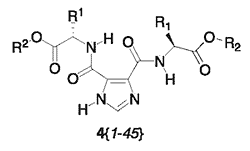
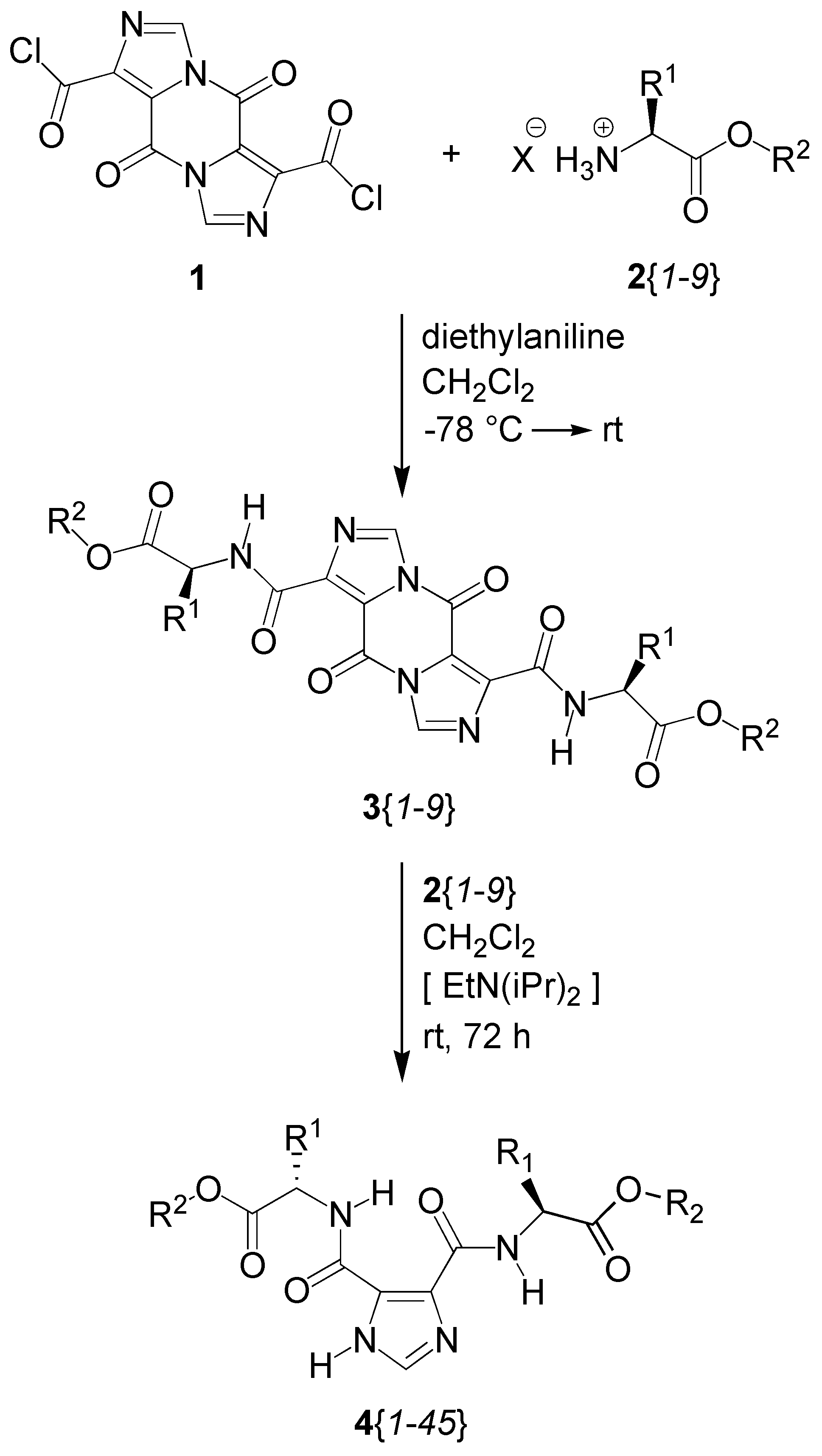
Parallel Synthesis of a Library of Symmetrically- and Dissymmetrically-disubstituted Imidazole-4,5-dicarboxamides Bearing Amino Acid Esters
Abstract
Introduction
Results and Discussion
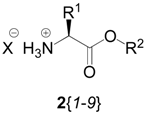 | |||
|---|---|---|---|
| Member | R1 | R2 | X  |
| 2{1} | H | C(CH3)3 | Cl |
| 2{2} | H | CH2Ph | Cl |
| 2{3} | CH3 | C(CH3)3 | Cl |
| 2{4} | CH3 | CH2Ph | Cl |
| 2{5} | CH2CH(CH3)2 | C(CH3)3 | Cl |
| 2{6} | CH2CH(CH3)2 | CH2Ph | OSO2C6H4CH3 |
| 2{7} | CH2Ph | C(CH3)3 | Cl |
| 2{8} | CH2Ph | CH2Ph | Cl |
| 2{9} | (CH2)4NH2 | C(CH3)3 | Cl |
| Cmpd. | Reactants | Yield (%) | |
|---|---|---|---|
| 4{1} | 2{1} | 2{1} | 79 |
| 4{2} | 2{2} | 2{2} | 22 |
| 4{3} | 2{3} | 2{3} | 69 |
| 4{4} | 2{4} | 2{4} | 12 |
| 4{5} | 2{5} | 2{5} | 83 |
| 4{6} | 2{6} | 2{6} | 97 |
| 4{7} | 2{7} | 2{7} | 62 |
| 4{8} | 2{8} | 2{8} | 73 |
| 4{9} | 2{9} | 2{9} | 86 |
| Cmpd. | Reactants | Yield (%) | Cmpd. | Reactants | Yield (%) | ||
|---|---|---|---|---|---|---|---|
| 4{10} | 2{1} | 2{2} | 83 | 4{28} | 2{3} | 2{7} | 54 |
| 4{11} | 2{1} | 2{3} | 86 | 4{29} | 2{3} | 2{8} | 56 |
| 4{12} | 2{1} | 2{4} | 85 | 4{30} | 2{3} | 2{9} | 77 |
| 4{13} | 2{1} | 2{5} | 91 | 4{31} | 2{4} | 2{5} | 86 |
| 4{14} | 2{1} | 2{6} | 83 | 4{32} | 2{4} | 2{6} | 97 |
| 4{15} | 2{1} | 2{7} | 78 | 4{33} | 2{4} | 2{7} | 59 |
| 4{16} | 2{1} | 2{8} | 84 | 4{34} | 2{4} | 2{8} | 90 |
| 4{17} | 2{1} | 2{9} | 61 | 4{35} | 2{4} | 2{9} | 63 |
| 4{18} | 2{2} | 2{3} | 56 | 4{36} | 2{5} | 2{6} | 94 |
| 4{19} | 2{2} | 2{4} | 70 | 4{37} | 2{5} | 2{7} | 87 |
| 4{20} | 2{2} | 2{5} | 69 | 4{38} | 2{5} | 2{8} | 85 |
| 4{21} | 2{2} | 2{6} | 55 | 4{39} | 2{5} | 2{9} | 75 |
| 4{22} | 2{2} | 2{7} | 67 | 4{40} | 2{6} | 2{7} | 88 |
| 4{23} | 2{2} | 2{8} | 64 | 4{41} | 2{6} | 2{8} | 96 |
| 4{24} | 2{2} | 2{9} | 62 | 4{42} | 2{6} | 2{9} | 95 |
| 4{25} | 2{3} | 2{4} | 57 | 4{43} | 2{7} | 2{8} | 48 |
| 4{26} | 2{3} | 2{5} | 76 | 4{44} | 2{7} | 2{9} | 59 |
| 4{27} | 2{3} | 2{6} | 94 | 4{45} | 2{8} | 2{9} | 56 |
| Member | Low | High | Average | Median |
|---|---|---|---|---|
| 2{1} | 61 | 91 | 81 | 83 |
| 2{2} | 22 | 83 | 61 | 64 |
| 2{3} | 54 | 94 | 69 | 69 |
| 2{4} | 12 | 97 | 69 | 70 |
| 2{5} | 69 | 94 | 83 | 85 |
| 2{6} | 55 | 97 | 89 | 94 |
| 2{7} | 48 | 88 | 67 | 62 |
| 2{8} | 48 | 96 | 72 | 73 |
| 2{9} | 56 | 95 | 70 | 63 |
| Cmpd. | PubChem SID‡ | Cmpd. | PubChem SID‡ |
|---|---|---|---|
| 4{1} | 26732547 | 4{24} | 49733438 |
| 4{2} | 49713852 | 4{25} | 49713850 |
