Polyphenols in Cocoa and Cocoa Products: Is There a Link between Antioxidant Properties and Health?
Abstract
:Introduction
Polyphenols and other components in cocoa beans and cocoa-based products
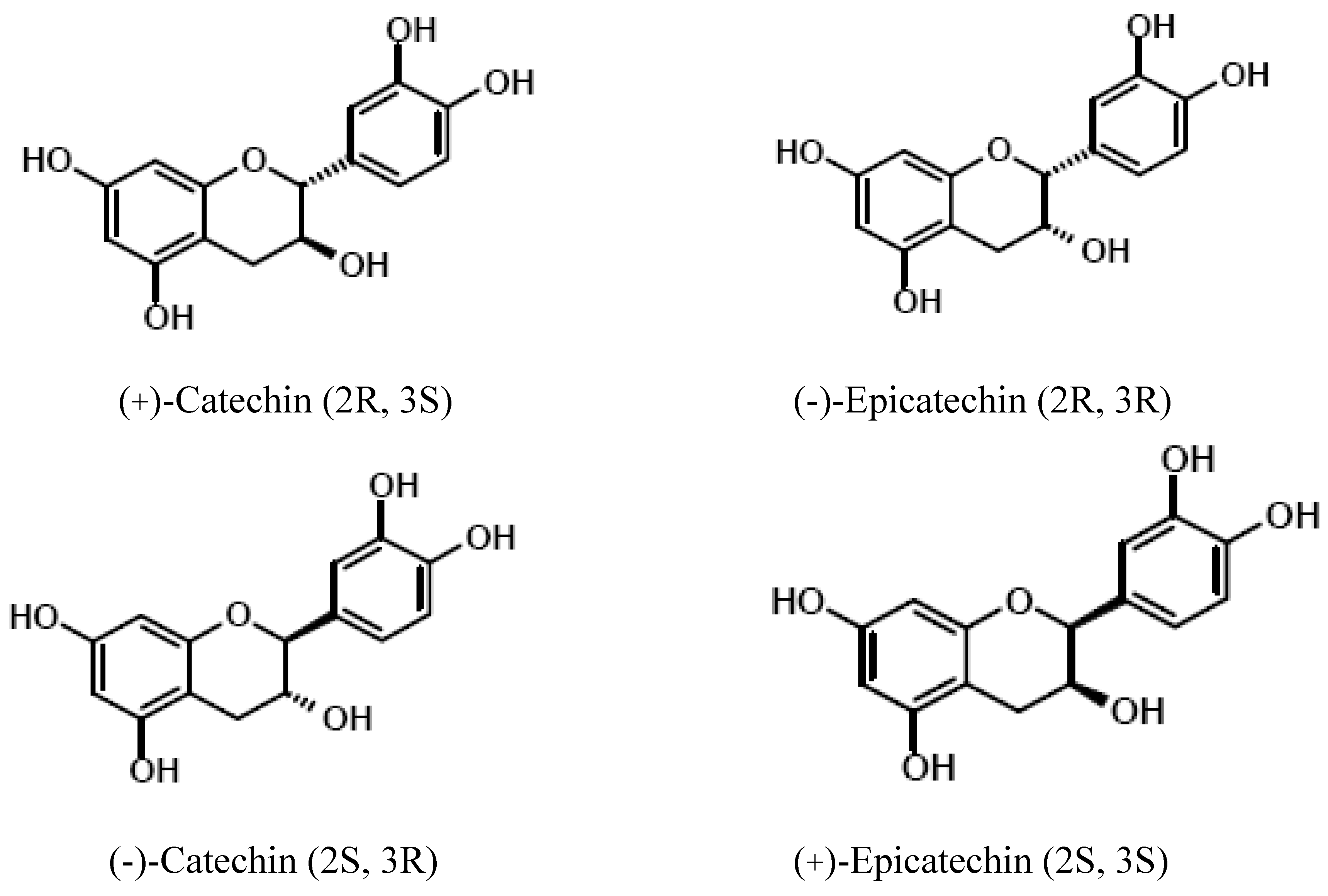
Polyphenols
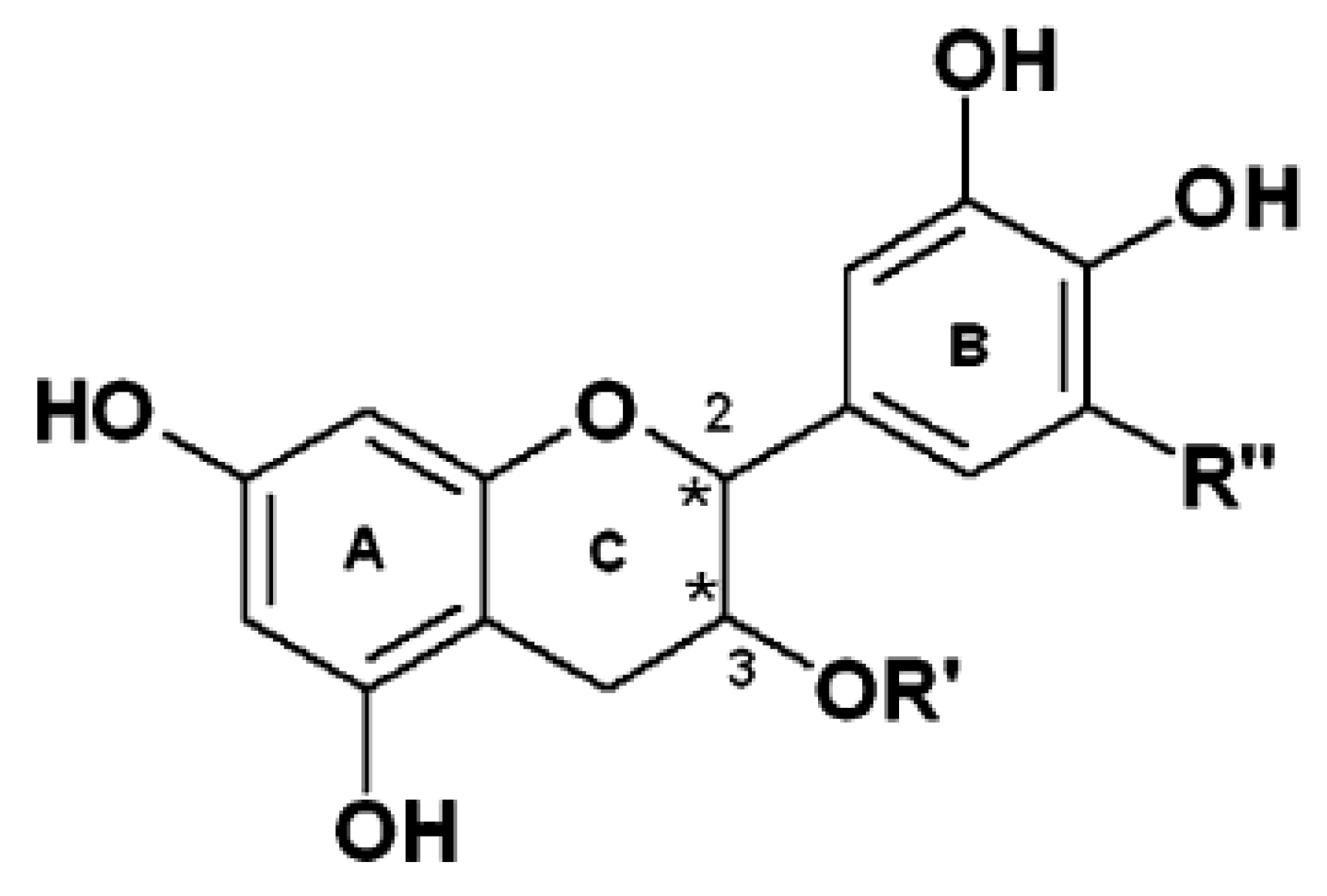
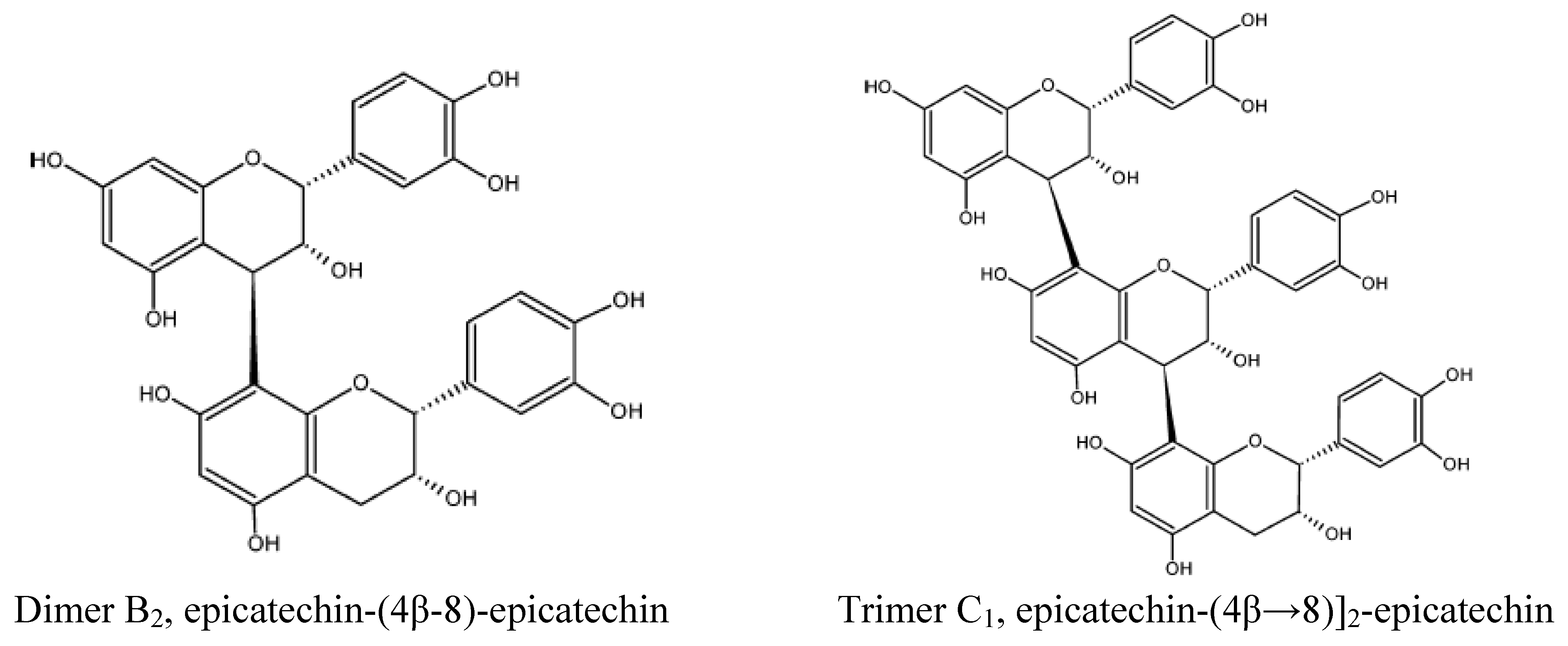
Methylxanthines

Peptides
Minerals
Factors affecting the quantity and quality of polyphenols in cocoa beans and cocoa-based products
Countries of origin
Fermentation
Manufacturing process
Bioavailability of cocoa polyphenols
Conclusions
References and Notes
- Barry Callebaut. History of chocolates. 2008. http://www.barry-callebaut.com/1589.
- Dillinger, T.L.; Barriga, P.; Escarcega, S.; Jimenez, M.; Lowe, D.S.; Grivetti, L.E. Food of the Gods: Cure for humanity? A cultural history of the medicinal and ritual use of chocolate. J. Nutr. 2000, 130, 2057S–2072S. [Google Scholar]
- Kelm, M.A.; Johnson, J.C.; Robbins, R.J.; Hammerstone, J.F.; Schmitz, H.H. High-performance liquid chromatography separation and purification of cacao (Theobroma cacao L.) procyanidins according to degree of polymerization using a diol stationary phase. J. Agric. Food Chem. 2006, 54, 1571–1576. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Cienfuegos-Jovellanos, E.; Marin, A.; Muguerza, B.; Gil Izquierdo, A.; Cerdaa, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; Pasamar, M.; Ramoan, D.; Espin, J.C. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007, 55, 3926–3935. [Google Scholar] [CrossRef]
- Cooper, K.A.; Donovan, J.L.; Waterhouse, A.L.; Williamson, G. Cocoa and health: a decade of research. Bri. J. Nutr. 2008, 99, 1–11. [Google Scholar]
- Ramljak, D.; Romanczyk, L.J.; Metheny-Barlow, L.J.; Thompson, N.; Knezevic, V.; Galperin, M.; Ramesh, A.; Dickson, R.B. Pentameric procyanidin from Theobroma cacao selectively inhibits growth of human breast cancer cells. Mol. Cancer Ther. 2005, 4, 537–546. [Google Scholar]
- Ramiro, E.; Franch, A.; Castellote, C.; Andres-Lacueva, C. Effect of Theobroma cacao flavonoids on immune activation of a lymphoid cell line. Bri. J. Nutr. 2005, 93, 859–866. [Google Scholar] [CrossRef]
- Matsui, N.; Ito, R.; Nishimura, E.; Yoshikawa, M. Ingested cocoa can prevent high-fat diet-induced obesity by regulating the expression of genes for fatty acid metabolism. Nutrition 2005, 21, 594–601. [Google Scholar] [CrossRef]
- Kurosawa, T.; Itoh, F.; Nozaki, A.; Nakano, Y. Suppressive effects of cacao liquor polyphenols (CLP) on LDL oxidation and the development of atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic rabbits. Atherosclerosis 2005, 179, 237–246. [Google Scholar] [CrossRef]
- Vinson, J.A.; Proch, J.; Bose, P.; Muchler, S.; Taffera, P.; Shuta, D.; Samman, N.; Agbor, G.A. Chocolate is a powerful ex vivo and in vivo antioxidant, an antiatherosclerotic agent in an animal model, and a significant contributor to antioxidants in the European and American Diets. J. Agric. Food Chem. 2006, 54, 8071–8076. [Google Scholar]
- Rios, L.Y.; Gonthier, M.P.; Remesy, C.; Mila, I.; Lapierre, C.; Lazarus, S.A.; Williamson, G.; Scalbert, A. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am. J. Clin. Nutr. 2003, 77, 912–918. [Google Scholar]
- Greer, F.; Hudson, R.; Ross, R.; Graham, T. Caffeine ingestion decrease glucose disposal during a hyperinsulinemic-euglycemic clamp in sedentary humans. Diabetes 2001, 50, 2349–2354. [Google Scholar] [CrossRef]
- Wang, Y.; Waller, D.P.; Amiya, P.; Hikim, S.; Russell, L.D. Reproductive toxicity of theobromine and cocoa extract in male rats. Reprod. Toxicol. 1992, 6, 347–353. [Google Scholar] [CrossRef]
- Pencek, R.R.; Battram, D.; Shearer, J.; James, F.D.; Lacy, D.B.; Jabbour, K.; Williams, P.E.; Graham, T.E.; Wasserman, D.H. Portal vein caffeine infusion enhances net hepatic glucose uptake during a glucose load in conscious dogs. J. Nutr. 2004, 134, 3042–3046. [Google Scholar]
- USDA. United States Department of Agriculture, Nutrient Data Laboratory. 2008. http://www.nal.usda.gov/fnic/foodcomp/search/. [Google Scholar]
- Joo, S.; Kies, C.; Schnepf, M. Chocolate and chocolate-like products: impact on copper status of humans. J. Appl. Nutr. 1995, 47, 67–77. [Google Scholar]
- Schroeter, H.; Holt, R.R.; Orozco, T.J.; Schmitz, H.H.; Keen, C.L. Nutrition: milk and absorption of dietary flavanols. Nature 2003, 426, 787–788. [Google Scholar]
- Tabernero, M.; Serrano, J; Saura-Calixto, F. The antioxidant capacity of cocoa products: contribution to the Spanish diet. Int. J Food Sci. Tech. 2006, 41, 28–32. [Google Scholar] [CrossRef]
- Ultee, A.J.; van Dorsen, J. Bijdrage tot de kennis der op Java gecultiveerde cacaosooten. Java Agric Station Report 1909, 33. [Google Scholar]
- Adam, W.B.; Hardy, F.; Nierenstein, M. The catechin of the cocoa bean. J. Am. Chem. Soc. 1931, 53, 727–728. [Google Scholar] [CrossRef]
- Freudenberg, K.; Cox, R.F.B.; Braun, E. The catechin of the cacao bean. J. Am. Chem. Soc. 1932, 54, 1913–1917. [Google Scholar] [CrossRef]
- Forsyth, W.G.C. Cacao polyphenolic substances. III. Separation and estimation on paper chromatograms. Biochem. J. 1955, 60, 108–111. [Google Scholar]
- Adam, W.B. Determination of the color-producing constituents of the cacao bean. The Analyst 1928, 53, 369–372. [Google Scholar] [CrossRef]
- Forsyth, W.G.C.; Quesnel, V.C. The mechanism of cacao curing. Adv. Enzymol. 1963, 25, 457–492. [Google Scholar]
