2Cl2 (3 mol%, 80 mg), powdered K2CO3 (1.4 g, 10 mmol) and Bu4NBr (1.3 g, 4 mmol) in water (3 mL) was heated with stirring at 75-80 ºC for 5-8 h. Thereafter, the reaction mixture was cooled, poured into water (25 mL) and extracted with dichloromethane (2 x 30 mL). The combined organic extracts were dried over anhydrous sodium sulfate and the solvent was removed under reduced pressure. The residue was purified by column chromatography with chloroform-methanol (95:5 v/v) to afford the respective compounds 5a-f.
1-Methyl-4-nitro-5-phenyl-1H-imidazole (5a): Yield 0.73 g (70%); mp 177-179 °C (Lit. [23] 178-180 oC). 1H-NMR (CDCl3): δ 3.51 (s, 3H, N-CH3), 7.36 (m, 2H, 2'-H + 6'-H ), 7.46 (s, 1H, 2-H), 7.50 (m, 3H, 3'-H + 4'-H+ 5'-H ); 13C-NMR (CDCl3): δ 33.1 (CH3), 126.5 (C), 128.9 (C-2'+ C-6'), 130.1 (C-3'+ C-5'), 130.2 (C-4'), 132.6 (C-5), 135.6 (C-2), 140.1 (C-4); HRMS ((+ve)-ESI): m/z calcd. for C10H9N3O2Na [M+Na]+: 226.05870, found: 226.05870; Anal. Calcd. for C10H9N3O2 (203.20): C, 59.11; H, 4.46; N, 20.68. Found: C, 59.50; H, 4.77; N, 20.54.
1-Methyl-5-(4-methylphenyl)-4-nitro-1H-imidazole (5b): Yield 0.57 g (65%); mp 179-181 °C. 1H-NMR (CDCl3): δ 2.41 (s, 3H, 4'-CH3), 3.50 (s, 3H, N-CH3), 7.25 (d, J = 8 Hz, 2H, 2'-H+ 6'-H), 7.31 (d, J = 8 Hz, 2H, 3'-H+ 5'-H), 7.45 (s, 1H, 2-H); 13C-NMR (CDCl3): δ 21.6 (4'-CH3), 33.1 (N-CH3), 115.0 (C-1'), 123.4 (C-4'), 129.6 (C-3'+ C-5'), 129.9 (C-2'+ C-6'), 130.6 (C-5), 135.6 (C-2), 140.5 (C-4); HRMS ((+ve)-ESI): m/z calcd. for C11H11N3O2Na [M+Na]+: 240.07435, found: 240.07435; Anal. Calcd. for C11H11N3O2 (217.22): C, 60.82; H, 5.01; N, 19.34. Found: C, 61.03; H, 5.15; N, 19.05.
5-(4-Methoxyphenyl)-1-methyl-4-nitro-1H-imidazole (5c): Yield 0.70 g (75%); mp 182-184 °C. 1H-NMR (CDCl3): δ 3.51 (s, 3H, N-CH3), 3.86 (s, 3H, O-CH3), 7.01 (d, J = 8.8 Hz, 2H, 3'-H+ 5'-H ), 7.31 (d, J = 8.8 Hz, 2H, 2'-H+ 6'-H), 7.45 (s, 1H, 2-H); 13C-NMR (CDCl3): δ 33.1 (N-CH3), 55.5 (O-CH3), 114.4 (C-3'+ C-5'), 118.2 (C-1'), 131.6 (C-2'+ C-6'), 132.6 (C-5), 135.4 (C-2), 141.0 (C-4), 160.9 (C-4'); ); HRMS ((+ve)-ESI): m/z calcd. for C11H12N3O3 [M+H]+: 234.08732, found: 234.08732; Anal. Calcd. for C11H11N3O3 (233.22): C, 56.65; H, 4.75; N, 18.02. Found: C, 56.72; H, 4.82; N, 17.78.
5-(4-Fluorophenyl)-1-methyl-4-nitro-1H-imidazole (5d): Yield 0.65 g (73%); mp 137-139 °C. 1H-NMR (CDCl3): δ 3.52 (s, 3H, N-CH3), 7.20 (m, 2H, 2'-H + 6'-H), 7.38 (m, 2H, 3'-H + 5'-H), 7.47 (s, 1H, H-2); 13C-NMR (CDCl3): δ 33.1 (CH3), 116.3 (d, 2JC-F = 22 Hz, C-3'+ C-5'), 122.4 (d, 4JC-F = 3.6 Hz, C-1'), 131.0 (C-5), 132.3 (d, 3JC-F = 8.6 Hz, C-2'+ C-6'), 135.6 (C-2), 141.0 (C-4), 163.7(d, 1JC-F = 250 Hz, C-4'); HRMS ((+ve)-ESI): m/z calcd. for C10H8FN3O2Na [M+Na]+: 244.04928, found: 244.04928; Anal. Calcd. for C10H8FN3O2 (221.19): C, 54.30; H, 3.65; N, 19.00. Found: C, 53.98; H, 3.49; N, 18.80.