| 4{3} | 26732537 | 4{26} | 26732546 |
| 4{4} | 49733436 | 4{27} | 26732531 |
| 4{5} | 26732515 | 4{28} | 26732516 |
| 4{6} | 49734139 | 4{29} | 49713848 |
| 4{7} | 26732535 | 4{30} | 26732538 |
| 4{8} | 49733439 | 4{31} | 49713847 |
| 4{9} | 26732539 | 4{32} | 50096426 |
| 4{10} | 49713854 | 4{33} | 49734319 |
| 4{11} | 26732541 | 4{34} | 49714451 |
| 4{12} | 49713857 | 4{35} | 49713851 |
| 4{13} | 26732520 | 4{36} | 49713845 |
| 4{14} | 26732540 | 4{37} | 26732533 |
| 4{15} | 26732542 | 4{38} | 49731974 |
| 4{16} | 49713858 | 4{39} | 26732534 |
| 4{17} | 26732514 | 4{40} | 49713844 |
| 4{18} | 49713855 | 4{41} | 49713843 |
| 4{19} | 49731975 | 4{42} | 49713846 |
| 4{20} | 49713856 | 4{43} | 49713849 |
| 4{21} | 49713853 | 4{44} | 26732536 |
| 4{22} | 49731976 | 4{45} | 50096427 |
| 4{23} | 49733857 |
Experimental
General
LC-MS Analysis
Synthesis
| cmpd | Name |
|---|---|
| 4{1} | 4,5-bis[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 4{2} | 4,5-bis[(benzyloxyglycyl)carbonyl]-1 H-imidazole |
| 4{3} | 4,5-bis[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 4{4} | 4,5-bis[(benzyloxy-S-alanyl)carbonyl]-1H-imidazole |
| 4{5} | 4,5-bis[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 4{6} | 4,5-bis[(benzyloxy-S-leucyl)carbonyl]-1H-imidazole |
| 4{7} | 4,5-bis[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 4{8} | 4,5-bis[(benzyloxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 4{9} | 4,5-bis[(tert-butoxy-S-[Nε-(tert-butoxy)carbonyl]lysyl)carbonyl]-1H-imidazole |
| 4{10} | 4-[(benzyloxyglycyl)carbonyl]-5-[( tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 4{11} | 4-[(tert-butoxy-S-alanyl)carbonyl]-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 4{12} | 4-[(benzyloxy-S-alanyl)carbonyl]-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 4{13} | 4-[(tert-butoxy-S-leucyl)carbonyl]-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 4{14} | 4-[(benzyloxy-S-leucyl)carbonyl]-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 4{15} | 4-[(tert-butoxy-S-phenylalanyl)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 4{16} | 4-[(benzyloxy-S-phenylalanyl)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 4{17} | 4-[(tert-butoxy-S-[Nε-(tert-butoxy)carbonyl]lysyl)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 4{18} | 4-[(tert-butoxy-S-alanyl)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 4{19} | 4-[(benzyloxy-S-alanyl)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 4{20} | 4-[(tert-butoxy-S-leucyl)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 4{21} | 