- Quesnel, V.C. Fractionation and properties of the polymeric of the polymeric leucocyanidin of the seeds of Theobroma cacao. Phytochemistry 1968, 7, 1583–1592. [Google Scholar] [CrossRef]
- Jalal, M.A.F.; Collin, H.A. Polyphenols of mature plant, seedling, and tissue cultures of Theobroma cacao. Phytochemistry 1977, 16, 1377–1380. [Google Scholar] [CrossRef]
- Thompson, R.S.; Jacques, D.; Haslam, E.; Tanner, R.J.N. Plant proanthocyanidins. Part I. Introduction; the isolation, structure, and distribution in nature of plant procyanidins. J. Chem Soc [Perkin 1] 1972, I, 1387–1399. [Google Scholar]
- Natsume, M.; Osakabe, N.; Yamagishi, M.; Takizawa, T.; Nakamura, T.; Miyatake, H.; Hatano, T.; Yoshida, T. Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC. Biosci. Biotechnol. Biochem. 2000, 64, 2581–2587. [Google Scholar] [CrossRef]
- Natsume, M.; Osakabe, N.; Takizawa, T.; Nakamura, T.; Miyatake, H.; Hatano, T.; Yoshida, T. Analysis of polyphenol constituents in cocoa and chocolate. In Quality Management of Nutraceuticals; Ho., C-T., Zeng, Q.Y., Eds.; ACS Symposium Series 803; American Chemical Society: Washington, DC, USA, 2002; pp. 180–198. [Google Scholar]
- Abbe Maleyki, M.J.; Amin, I. Antioxidant properties of cocoa powder. J. Food Biochem. 2008. (In press).
- Cooper, K.A.; Campos-Gimenez, E.; Alvarez, D.J.; Nagy, K.; Donovan, J.L.; Williamson, G. Rapid reverse-phase ultra-performance liquid chromatography analysis of the major cocoa polyphenols and inter-relationship of their concentration in chocolate. J. Agric. Food Chem. 2007, 55, 2841–2847. [Google Scholar] [CrossRef]
- Stafford, H.A.; Lester, H.H. Procyanidins (condensed tannins) in green cell suspension cultures of Douglas Fir compared with those in strawberry and avocado leaves by means of C8-reversed-phase chromatography. Plant Physiol. 1980, 66, 1085–1090. [Google Scholar] [CrossRef]
- Rios, L.Y.; Bennett, R.N.; Lazarus, S.A.; Remesy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar]
- Kofink, M.; Papagiannopoulos, M.; Galensa, R. Enantioseparation of catechin and epicatechin in plant food by chiral capillary electrophoresis. Eur. Food Res. Technol. 2007, 225, 569–577. [Google Scholar] [CrossRef]
- Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Membrane effects of cocoa procyanidins in liposomes and Jurkat T cells. Biol. Res. 2004, 37, 293–300. [Google Scholar]
- Verstraeten, S.V.; Hammerstone, J.F.; Keen, C.L.; Fraga, C.G.; Oteiza, P.I. Antioxidant and membrane effects of procyanidin dimers and trimers isolated from peanut and cocoa. J. Agric. Food Chem. 2005, 53, 5041–5048. [Google Scholar] [CrossRef]
- Porter, L.J.; Ma, Z.; Chan, B.G. Cacao procyanidins: major flavanoids and identification of some minor metabolites. Phyrochemistry 1991, 30, 1657–1663. [Google Scholar] [CrossRef]
- Richelle, M.; Tavazzi, I.; Offord, E. Comparison of antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. J. Agric. Food Chem. 2001, 49, 3438–3442. [Google Scholar] [CrossRef]
- Dreosti, I.E. Antioxidant polyphenols in tea, cocoa, and wine. Nutrition 2000, 16, 692–694. [Google Scholar] [CrossRef]
- Kim, H.; Keeney, P.G. (-)-epicatechin content in fermented and unfermented cocoa beans. J. Food Sci. 1984, 49, 1090–1092. [Google Scholar] [CrossRef]
- Hammerstone, J.F.; Lazarus, S.A.; Mitchell, A.E.; Rucker, R.; Schmitz, H.H. Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-perfomance liquid chromatography/ mass spectrometry. J. Agric. Food Chem. 1999, 47, 490–496. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Hollman, P.C.H.; Kromhout, D. Chocolate as a source of tea flavonoids. The Lancet. 1999, 354, 488. [Google Scholar]
- Arts, I.C.W.; van de Putte, B.; Hollman, P.C.H. Catechin contents of foods commonly consumed in the Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food Chem. 2000, 48, 1746–1751. [Google Scholar] [CrossRef]
- Kim, H.; Keeney, P.G. Methods of analysis for (-)-epicatechin in cocoa beans by high-performance liquid chromatography. J. Food Sci. 1983, 48, 548–551. [Google Scholar] [CrossRef]
- Sanbongi, C.; Osakabe, N.; Natsume, M.; Takizawa, T.; Gomi, S.; Osawa, T. Antioxidative polyphenols isolated from Theobroma Cacao. J. Agric. Food Chem. 1998, 46, 454–457. [Google Scholar] [CrossRef]
- Kofink, M.; Papagiannopoulos, M.; Galensa, R. (-)-catechin in cocoa and chocolate: occurence and analysis of an atypical flavan-3-ol enantiomer. Molecules 2007, 12, 1274–1288. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Holt, R.R.; Lazarus, S.A.; Orozco, T.J.; Keen, C.L. Inhibitory effects of cocoa flavanols and procyanidin oligomers on free radical-induced erythrocyte hemolysis. Exp. Biol. Med. 2002, 227, 321–329. [Google Scholar]
- Adamson, G.E.; Lazarus, S.A.; Mitchell, A.E.; Prior, R.L.; Cao, G.; Jacobs, P.H.; Kremers, B.G.; Hammerstone, J.F.; Rucker, R.B.; Ritter, K.A.; Schmitz, H.H. HPLC method for the quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacity. J. Agric. Food Chem. 1999, 47, 4184–4188. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Natsume, M.; Yasuda, A.; Takizawa, T.; Nakamura, T.; Terao, J. Cocoa powder enhances the level of antioxidative activity in rat plasma. Bri. J. Nutr. 2000, 84, 673–680. [Google Scholar]