5-(4-Chlorophenyl)-1-methyl-4-nitro-1H-imidazole (5e): Yield 0.54 g (57%); mp 237-239 °C (Lit. [24] 238-240 °C).1H-NMR (CDCl3): δ 3.52 (s, 3H, N-CH3), 7.32 (d, J = 8.6 Hz, 2H, 2'-H + 6'-H), 7.48 (s, 1H, 2-H), 7.49 (d, J = 8.6 Hz, 2H, 3'-H + 5'-H); 13C-NMR (CDCl3): δ 33.2 (CH3), 116.6 (C-1'), 124.9 (C-4'), 129.3 (C-2'+ C-6'), 130.2 (C-5), 131.5 (C-3'+ C-5'), 135.8 (C-2), 141.1 (C-4); HRMS ((+ve)-ESI): m/z calcd. for C10H9ClN3O2 [M+H]+: 238.03778, found: 238.03778; Anal. Calcd. for C10H8ClN3O2 (237.64): C, 50.54; H, 3.39; N, 17.68. Found: C, 50.33; H, 3.28; N, 17. 32.
5-(3-Chlorophenyl)-1-methyl-4-nitro-1H-imidazole (5f): Yield 0.60 g (63%); mp 170-172 °C. 1H-NMR (CDCl3): δ 3.50 (s, 3H, N-CH3), 7.26 (m, 1H), 7.36 (m, 1H), 7.45 (s, 1H, 2-H), 7.48 (m, 2H); 13C-NMR (CDCl3): δ 33.2 (CH3), 120.3 (C-1'), 128.4 (C-6'), 130.1 (C-2'), 130.3 (C-4'), 130.5 (C-5'), 130.9 (C-5), 134.9 (C-3'), 135.8 (C-2), 141.4 (C-4); HRMS ((+ve)-ESI): m/z calcd. for C10H9ClN3O2 [M+H]+: 238.03778, found: 238.03778; Anal. Calcd. for C10H8ClN3O2 (237.64): C, 50.50; H, 3.39; N, 17.68. Found: C, 50.90; H, 3.18; N, 17.75.
Biological Activity
Test organisms
Entamoeba histolytica HK-9 strain (ATCC number 30015) was cultured in LYI-S-2 medium supplemented with antibiotics. Giardia intestinalis WB strain (ATCC number 30957) was grown in a modified YI-S medium with antibiotics. Both parasites were grown as described [35]. Briefly, the parasites were cultivated and maintained in 15-mL screw-capped borosilicate glass tubes. Entamoeba and Giardia were harvested from confluent cultures by chilling of the tubes on ice, followed by centrifugation.
Antiamoebic and antigiardial activity
The antiamoebic and antigiardial activities of the prepared molecules and metronidazole as the standard antiamoebic and antigiardial drug were tested as described [36]. Briefly, the tested compounds and metronidazole were dissolved in dimethyl sulfoxide (DMSO) then in medium and filter-sterilized. Two-fold dilutions starting at 15 μg/mL were prepared in a final volume of 15 mL to exclude air from the tube. Each tube was inoculated with 20,000 cells of the parasite under testing (Entamoeba or Giardia). Each compound was assayed in duplicate in each of three independent experiments. In each assay, the appropriate controls were performed, including the one without any compound and another with metronidazole as the positive control. The biological activity of the compounds was evaluated by counting the parasites in each tube using the standard hemacytometer. In each count, trypan blue was employed to distinguish live from dead parasites [37].
Cytotoxicity assay
The cytotoxicity of the reported compounds and the reference drug, metronidazole, was investigated on Hep-2 and Vero cells using the standard cytotoxicity assay and the trypan blue exclusion method as described before [36]. Briefly, 100 μL portions of each cell suspension were added to the wells of 96-well plates, incubated for 24 h, and the medium in each well was then replaced with 150 μL fresh medium. Solutions of the compounds or the reference drug were dissolved in DMSO, prepared in medium, and filter sterilized. Then, 150 μL-two fold serial dilutions of each of the compounds and the reference drug starting at a concentration of 2,000 μg/mL in culture medium were prepared in the plates. After 48 hour incubation, the number of cells in each well was determined a hemacytometer. Each compound was assayed in duplicate in each of three independent experiments. In each assay the negative controls (without any compound or reference drug) were included in duplicates.