4-[(benzyloxy-S-leucyl)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 4{22} | 4-[(tert-butoxy-S-phenylalanyl)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 4{23} | 4-[(benzyloxy-S-phenylalanyl)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 4{24} | 4-[(tert-butoxy-S-[Nε-(tert-butoxy)carbonyl]lysyl)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 4{25} | 4-[(benzyloxy-S-alanyl)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 4{26} | 4-[(tert-butoxy-S-leucyl)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 4{27} | 4-[(benzyloxy- S-leucyl)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 4{28} | 4-[(tert-butoxy-S-phenylalanyl)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 4{29} | 4-[(benzyloxy-S-phenylalanyl)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 4{30} | 4-[(tert-butoxy-S-[Nε-(tert-butoxy)carbonyl]lysyl)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 4{31} | 4-[(tert-butoxy-S-leucyl)carbonyl]-5-[(benzyloxy-S-alanyl)carbonyl]-1H-imidazole |
| 4{32} | 4-[(benzyloxy-S-leucyl)carbonyl]-5-[(benzyloxy-S-alanyl)carbonyl]-1H-imidazole |
| 4{33} | 4-[(tert-butoxy-S-phenylalanyl)carbonyl]-5-[(benzyloxy-S-alanyl)carbonyl]-1H-imidazole |
| 4{34} | 4-[(benzyloxy-S-phenylalanyl)carbonyl]-5-[(benzyloxy-S-alanyl)carbonyl]-1H-imidazole |
| 4{35} | 4-[(tert-butoxy-S-[Nε-(tert-butoxy)carbonyl]lysyl)carbonyl]-5-[(benzyloxy-S-alanyl)carbonyl]-1H-imidazole |
| 4{36} | 4-[(benzyloxy-S-leucyl)carbonyl]-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 4{37} | 4-[(tert-butoxy-S-phenylalanyl)carbonyl]-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 4{38} | 4-[(benzyloxy- S-phenylalanyl)carbonyl]-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 4{39} | 4-[(tert-butoxy-S-[Nε-(tert-butoxy)carbonyl]lysyl)carbonyl]-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 4{40} | 4-[(tert-butoxy-S-phenylalanyl)carbonyl]-5-[(benzyloxy-S-leucyl)carbonyl]-1H-imidazole |
| 4{41} | 4-[(benzyloxy- S-phenylalanyl)carbonyl]-5-[(benzyloxy-S-leucyl)carbonyl]-1H-imidazole |
| 4{42} | 4-[(tert-butoxy-S-[Nε-(tert-butoxy)carbonyl]lysyl)carbonyl]-5-[(benzyloxy-S-leucyl)carbonyl]-1H-imidazole |
| 4{43} | 4-[(benzyloxy-S-phenylalanyl)carbonyl]-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 4{44} | 4-[(tert-butoxy-S-[Nε-(tert-butoxy)carbonyl]lysyl)carbonyl]-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 4{45} | 4-[(tert-butoxy-S-[Nε-(tert-butoxy)carbonyl]lysyl)carbonyl]-5-[(benzyloxy-S-phenylalanyl)carbonyl]-1H-imidazole |
Supplementary Files