- Rein, D.; Lotito, S.; Holt, R.R.; Keen, C.L.; Schmitz, H.H.; Fraga, C.G. Epicatechin in human plasma: in vivo determination and effect of chocolate consumption on plasma oxidation status. J. Nutr. 2000, 130, 2109S–2114S. [Google Scholar]
- Wang, J.F.; Schramm, D.D.; Holt, R.R.; Ensunsa, J.L.; Fraga, C.G.; Schmitz, H.H.; Keen, C.L. A dose-response effect from chocolate consumption on plasma epicatechin and oxidative damage. J. Nutr. 2000, 130, 2115S–2119S. [Google Scholar]
- Murphy, K.J.; Chronopoulos, A.K.; Singh, I.; Francis, M.A.; Moriarty, H.; Pike, M.J.; Turner, A.H.; Mann, N.J.; Sinclair, A.J. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am. J. Clin Nutr. 2003, 77, 1466–1473. [Google Scholar]
- Mathur, S.; Devaraj, S.; Grundy, S.M.; Jialal, I. Cocoa products decrease low density lipoprotein oxidative susceptibility but do not affect biomarkers of inflammation in humans. J. Nutr. 2002, 132, 3663–3667. [Google Scholar]
- Mursu, J.; Voutilainen, S.; Nurmi, T.; Rissanez, T.H.; Virtanen, J.K.; Kaikkonen, J.; Nyyssonen, K.; Salonen, J.T. Dark chocolate consumption increases HDL cholesterol concentration and chocolate fatty acids may inhibit lipid peroxidation in healthy humans. Free Rad. Biol. Med. 2004, 37, 1351–1359. [Google Scholar] [CrossRef]
- Ying, Wan; Joe A, Vinson; Terry D, Etherton; John, Proch; Sheryl A, Lazarus; Penny M, Kris-Etherton. Effects of cocoa powder and dark chocolate on LDL oxidative susceptibility and prostaglandin concentrations in humans. Am. J. Clin. Nutr. 2001, 74, 596–602. [Google Scholar]
- Kris-Etherton, P.M.; Derr, J.A.; Mustad, V.A.; Seligson, F.H.; Pearson, T.A. Effects of a milk chocolate bar per day substituted for a high-carbohydrate snack in young men on an NCEP/AHA Step 1 Diet. Am. J. Clin. Nutr. 1994, 60, 1037S–1342S. [Google Scholar]
- Osakabe, N.; Baba, S.; Yasuda, A.; Iwamoto, T.; Kamiyama, M.; Tokunaga, T.; Kondo, K. Dose-response study of daily cocoa intake on the oxidative susceptibility of low-density-lipoprotein. J.Health Sci. 2004, 50, 679–684. [Google Scholar]
- Taubert, D.; Roesen, R.; Lehmann, C.; Jung, N.; Schomig, E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide. JAMA. 2007, 298, 49–60. [Google Scholar] [CrossRef]
- Heiss, C.; Kleinbongard, P.; Dejam, A.; Perre, S.; Schroeter, H.; Sies, H.; Kelm, M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J. Am. Coll. Cardiol. 2005, 46, 1276–1283. [Google Scholar] [CrossRef]
- Keen, C.L.; Holt, R.R.; Polagruto, J.A.; Wang, J.F.; Schmitz, H.H. Cocoa flavanols and cardiovascular health. Phytochemistry Reviews 2002, 1, 231–240. [Google Scholar] [CrossRef]
- Engler, M.B.; Engler, M.M. The vasculoprotective effects of flavonoid-rich cocoa and chocolate. Nutr. Res. 2004, 24, 695–706. [Google Scholar] [CrossRef]
- Heiss, C.; Dejam, A.; Kleinbongard, P.; Schewe, T.; Sies, H.; Kelm, M. Vascular effects of cocoa rich in flavan-3-ols. JAMA 2003, 290, 1030–1031. [Google Scholar] [CrossRef]
- Balzer, J.; Rassaf, T.; Heiss, C.; Kleinbongard, P.; Lauer, T.; Merx, M.; Heussen, N.; Gross, H.B.; Keen, C.L.; Schroeter, H.; Kelm, M. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients. J. Am. Coll. Cardiol. 2008, 51, 2141–2149. [Google Scholar] [CrossRef]
- Karim, M.; McCormick, K.; Kappagoda, C.T. Effects of cocoa extracts on endothelium-dependent relaxation. J. Nutr. 2000, 130, 2105S–2108S. [Google Scholar]
- Engler, M.B.; Engler, M.M.; Chen, C.Y.; Malloy, M.J.; Browne, A.; Chiu, E.Y.; Kwak, H-K.; Milbury, P.; Paul, S.M.; Blumberg, J.; Mietus-Snyder, M.L. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004, 23, 197–204. [Google Scholar] [CrossRef]
- Farouque, H.M.O.; Leung, M.; Hope, S.A.; Baldi, M.; Schechter, C.; Cameron, J.D.; Meredith, I.T. Acute and chronic effects of flavanol-rich cocoa on vascular function in subjects with coronary artery disease: a randomized double-blind placebo-controlled study. Clin. Sci. 2006, 111, 71–80. [Google Scholar] [CrossRef]
- Baba, S.; Natsume, M.; Yasuda, A.; Nakamura, Y.; Tamura, T.; Osakabe, N.; Kanegae, M.; Kondo, K. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo and hypercholesterolemic humans after intake of different levels of cocoa powder. J. Nutr. 2007, 137, 1436–1441. [Google Scholar]
- Baba, S.; Osakabe, N.; Kato, Y.; Natsume, M.; Yasuda, A.; Kido, T.; Fukuda, K.; Muto, Y.; Kondo, K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am. J. Clin. Nutr. 2007, 85, 709–717. [Google Scholar]
- Allen, R.R.; Carson, L.A.; Kwik-Uribe, C.; Evans, E.M.; Erdman, J.W. Daily consumption of a dark chocolate containing flavanols and added sterol esters affects cardiovascular risk factors in a normotensive population with elevated cholesterol. J. Nutr. 2008, 138, 725–731. [Google Scholar]
- Tomaru, M.; Takano, H.; Osakabe, N.; Yasuda, A.; Inouse, K-I.; Yanigisawa, R.; Ohwatari, T.; Uematsu, H. Dietary supplementation with cacao liquor proanthocyanidins prevents elevation of blood glucose levels in diabetic obese mice. Nutrition 2007, 23, 351–355. [Google Scholar] [CrossRef]
- Ruzaidi, A.M.M; Amin, I.; Nawalyah, A.G.; Hamid, M.; Faizul, H.A. The effect of Malaysian cocoa extract on glucose levels and lipid profiles in diabetic rats. J. Ethnopharmacol. 2005, 98, 55–60. [Google Scholar] [CrossRef]
- Ruzaidi, A.M.M; Abbe, M.M.J; Amin, I.; Nawalyah, A.G.; Muhajir, H. Protective effect of polyphenol-rich extract prepared from Malaysian cocoa (Theobroma cacao) on glucose levels and lipid profiles in streptozotocin-induced diabetic rats. J. Sci. Food Agric. 2008, 88, 1442–1447. [Google Scholar] [CrossRef]