Conclusions
In conclusion, we have described the synthesis of a number of 5-aryl-1-methyl-4-nitroimidazoles 5a-f with promising antiparasitic activity. Bioassay of these compounds indicated significant antiparasitic activities against Entamoeba histolytica and Giardia intestinalis that they could be used as lead structures for the development of antiparasitic drugs. The IC50 of the hybrid molecule 5f was found to be about three times lower than that of the standard drug metronidazole against those parasites and it could therefore be considered as a good drug candidate to be tested against metronidazole-resistant parasites and possibly anaerobic bacteria.
Acknowledgements
H. A. Saadeh and M. M. El-Abadelah are grateful to the Deanship of Scientific Research at the University of Jordan for financial support.
- Samples Availability: Samples of compounds 5a-f are available from the authors.
References and Notes
- Boiani, M.; Gonzalez, M. Imidazole and benzimidazole derivatives as chemotherapeutic agents. Mini-Rev. Med. Chem. 2005, 5, 409–424. [Google Scholar] [CrossRef]
- De Luca, L. Naturally occurring and synthetic imidazoles: Their chemistry and their biological activities. Curr. Med. Chem. 2006, 13, 1–23. [Google Scholar]
- Du, H.; He, Y.; Rasapalli, S.; Lovely, C.J. Newmethods of imidazole functionalization from imidazole to marine alkaloids. Synlett 2006, 7, 965–992. [Google Scholar]
- Grimmett, M.R. Imidazoles and their benzo derivatives. In Comprehesive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Potts, K.T., Eds.; Pergamon Press: Oxford, UK, 1984; Vol. 5 (Part 4A), pp. 345–497. [Google Scholar]
- Ganellin, C.R. Discovery of the antiulcer drug Tagamet. Drug Discov. Dev. 2006, 1, 295–311. [Google Scholar] [CrossRef]
- Silverman, R.A. The Organic Chemistry of Drug Design and Drug Action; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; p. 159. [Google Scholar]
- Yokoyama, M.; Aono, H.; Takeda, A.; Morita, K. Cimetidine for chronic calcifying tendinitis of the shoulder. Reg. Anesth. Pain Med. 2003, 28, 248–252. [Google Scholar]
- Matsuo, Y. Pharmacology of cimetidine. Kansen Ensho Men'eki 1983, 13, 217–228. [Google Scholar]
- Muller, C.E. Basic chemistry of 2-nitroimidazoles (azomycin derivatives). Dev. Nucl. Med. 1999, 33, 47–59. [Google Scholar]
- Hori, H.; Jin, C.Z.; Kiyono, M.; Kasai, S.; Shimamura, M.; Inayama, S. Design, synthesis, and biological activity of anti-angiogenic hypoxic cell radiosensitizer haloacetylcarbamoyl-2-nitroimidazoles. Bioorg. Med. Chem. 1997, 5, 591–599. [Google Scholar] [CrossRef]
- Lawton, C.A.; Coleman, C.N.; Buzydlowski, J.W.; Forman, J.D.; Marcial, V.A.; DelRowe, J.D.; Rotman, M. Results of a phase II trial of external beam radiation with etanidazole (SR 2508) for the treatment of locally advanced prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 673–680. [Google Scholar] [CrossRef]
- Maurin, M.B.; Rowe, S.M.; Field, K.S.; Swintosky, R.C.; Hussain, M.A. Solubility behavior, phase transition, and structure-based nucleation inhibition of etanidazole in aqueous solutions. Pharm. Res. 1996, 13, 1401–1405. [Google Scholar] [CrossRef]
- Bendesky, A.; Menendez, D. Metronidazole: A comprehensive review. Rev. Fac. Med. U.N.A.M. 2001, 44, 255–259. [Google Scholar]
- Freeman, C.D.; Klutman, N.E.; Lamp, K.C. Metronidazole: A therapeutic review and update. Drugs 1997, 54, 679–708. [Google Scholar] [CrossRef]
- Goldman, P.; Wuest, J.D. Reactions of nitroimidazoles. Nucleophilic substitution of the nitro group. J. Am. Chem. Soc. 1981, 103, 6224–6226. [Google Scholar] [CrossRef]
- Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Metronidazole in anaerobic infections: A review of its activity, pharmacokinetics and therapeutic use. Drugs 1978, 16, 387–417. [Google Scholar] [CrossRef]
- Mukherjee, A.; Kumar, S.; Seth, M.; Bhaduri, A.P. Synthesis of 1-methyl-4-nitro-5- substituted imidazole and substituted imidazolothiazole derivatives as possible antiparasitic agents. Indian J. Chem. Section B: Org. Chem. Incl. Med. Chem. 1989, 28B, 391–396. [Google Scholar]