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Nielsen, T.E.; Schreiber, S. L. Towards the optimal screening collection: a synthesis strategy. Angew. Chem. Int. Ed. 2008, 47, 48–56. [Google Scholar] [CrossRef]
- Kaiser, M.; Wetzel, S.; Kumar, K.; Waldmann, H. Biology-inspired synthesis of compound libraries. Cell. Mol. Life Sci. 2008, 65, 1186–1201. [Google Scholar] [CrossRef]
- Huryn, D. M.; Cosford, N. D. P. The Molecular Libraries Screening Center Network (MLSCN): Identifying chemical probes of biological systems. Ann. Rep. Med. Chem. 2007, 42, 401–406. [Google Scholar] [CrossRef]
- Winssinger, N.; Pianowski, Z.; Debaene, F. Probing biology with Small Molecule Microarrays (SMM). Top. Curr. Chem. 2007, 278, 311–342. [Google Scholar] [CrossRef]
- Haggarty, S. J.; Schreiber, S. L. Forward chemical genetics. In Chemical Biology From Small Molecules to Systems Biology and Drug Design; Schreiber, S. L., Kapoor, T.M., Günther, W., Eds.; Wiley-VCH Verlag GmbH & Co: KGaA, Weinheim, 2007; pp. 299–354. [Google Scholar]
- Zerhouni, E. Medicine. The NIH Roadmap. Science 2003, 302, 63–72. [Google Scholar] [CrossRef]
- Schnur, D. M. Recent trends in library design: 'rational design' revisited. Curr. Opin. Drug Discov. Devel. 2008, 11, 375–380. [Google Scholar]
- Olah, M. M.; Bologa, C. G.; Oprea, T. I. Strategies for compound selection. Curr. Drug Discov. Technol. 2004, 1, 211–220. [Google Scholar] [CrossRef]
- Vistoli, G.; Pedretti, A.; Testa, B. Assessing drug-likeness-what are we missing? Drug Discov. Today 2008, 13, 285–294. [Google Scholar] [CrossRef]
- Muegge, I. Selection criteria for drug-like compounds. Med. Res. Rev. 2003, 23, 302–321. [Google Scholar] [CrossRef]
- Baures, P. W. Heterocyclic HIV-1 protease inhibitors. Org. Lett. 1999, 1, 249–252. [Google Scholar] [CrossRef]
- Van Compernolle, S.; Wiznycia, A. V.; Rush, J. R.; Dhanasekaran, M.; Baures, P. W.; Todd, S. C. Small molecule inhibition of Hepatitis C virus E2 binding to CD81. Virology 2003, 314, 371–380. [Google Scholar] [CrossRef]
- Perchellet, E. M.; Perchellet, J. P.; Baures, P. W. Imidazole-4,5-dicarboxamides with antiproliferative activity against HL-60 cells. J. Med. Chem. 2005, 48, 5955–5965. [Google Scholar] [CrossRef]
- Solinas, R.; DiCesare, J. C.; Baures, P. W. Parallel synthesis of an imidazole-4,5-dicarboxylic acid library bearing amino acid esters and alkanamines. Molecules 2008, 13, 3149–3170. [Google Scholar] [CrossRef]
- Rush, J. R.; Sandstrom, S. L.; Yang, J.; Davis, R.; Prakash, O.; Baures, P. W. Intramolecular hydrogen bond strength and pKa determination of N,N’-disubstituted imidazole-4,5-dicarboxamides. Org. Lett. 2005, 7, 135–138. [Google Scholar] [CrossRef]