- Grassi, D.; Lippi, C.; Necozione, S.; Desideri, G.; Ferri, C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr. 2005, 81, 611–614. [Google Scholar]
- Grassi, D.; Necozione, S.; Lippi, C.; Croce, G.; Valeri, L.; Pasqualetti, P.; Desideri, G.; Blumberg, J.B.; Ferri, C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 2005, 46, 398–405. [Google Scholar] [CrossRef]
- Brand-Miller, J.; Holt, S.H.A.; de Jong, V.; Petocz, P. Cocoa powder increases postprandial insulinemia in lean young adults. J. Nutr. 2003, 133, 3149–3152. [Google Scholar]
- Amin, I.; Koh, B.K.; Asmah., R. Effect of cacao liquor extract on tumor marker enzymes during chemical hepatocarcinogenesis in rats. J. Med. Food 2004, 7, 7–12. [Google Scholar] [CrossRef]
- Bisson, J.-F.; Guardia-Llorens, M.-A.; Hidalgo, S.; Rozan, P.; Messaoudi, M. Protective effect of Acticoa powder, a cocoa polyphenolic extract, on prostate carcinogenesis in Wistar-Unilever rats. Eur. J. Can. Prev. 2008, 17, 54–61. [Google Scholar] [CrossRef]
- Bisson, J.-F.; Hidalgo, S.; Rozan, P.; Messaoudi, M. Therapeutic effect of ACTICOA powder, a cocoa polyphenolic extract, on experimentally induced prostate hyperplasia in Wistar-Unilever rats. J. Med. Food 2007, 10, 628–635. [Google Scholar] [CrossRef]
- Bisson, J.-F.; Nejdi, A.; Rozan, P.; Hidalgo, S.; Lalonde, R.; Messaoudi, M. Effects of long-term administration of a cocoa polyphenolic extract (Acticoa powder) on cognitive performances in aged rats. Bri. J. Nutr. 2008, 100, 94–101. [Google Scholar]
- Rozan, P.; Hidalgo, S.; Nejdi, A.; Bisson, J.-F.; Lalonde, R.; Messaoudi, M. Preventive antioxidant effects of cocoa polyphenolic extract on free radical production and cognitive performances after heat exposure in Wistar rats. J. Food Sci. 2006, 72, S203–S206. [Google Scholar]
- Smit, H.J; Blackburn, R. J. Reinforcing effects of caffeine and theobromine as found in chocolate. Psychopharmacology 2005, 181, 101–106. [Google Scholar] [CrossRef]
- Forsyth, W.G.C. Cacao polyphenolic substances. II. Changes during fermentation. Biochem. J. 1952, 51, 516, 520. [Google Scholar]
- Timbie, D.J.; Sechrist, L.; Kenney, P.G. Application of HPLC to the study of variables affecting theobromine and caffeine concentrations in cocoa beans. J. Food Sci. 1978, 43, 560–562. [Google Scholar] [CrossRef]
- Pura Naik, J. Improved high-performance liquid chromatography method to determine theobromine and caffeine in cocoa and cocoa products. J. Agric. Food Chem. 2001, 49, 3579–3583. [Google Scholar] [CrossRef]
- Franzke, C.; Grunet, K.S.; Griehl, H. Uber die bestimmung und den gehalt von theobromin und theophyllin in Mate, Kola und Kakao. Z. Lebensm. Unters.Forsch. 1969, 139, 85. [Google Scholar] [CrossRef]
- Thomas, J.B.; James, J.H.; Schantz, M.M.; Porter, B.J.; Sharpless, K.E. Determination of caffeine, theobromine, and theophylline in standard reference material 2384, baking chocolate, using reversed-phase liquid chromatography. J. Agric. Food Chem. 2004, 52, 3259–3263. [Google Scholar]
- Kelly, C.J. Effects of theobromine should be considered in future studies. Am. J. Clin. Nutr. 2005, 82, 483–489. [Google Scholar]
- Lee, C. Antioxidant ability of caffeine and its metabolites based on the study of oxygen radical absorbing capacity and inhibition of LDL peroxidation. Clin. Chim. Acta. 2000, 295, 141–154. [Google Scholar] [CrossRef]
- Vinson, J.A.; Proch, J.; Zubik, L. Phenol antioxidant quantity and quality in foods: cocoa, dark chocolate, and milk chocolate. J. Agric. Food Chem. 1999, 47, 4821–4824. [Google Scholar] [CrossRef]
- Lee, S.; Hudson, R.; Kilpatrick, K.; Graham, T.E.; Ross, R. Caffeine ingestion is associated with reductions in glucose uptake independent of obesity and Type 2 Diabetes before and after exercise training. Diabetes Care 2005, 28, 566–572. [Google Scholar] [CrossRef]
- Moisey, L.L.; Kacker, S.; Bickerton, A.C.; Robinson, L.E.; Graham, T.E. Caffeinated coffee consumption impairs blood glucose homeostasis in response to high and low glycemic index meals in healthy men. Am. J. Clin. Nutr. 2008, 87, 1254–1261. [Google Scholar]
- Lane, J.D.; Feinglos, M.N.; Surwit, R.S. Caffeine increases ambulatory glucose and postprandial responses in coffee drinkers with type 2 diabetes. Diabetes Care 2008, 31, 221–222. [Google Scholar]
- Graham, T.E.; Sathasivam, P.; Rowland, M.; Marko, N.; Greer, F.; Battram, D. Caffeine ingestion elevates plasma insulin response in humans during an oral glucose tolerance test. Can. J. Physiol. Pharmacol. 2001, 79, 559–565. [Google Scholar] [CrossRef]
- Cerasi, E.; Luft, R. The effect of an adenosine-3'5'-monophosphate diesterase inhibitor (aminophylline) on the insulin response to glucose infusion in prediabetic and diabetic subjects. Horm. Metab. Res. 1969, 1, 162–168. [Google Scholar] [CrossRef]
- Cerasi, E.; Luft, R. The effect of an adenosine-3'5'-monophosphate diesterase inhibitor (aminophylline) on the insulin response to glucose infusion in prediabetic and diabetic subjects. Horm. Metab. Res. 1969, 1, 162–168. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Caviezel, F.; Alberetto, M. Secondary failure of oral hypoglycaemic agents in lean patients with type 2 diabetes mellitus: Insulin sensitivity, insulin response to different stimuli, and the role of cyclic-AMP. Diabetes Metab. 1992, 18, 25–31. [Google Scholar]