- Thomas, A.H. Suggested mechanisms for the antimycotic activity of the polyene antibiotics and the N-substituted imidazoles. J. Antimicrob. Chemother. 1986, 17, 269–279. [Google Scholar] [CrossRef]
- Egolf, R.A.; Heindel, N.D. The synthesis of aryl 4-nitro-5-imidazolyl sulfone radiation sensitizers sterically protected against glutathione reaction. J. Heterocycl. Chem. 1991, 28, 577–582. [Google Scholar] [CrossRef]
- Shafiee, A.; Shahocini, S. Nitroimidazoles. V. Synthesis of 1-methyl-2-(2-methyl-4-thiazolyl)-nitroimidazoles. J. Heterocycl. Chem. 1989, 26, 1627–1629. [Google Scholar]
- Boechat, N.; Carvalho, A.S.; Fernandes-Ferreira, E.; Soares, R.O.A.; Souza, A.S.; Gibaldi, D.; Bozza, M.; Pinto, A.C. Novel nitroimidazoles with trypanocidal and cell growth inhibition activities. Cytobios 2001, 105, 83–90. [Google Scholar]
- Carvalho, A.S.; Gibaldi, D.; Pinto, A.C.; Bozza, M.; Boechat, N. Synthesis and trypanocidal evaluation of news 5-[N-(3-(5-substituted)-1,3,4-thiadiazolyl)]amino-1- methyl-4-nitroimidazoles. Lett. Drug Design Discov. 2006, 3, 98–101. [Google Scholar] [CrossRef]
- Ehlhardt, W.J.; Beaulieu, B.B.; Goldman, P. Nitrosoimidazoles: Highly bactericidal analogs of 5-nitroimidazole drugs. J. Med. Chem. 1988, 31, 323-329, and references cited therein. [Google Scholar] [CrossRef]
- Fitzmaurice, C.; Lord, G.H. Substituted imidazoles. Brit. Pat. GB 1 046 248, 1966. [Chem. Abstr. 1967, 66, 37927]. [Google Scholar]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Stanforth, S.P. Catalytic cross-coupling reactions in biaryl synthesis. Tetrahedron 1998, 54, 263–303. [Google Scholar] [CrossRef]
- Chemeler, S.R.; Trauner, D.; Danishefsky, S.J. The B-alkyl Suzuki-Miyaura cross- coupling reaction: Development, mechanistic study, and applications in natural product synthesis. Angew. Chem. Int. Ed. Engl. 2001, 40, 4544–4568. [Google Scholar] [CrossRef]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef]
- Kotha, S.; Lahiri, K.; Kashinath, D. Recent applications of the Suzuki-Miyaura cross-coupling reaction in organic synthesis. Tetrahedron 2002, 58, 9633–9695. [Google Scholar] [CrossRef]
- Zapf, A. Coupling of aryl and alkyl halides with organoboron reagents (Suzuki Reaction). In Transition Metals for Organic Synthesis, 2nd; Beller, M., Bolm, C., Eds.; Wiley-VCH: Weinheim, Germany, 2004; pp. 211–229. [Google Scholar]
- Shortly after completion of the present work, a synthesis of 4,5-diaryl-1-methylimidazoles by Pd-catalyzed direct coupling reaction has been reported: Bellina, F.; Cauteruccio, S.; Di Fiore, A.; Rossi, R. Regioselective synthesis of 4,5-diaryl-1-methyl-1H-imidazoles including highly cytotoxic derivatives by Pd-catalyzed direct C-5 arylation of 1-methyl-1H-imidazole with aryl bromides. Eur. J. Org. Chem. 2008, 32, 5436–5445.
- Reetz, M.T.; Westermann, E. Phosphane-free palladium-catalyzed coupling reactions: The decisive role of Pd nanoparticles. Angew. Chem. Int. Ed. Engl. 2000, 39, 165–168. [Google Scholar] [CrossRef]
- Knight, R. The chemotherapy of amebiasis. J.Antimicrob. Chemother. 1980, 6, 577–593. [Google Scholar] [CrossRef]
- Majewska, A.C.; Kasprzak, W.; De Jonckheere, J.F.; Kaczmarek, E. Heterogeneity in the sensitivity of stocks and clones of Giardia to metronidazole and ornidazole. Trans. R. Soc. Trop. Med. Hyg. 1991, 85, 67–69. [Google Scholar] [CrossRef]
- Clark, C.G.; Diamond, L.S. Methods for cultivation of luminal parasitic protists of clinical importance. Clin. Microbiol. Rev. 2002, 15, 329–341. [Google Scholar] [CrossRef]
- Saadeh, H.A.; Mosleh, I.M.; Mubarak, M.S. Synthesis of novel hybrid molecules from precursors with known antiparasitic activity. Molecules 2009, 14, 1483–1494. [Google Scholar] [CrossRef]
- Aley, S.B.; Zimmerman, M.; Hetsko, M.; Selsted, M.E.; Gillin, F.D. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect. Immun. 1994, 62, 5397–5403. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).