- Baures, P. W. Imidazole-4,5-dicarboxylic acid: A versatile scaffold for drug discovery and materials research. Trends Heterocy. Chem. 2006, 11, 1–22. [Google Scholar]
- Lipinski, C. A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Veber, D. F.; Johnson, S. R.; Cheng, H. Y.; Smith, B. R.; Ward, K. W.; Kopple, K. D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Lipinski, C. A.; Lombardo, F.; Dominy, B. W.; Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- A website with data for these compounds and background information for the overall project is found at the following link: Available online: http://www.imidazole.utulsa.edu.
- Wiznycia, A. V.; Helfrich, B. A.; Baures, P. W. An improved method for the synthesis of dissymmetric N,N’-disubstituted imidazole-4,5-dicarboxamides. J. Org. Chem. 2002, 67, 7151–7154. [Google Scholar] [CrossRef]
- The details of this bioassay can be accessed at: Available online: http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?q=&pagefrom=BioAssaySrvice&aid=1236.
- The PubChem database can be accessed at Available online: http://www.ncbi.nlm.nih.gov/PubMed.
- Hur, J.; Wild, D. J. PubChemSR: A search and retrieval tool for PubChem. Chem. Cent. J. 2008, 2, 11. [Google Scholar] [CrossRef]
- Sample Availability: Please contact the corresponding author.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Solinas, R.; DiCesare, J.C.; Baures, P.W. Parallel Synthesis of a Library of Symmetrically- and Dissymmetrically-disubstituted Imidazole-4,5-dicarboxamides Bearing Amino Acid Esters. Molecules 2009, 14, 352-363. https://doi.org/10.3390/molecules14010352
Solinas R, DiCesare JC, Baures PW. Parallel Synthesis of a Library of Symmetrically- and Dissymmetrically-disubstituted Imidazole-4,5-dicarboxamides Bearing Amino Acid Esters. Molecules. 2009; 14(1):352-363. https://doi.org/10.3390/molecules14010352
Chicago/Turabian StyleSolinas, Rosanna, John C. DiCesare, and Paul W. Baures. 2009. "Parallel Synthesis of a Library of Symmetrically- and Dissymmetrically-disubstituted Imidazole-4,5-dicarboxamides Bearing Amino Acid Esters" Molecules 14, no. 1: 352-363. https://doi.org/10.3390/molecules14010352
APA StyleSolinas, R., DiCesare, J. C., & Baures, P. W. (2009). Parallel Synthesis of a Library of Symmetrically- and Dissymmetrically-disubstituted Imidazole-4,5-dicarboxamides Bearing Amino Acid Esters. Molecules, 14(1), 352-363. https://doi.org/10.3390/molecules14010352