- Hoffer, L.J.; Lowenstein, J.M. Effects of adenosine and adenosine analogues on glycogen metabolism in isolated rat hepatocytes. Biochem. Pharmacol. 1986, 35, 4529–4536. [Google Scholar] [CrossRef]
- Oetjen, E.C.; Schweickhardt, K.; Unthan-Fechner, K. Stimulation of glucose production from glycogen by glucagon, noradrenaline and non-degradable adenosine analogues is counteracted by adenosine and ATP in cultured rat hepatocytes. Biochem J. 1990, 271, 337–344. [Google Scholar]
- Bertrand, G.; Petit, P.; Bozem, M. Membrane and intracellular effects of adenosine in mouse pancreatic beta-cells. Am. J. Physiol. 1989, 257, E473–E478. [Google Scholar]
- Hillaire-Buys, D.; Chapal, J.; Bertrand, G. Purinergic receptors on insulin secreting cells. Fundam. Clin. Pharmacol. 1994, 8, 117–127. [Google Scholar] [CrossRef]
- Arias, A.M.P.; Bisschop, P.H.; Ackermans, M.T.; Nijpels, G.; Endert, E.; Romijn, J.A.; Sauerwein, H.P. Aminophylline stimulates insulin secretion in patients with type 2 diabetes mellitus. Metabolism 2001, 9, 1030–1035. [Google Scholar]
- Eteng, M.U.; Ettarh, R.R. Comparative effects of theobromine and cocoa extract on lipid profiles in rats. Nutr. Res. 2000, 20, 1513–1517. [Google Scholar] [CrossRef]
- Eteng, M.U.; Ibekwe, H.A.; Umoh, U.I.; Ebong, P.E.; Umoh, I.B.; Eyong, E.U. Theobromine rich cocoa powder induces weight loss and changes in lipid profile of obese Wistar rats. Discov. Innov. 2006, 18, 191–196. [Google Scholar]
- Granner, D.K. Hormone action. In Harper's Biochemistry, 22nd Edition; Murray, RK, Meyers, PA, Granner, DK, Rodwell, VW, Eds.; Prentice Hall International Inc.: Upper Saddle River, NJ, USA, 1990; pp. 467–473. [Google Scholar]
- Barcz, E.; Sommer, E.; Sokolnicka, I.; Gawrychowski, K.; Roszkowska-Purska, K.; Janik, P.; Skopinska-Roozewska, E. The influence of theobromine on angiogenic activity and proangiogenic cytokines production of human ovarian cancer cells. Oncol.Rep. 1998, 5, 517–520. [Google Scholar]
- Chang, C.C.; Chang, T.C.; Kao, S.C.; Kuo, Y.F.; Chien, C.F. Pentoxyfylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Grave's ophthalmopathy and pretibial myoedema. Acta Endocrinol. Copehn. 1993, 129, 322–327. [Google Scholar]
- Gil, M.; Skopinska, R.E.; Radomska, D.; Demkon, U.; Skurzak, H.; Rochowska, M.; Beauth, J.; Roszkowski, K. Effect of purinergic receptor antagonists, suramin and theobromine on tumour-induced angiogenesis in BALB/C mice. Folia. Biol. Praha. 1993, 39, 63–68. [Google Scholar]
- Clark, E.; Rice, G.C.; Weeks, R.S.; Jenkins, N.; Nelson, R.; Bianco, J.A.; Singer, J.W. Lisofylline inhibits transforming growth factor beta release and enhances trilineage hematopoietic recovery after 5- fluorouracil. Cancer Res. J. 1996, 56, 105–112. [Google Scholar]
- Slattery, M.L.; West, D.W. Smoking, alcohol, coffee, tea, caffeine, and theobromine: risk of prostate cancer in Utah (United States). Cancer Causes Control 1993, 4, 559–63. [Google Scholar] [CrossRef]
- Soffietti, M.G.; Nebbia, C.; Valenza, F.; Amedeo, S.; Re, G. Effects of theobromine in mature and immature rabbits. J. Comp. Pathol. 1989, 100, 47–68. [Google Scholar] [CrossRef]
- Strachan, T.R.; Bennett, A. Theobromine poisoning in dogs. Vet. Rec. 1994, 134, 284–289. [Google Scholar]
- Kimmel, C.A.; Kimmel, G.L.; White, C.; Grasto, T.E.; Young, J.F.; Nelson, G.J. Blood flow changes and conceptual development in pregnant rats in response to caffeine. Fund. Applied Toxicol. 1984, 4, 240–247. [Google Scholar] [CrossRef]
- Abdi, F.B.; Pollard, I.; Wilkinson, J.M. Placental transfer and foetal disposition of caffeine and its metabolites in 20 day pregnant rat: A function of dose. Xenobiotica 1993, 23, 449–456. [Google Scholar] [CrossRef]
- Biehl, B.; Wewetzer, C.; Passern, D. Vacuolar (storage) proteins of cocoa seeds and their degradation during germination and fermentation. J. Sci. Food Agric. 1982, 33, 1291–1304. [Google Scholar] [CrossRef]
- Voigt, J.; Biehl, B.; Heinrichs, H.; Kamaruddin, S.; Marsoner, G.G.; Hugi, A. In-vitro formation of cocoaspecific aroma precursors: aroma related peptides generated from cocoa-seed protein by co-operation of an aspartic endoprotease and a carboxypeptidase. Food Chem. 1994, 49, 173–180. [Google Scholar] [CrossRef]
- Voigt, J.; Heinrichs, H.; Voigt, G.; Biehl, B. Cocoa specific aroma precursors are generated by proteolytic digestion of the vicilin-like globulin of cocoa seeds. Food Chem. 1994, 50, 177–184. [Google Scholar] [CrossRef]
- Zak, D.K.; Keeney, P.G. Extraction and fractionation of cocoa proteins as applied to several varieties of cocoa beans. J. Agric. Food Chem. 1976, 24, 478–482. [Google Scholar]
- Zak, D.K.; Keeney, P.G. Changes in cocoa proteins during ripening of fruit, fermentation, and further processing of cocoa beans. J. Agric. Food Chem. 1976, 24, 483–486. [Google Scholar] [CrossRef]
- Voigt, J.; Biehl, B.; Syed Kamaruddin, S.W. The major seed proteins of Theobroma cacao L. Food Chem. 1993, 47, 145–151. [Google Scholar] [CrossRef]
- Amin, I.; Jinap, S.; Jamilah, B. Vicilin-class globulins and their degradation during cocoa fermentation. Food Chem. 1997, 59, 1–5. [Google Scholar] [CrossRef]
- Buyukpamukcu, E.; David, Goodall M.; Hansen, C-E.; Keely, B.J.; Kochhar, S.; Wille, H. Characterization of peptides formed during fermentation of cocoa bean. J. Agric. Food Chem. 2001, 49, 5822–5827. [Google Scholar] [CrossRef]
- Erdmann, K.; Cheung, B.W.Y.; Schroder, H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J. Nutr. Biochem. 2008. (in press).
- Marcuse, R. Antioxidative effect of amino acids. Nature 1960, 186, 886–887. [Google Scholar] [CrossRef]
- Yong, S.H.; Karel, M. Reaction of histidine with methyl linoleate: characterization of the histidine degradation product. J. Am. Oil Chem. Soc. 1978, 55, 352–356. [Google Scholar] [CrossRef]
- Chen, H.M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef]
- Steinberg, F.M.; Bearden, M.M.; Keen, C.L. Cocoa and chocolate flavonoids: Implications for cardiovascular health. J. Am. Diet Assoc. 2003, 103, 215–223. [Google Scholar] [CrossRef]
- Champagne, C.M. Magnesium in hypertension, cardiovascular disease, metabolic syndrome, and other conditions: A Review. Nutr. Clin. Prac. 2008, 23, 142–151. [Google Scholar] [CrossRef]
- Al-Delaimy, W.K.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Magnesium intake and risk of coronary heart disease among men. J. Am. Coll. Nutr. 2004, 23, 63–70. [Google Scholar] [CrossRef]
- Ma, B.; Lawson, A.B.; Liese, A.D.; Bell, R.A.; Mayer-Davis, E.J. Dairy, magnesium, and calcium intake in relation to insulin sensitivity: approaches to modeling a dose-dependent association. Am. J. Epidemiol. 2006, 164, 449–458. [Google Scholar] [CrossRef]
- He, K.; Liu, K.; Daviglus, M.L.; Morris, S.J.; Loria, C.M.; Van Horn, L.; Jacobs, D.R.; Savage, P.J. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation 2006, 13, 1675–1682. [Google Scholar]
- Song, Y.; Ridker, P.M.; Manson, J.E.; Cook, N.R.; Buring, J.E.; Liu, S. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care 2005, 28, 1438–1444. [Google Scholar] [CrossRef]
- Schulze, Matthias B.; Schulz, M.; Heidemann, C.; Schienkiewitz, A.; Hoffmann, K.; Boeing, H. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch. Intern. Med. 2007, 167, 956–965. [Google Scholar] [CrossRef]
- Kandeel, F.R.; Balon, E.; Scott, S.; Nadler, J.L. Magnesium deficiency and glucose metabolism in rat adipocytes. Metabolism 1996, 45, 838–843. [Google Scholar] [CrossRef]
- Colak, E.; Dimitrijevic-Sreckovic, V.; Djordjevic, P.B.; Stankovic, S.; Glisic, B.; Sreckovic, B.; Majkic-Singh, N. Biomarkers of enzymatic and non-enzymatic antioxidative defense in type 2 diabetes mellitus–comparative analysis. Biochem. Med. 2008, 18, 42–51. [Google Scholar]
- Malstrom, B.; Andreasson, L.; Reinhammer, B. The Enzymes, XIIB; Boyer, P, Ed.; Academic Press: New York, USA, 1975; p. 533. [Google Scholar]
- Joo, S-J.; Betts, N.M. Copper intakes and consumption patterns of chocolate foods as sources of copper for individuals in the 1987-1988 nationwide food consumption survey. Nutr. Res. 1996, 16, 41–52. [Google Scholar] [CrossRef]
- Letavayova, L.; Vlckova, V.; Brozmanova, J. Selenium: from cancer prevention to DNA damage. Toxicology 2006, 227, 1–14. [Google Scholar] [CrossRef]
- Kornhauser, C.; Garcia-Ramirez, J.R.; Wrobel, K.; Perez-Luque, E.L.; Garay-Sevilla, M.E.; Wrobel, K. Serum selenium and glutathione peroxidase concentrations in type 2 diabetes mellitus patients. Prim. Care Diabetes 2008, 2, 81–85. [Google Scholar] [CrossRef]
- Talas, Z.S.; Ozdemir, I.; Yilmaz, I.; Gok, Y.; Orun, I. The investigation of the antioxidative properties of the novel synthetic organoselenium compounds in some rat tissues. Exp. Biol. Med. 2008, 233, 575–579. [Google Scholar] [CrossRef]
- Luz Mora, M.D.L.; Pinilla, L.; Rosas, A.; Cartes, P. Selenium uptake and its influence on the antioxidative system of white clover as affected by lime and phosphorus fertilization. Plant Soil 2008, 303, 139–149. [Google Scholar] [CrossRef]
- Krawczyk, T. Chocolates’s hidden treasure. Inform 2000, 11, 1265–1272. [Google Scholar]
- Clapperton, J.; Lockwood, R.; Romanczyk, L.; Hammerstone, J. F. Contribution of genotype to cocoa (Theobroma cacao L.) flavour. Trop. Agric. (Trinidad) 1994, 71, 303–308. [Google Scholar]
- Caligiani, A.; Cirlini, M.; Palla, G.; Ravaglia, R.; Arlorio, M. GC-MS detection of chiral markers in cocoa beans of different quality and geographic origin. Chirality 2007, 19, 329–334. [Google Scholar] [CrossRef]
- Azizah, O.; Amin, I.; Nawalyah, a.G.; Ilham, A. Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 2007, 100, 1523–1530. [Google Scholar] [CrossRef]
- Rohan, T.A. The precursors of chocolate aroma: application of gas chromatography in following formation during fermentation of cocoa beans. J. Food Sci. 1963, 32, 402–404. [Google Scholar] [CrossRef]
- Rohan, T.A.; Stewart, T. The precursors of chocolate aroma: production of free amino acids during fermentation of cocoa beans. J. Food Sci. 1966, 32, 395–398. [Google Scholar] [CrossRef]
- Biehl, B.; Passern, D. Proteolysis during fermentation-like incubation of cocoa seeds. J. Sci. Food Agric. 1982, 33, 1280–1290. [Google Scholar] [CrossRef]
- Biehl, B.; Voigt, J. Biochemistry of chocolate flavour precursors. In International Cocoa Conference, Salvadorde Bahia, Brazil, November 1996.
- Robinson, T.; Ranalli, A.W.; Phillips, A.W. Changes in cocoa tannins during processing. J. Agric. Food Chem. 1961, 9, 295–298. [Google Scholar] [CrossRef]
- Jinap, S.; Dimick, P.S. Acidic characteristics of fermented dried cocoa beans from different countries of origin. J. Food Sci. 1990, 55, 547–550. [Google Scholar] [CrossRef]
- Haslam, E.; Lilley, T.H. Polyphenol complexation: a study in molecular recognition. In Phenolic compounds in food and their effects on health; Ho, C.T., Lee, C.Y., Huang, M.T., Eds.; ACS Symposium Series 506; American Chemical Society: Washington, DC, USA, 1992; pp. 8–47. [Google Scholar]
- Lee, C.Y. Enzymatic oxidation of phenolic compounds in fruits. In Phenolic compounds in food and their effects on health; Ho, C.T., Lee, C.Y., Huang, M.T., Eds.; ACS Symposium Series 506; American Chemical Society: Washington, DC, USA, 1992; pp. 305–316. [Google Scholar]
- Bonvehi, J.S.; Coll, F.V. Evaluation of the bitterness and astringency of polyphenolic comppunds in cocoa powder. Food Chem. 1997, 60, 365–370. [Google Scholar] [CrossRef]
- Stark, T.; Bareuther, S.; Hofmann, T. Sensory-guided decomposition of roasted cocoa nibs (Theobroma cacao) and structure determination of taste-active polyphenols. J. Agric. Food Chem. 2005, 53, 5407–5418. [Google Scholar] [CrossRef]
- Pettipher, G.L. Analysis of cocoa pulp and the formulation of a standardised artificial cocoa pulp medium. J. Sci. Food Agric. 1986, 37, 297–309. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, E. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Hansen, C.E.; del Olmo, M.; Burri, C. Enzyme activities in cocoa beans during fermentation. J. Sci. Food Agric. 1998, 77, 273–281. [Google Scholar] [CrossRef]
- Bracco, U.; Grailhe, N.; Rostango, W.; Egli, R. Analytical evaluation of cocoa curing in the Ivory Coast. J. Sci. Food Agric. 1969, 20, 713–717. [Google Scholar] [CrossRef]
- Kyi, T.M.; Wan Ramli, W.D.; Abu Bakar, M.; Mohd. Wahid, S.; Abdul Amir, H.K.; Meor Zainal, M.T. The kinetics of polyphenol degradation during the drying of Malaysian cocoa beans. Int. J Food Sci. Technol. 2005, 40, 323–331. [Google Scholar] [CrossRef]
- De Brito, E.S.; Garcia, N.H.P.; Gallao, M.I.; Cortelazzo, A.L.; Fevereiro, P.S.; and Braga, M.R. Structural and chemical changes in cocoa (Theobroma cacao) during fermentation, drying and roasting. J. Sci. Food Agric. 2000, 81, 281–288. [Google Scholar]
- Miller, K.B.; Stuart, D.A.; Smith, N.L.; Lee, C.Y.; Mchale, N.L.; Flanagan, J.A.; Boxin, O.U.; Hurst, W.J. Antioxidant activity and polyphenol and procyanidin contents of selected commercially available cocoa-containing and chocolate products in the United States. J. Agric. Food Chem. 2006, 54, 4062–4068. [Google Scholar] [CrossRef]
- Gotti, R.; Furlanetto, S.; Pinzauti, S.; Cavrini, V. Analysis of catechins in Theobroma cacao beans by cyclodextrin-modified micellar electrokinetic chromatography. J. Chromatogr. Anal. 2006, 1112, 345–352. [Google Scholar]
- Bywaters, H.W. Modern methods of cocoa and chocolate manufacture. J. Franklin Inst. 1930, 209, 431–432. [Google Scholar] [CrossRef]
- Gu, L.; House, S.E.; Wu, X.; Ou, B.; Prior, R.L. Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J. Agric. Food Chem. 2006, 54, 4057–4061. [Google Scholar] [CrossRef]
- Cooper, K.A.; Campos-Gimenez, C.; Alvarez, D.J.; Rytz, A.; Nagy, K.; Williamson, G. Predictive relationship between polyphenol and nonfat cocoa solids content of chocolate. J. Agric. Food Chem. 2008, 56, 260–265. [Google Scholar]
- Nelson, B.C.; Sharpless, K.E. Quantification of the predominant monomeric catechins in baking chocolate standard reference material by LC/APCI-MS. J. Agric. Food Chem. 2003, 51, 531–537. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073–2085. [Google Scholar]
- Donovan, J.L.; Crespy, V.; Oliveira, M.; Cooper, K.A.; Gibson, B.B.; Williamson, G. (+)-Catechin is more bioavailable than (-)-catechin: Relevance to the bioavailability of catechin from cocoa. Free Rad. Res. 2006, 40, 1029–1034. [Google Scholar] [CrossRef]
- Fraga, C.G.; Martino, V.S.; Ferraro, G.E.; Coussio, J.D.; Boveris, A. Flavonoids as antioxidants evaluated by in vitro and in situ liver chemiluminescence. Biochem. Pharmacol. 1987, 36, 717–720. [Google Scholar] [CrossRef]
- Schramm, D.D.; Wang, J.F.; Holt, R.R.; Ensunsa, J.L.; Gonsalves, J.L.; Lazarus, S.A.; Schmitz, H.H.; German, J.B.; Keen, C.L. Chocolate procyanidins decrease the leukotriene-prostacyclin ratio in humans and human aortic endothelial cells. Am. J. Clin. Nutr. 2001, 73, 36–40. [Google Scholar]
- Holt, R.R.; Lazarus, S.A.; Sullards, M.C.; Zhu, Q.Y.; Schramm, D.D.; Hammerstone, J.F.; Fraga, C.G.; Schmitz, H.H.; Keen, C.L. Procyanidin dimmer B2 [epicatechin-(4β-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am. J. Clin. Nutr. 2002, 76, 798–804. [Google Scholar]
- Murphy, K.J.; Chronopoulos, A.K.; Singh, I.; Francis, M.A.; Moriarty, H.; Pike, M.J.; Turner, A.H.; Mann, N.J.; Sinclair, A.J. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am. J. Clin. Nutr. 2003, 77, 1466–1473. [Google Scholar]
- Baba, S.; Osakabe, N.; Yasuda, A.; Natsume, M.; Takizawa, T.; Nakamura, T.; Terao, J. Bioavailability of (-)-epicatechin upon intake of chocolate and cocoa in human volunteers. Free Rad. Res. 2000, 33, 635–641. [Google Scholar] [CrossRef]
- Richelle, M.; Tavazzi, I.; Enslen, M.; Offord, E. Plasma kinetics in man of epicatechin from black chocolate. Eur. J. Clin. Nutr. 1999, 53, 22–26. [Google Scholar]
- Baba, S.; Osakabe, N.; Natsume, M.; Terao, J. Absorption and urinary excretion of procyanidin B2 [epicatechin-(4β-8)-epicatechin] in rats. Free Rad. Biol. Med. 2002, 33, 142–148. [Google Scholar] [CrossRef]
- Depeint, F.; Gee, J.M.; Williamson, G.; Johnson, I.T. Evidence for consistent patterns between flavonoid structures and cellular activities. Proc. Nutr. Soc. 2002, 61, 97–103. [Google Scholar] [CrossRef]
- Spencer, J.P.; Schroeter, H.; Shenoy, B.; Srai, S.K.; Debnam, E.; Rice-Evans, C. Epicatechin is the primary bioavailable form of the procyanidin dimmers B2 and B5 after transfer across the small intestine. Biochem. Biophys. Res. Comm. 2001, 285, 588–593. [Google Scholar] [CrossRef]
- Da Silva, E.L.; Piskula, M.; Terao, J. Enhancement of antioxidative ability of rat plasma by oral administration of epicatechin. Free Rad. Biol. Med. 1998, 24, 1209–1216. [Google Scholar] [CrossRef]
- Natsume, M.; Osakabe, N.; Oyama, M.; Sasaki, M.; Baba, S.; Nakamura, Y.; Osawa, T.; Terao, J. Structures of (-)-epicatechin gkucuronide identified from plasma and urine after oral ingestion of (-)-epicatechin: differences between human and rat. Free Rad. Biol. Med. 2003, 34, 840–849. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Natsume, M.; Muto, Y.; Takizawa, T.; Terao, J. Absorption and urinary excretion of (-)-epicatechin after administration of different levels of cocoa powder or (-)-epicatechin in rats. J. Agric. Food Chem. 2001, 49, 6050–6056. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Green, R,J. Analysis of catechins from milk–tea beverages by enzyme assisted extraction followed by high performance liquid chromatography. Food Chem. 2006, 99, 484–491. [Google Scholar] [CrossRef]
- Serafini, M.; Bugianesi, R.; Maiani, G.; Valtuena, S.; De Santis, S.; Crozier, A. Plasma antioxidants from chocolate. Nature 2003, 424, 1013. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, E. Polyphenols in chocolate: Is there a contribution to human health? Food Res. Int. 2000, 33, 449–459. [Google Scholar] [CrossRef]
- Baxter, NJ; Lilley, T.H.; Haslam, E.; Williamson, M.P. Multiple interactions between polyphenols and a salivary protein-rich protein result in complexation and precipitation. Biochemistry 1997, 36, 5566–5577. [Google Scholar] [CrossRef]
- Brunet, M.J.; Blade, C.; Salvado, M.J.; Arola, L. Human Apo A-I and rat transferrin are the principal plasma proteins that bind wine catechins. J. Agric. Food Chem. 2002, 50, 2708–2712. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Hammerstone, J.F.; Lazarus, S.A.; Schmitz, H.H.; Keen, C.L. Stabilizing effect of ascorbic acid on flavan-3-ols and dimeric procyanidins from cocoa. J. Agric. Food Chem. 2003, 51, 828–833. [Google Scholar] [CrossRef]
- Klimczak, I.; Malecka, M.; Szlachta, M.; Gliszczynska-Swiglo, A. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J. Food Compos. Anal. 2007, 20, 313–322. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Holt, R.R.; Lazarus, S.A.; Ensunsa, J.L.; Hammerstone, J.F.; Schmitz, H.H.; Keen, C.L. Stability of the flavan-3-ols epicatechin and catechin and related dimeric procyanidins derived from cocoa. J. Agric. Food Chem. 2002, 50, 1700–1705. [Google Scholar] [CrossRef]
- Deprez, S.; Brezillon, C.; Rabot, S.; Philippe, C.; Mila, I.; Lapierre, C.; Scalbert, A. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J. Nutr. 2000, 130, 2733–2738. [Google Scholar]
- Spencer, J.P.E.; Chaudry, F.; Pannala, A.S.; Srai, S.K.; Debnam, E; Rice-Evans, C. Decomposition of cocoa procyanidins in the gastric milieu. Biochem. Biophys. Res. Comm. 2000, 272, 236–241. [Google Scholar] [CrossRef]
- Silberberg, M.; Gil-Izquierdo, A.; Combaret, L.; Remesy, C.; Scalbert, A.; Morand, C. Flavanone metabolism in healthy and tumor-bearing rats. Biomed. Pharmacother. 2006, 60, 529–535. [Google Scholar] [CrossRef]
- Kumazawa, T.; Seno, H.; Lee, X-P.; Ishii, A.; Watanabe-Suzuki, K.; Sato, K.; Suzuki, O. Extraction of methylxanthines from human body fluids by solid-phase microextraction. Anal. Chim. Acta 1999, 387, 53–60. [Google Scholar] [CrossRef]
- Lelo, A.; Miners, J.O.; Robson, R.; Birkett, D.J. Assessment of caffeine exposure: caffeine content of beverages, caffeine intake, and plasma concentrations of methylxanthines. Clin. Pharmacol. Ther. 1986, 39, 54–59. [Google Scholar] [CrossRef]
- Lelo, A.; Birkett, D.J.; Robson, R.A.; Miners, J.O. Comparative pharmacokinetics of caffeine and its primary demethylated metabolites paraxanthine, theobromine and theophylline in man. Brit. J. Clin. Pharmacol. 1986, 22, 177–182. [Google Scholar] [CrossRef]
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jalil, A.M.M.; Ismail, A. Polyphenols in Cocoa and Cocoa Products: Is There a Link between Antioxidant Properties and Health? Molecules 2008, 13, 2190-2219. https://doi.org/10.3390/molecules13092190
Jalil AMM, Ismail A. Polyphenols in Cocoa and Cocoa Products: Is There a Link between Antioxidant Properties and Health? Molecules. 2008; 13(9):2190-2219. https://doi.org/10.3390/molecules13092190
Chicago/Turabian StyleJalil, Abbe Maleyki Mhd, and Amin Ismail. 2008. "Polyphenols in Cocoa and Cocoa Products: Is There a Link between Antioxidant Properties and Health?" Molecules 13, no. 9: 2190-2219. https://doi.org/10.3390/molecules13092190
APA StyleJalil, A. M. M., & Ismail, A. (2008). Polyphenols in Cocoa and Cocoa Products: Is There a Link between Antioxidant Properties and Health? Molecules, 13(9), 2190-2219. https://doi.org/10.3390/molecules13092190