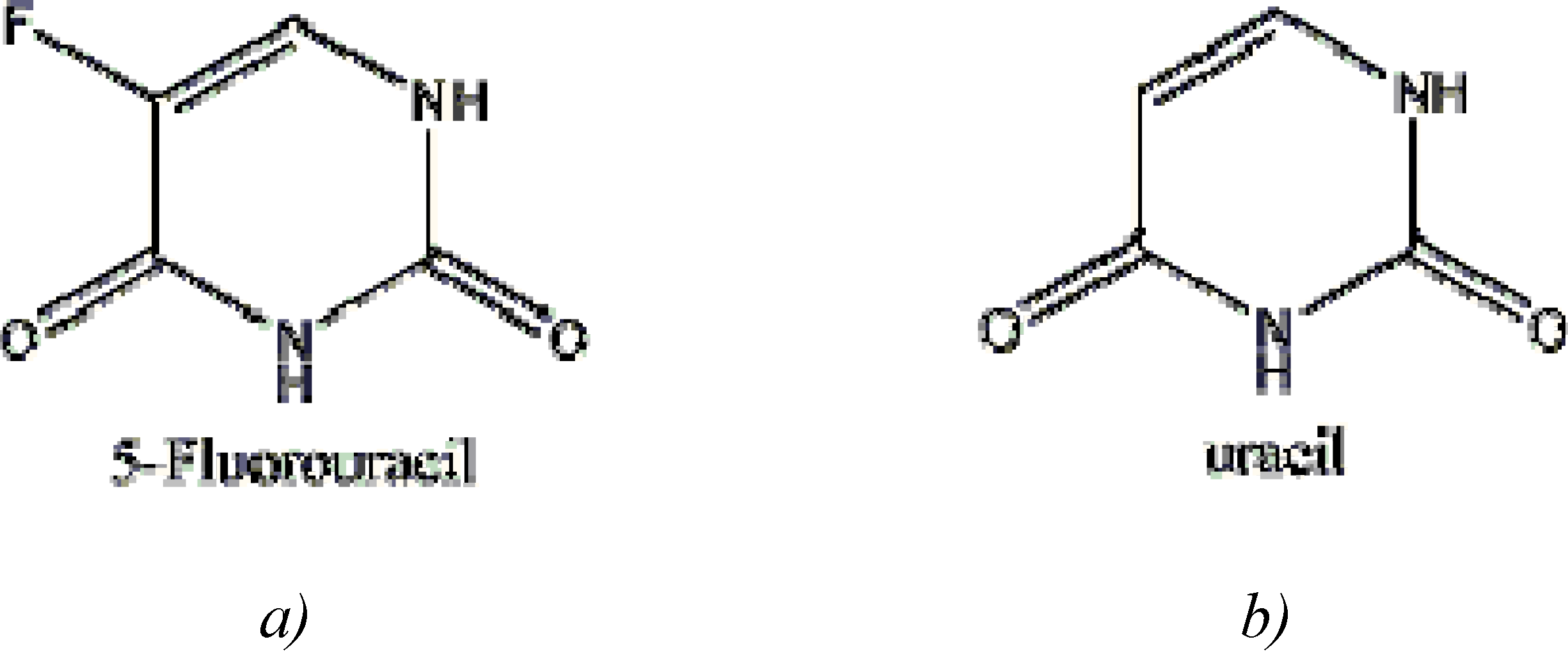
5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies
Abstract
:Introduction
Mechanism of action
TS inhibition
DNA and RNA misincorporation
Assisted modulation
Leucovorin
Interferons
Apoptosis
Cell cycle
L-Arginine
Others
Resistance
TS
DPD
DNA and RNA misincorporation
Anti-apoptosis
Cell cycle
NO
Mitochondria
Oxidative stress
Interferons
Other molecules
Gene arrays
Future perspectives
Therapeutic strategies
Reversal of resistance
Conclusions
References
- Grem, J.L. 5-Fluorouracil: forty-plus and still ticking. A review of its preclinical and clinical development. Invest New Drugs 2000, 18, 299–313. [Google Scholar] [CrossRef]
- Rutman, R.J.; Cantarow, A.; Paschkis, K.E. Studies on 2-acetylaminofluorene carcinogenesis: III. The utilization of uracil-2-C14 by pre–neoplastic rat liver. Cancer Res. 1954, 14, 119–123. [Google Scholar]
- Hulme, A.T.; Price, S.L.; Tocher, D.A. A New Polymorph of 5-Fluorouracil Found Following Computational Crystal Structure Predictions. J. Am. Chem. Soc. 2005, 127, 1116–1117. [Google Scholar] [CrossRef]
- Singh, U.P.; Ghose, R.; Ghose, A.K.; Sodhi, A.; Singh, S.M.; Singh, R.K. The effect of histidine on the structure and antitumor activity of metal-5-halouracil complexes. J. Inorg. Biochem. 1989, 37, 25–39. [Google Scholar]
- Thomas, D.M.; Zalcberg, J.R. 5-fluorouracil: a pharmacological paradigm in the use of cytotoxics. Clin. Exp. Pharmacol. Physiol. 1998, 25, 887–895. [Google Scholar]
- Noordhuis, P.; Holwerda, U.; Van der Wilt, C.L.; Van Groeningen, C.J.; Smid, K.; Meijer, S.; Pinedo, H.M.; Peters, G.J. 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal cancers. Ann. Oncol. 2004, 15, 1025–1032. [Google Scholar] [CrossRef]
- Giacchetti, S.; Perpoint, B.; Zidani, R.; Le Bail, N.; Faggiuolo, R.; Focan, C.; Chollet, P.; Llory, J.F.; Letourneau, Y.; Coudert, B.; Bertheaut-Cvitkovic, F.; Larregain-Fournier, D.; Le Rol, A.; Walter, S.; Adam, R.; Misset, J.L.; Lévi, F. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracilleucovorin as first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 2000, 18, 136–147. [Google Scholar]
- Douillard, J.Y.; Cunningham, D.; Roth, A.D.; Navarro, M.; James, R.D.; Karasek, P.; Jandik, P.; Iveson, T.; Carmichael, J.; Alakl, M.; Gruia, G.; Awad, L.; Rougier, P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000, 355, 1041–1047. [Google Scholar] [CrossRef]
- Parker, W.B.; Cheng, Y.C. Metabolism and mechanism of action of 5-fluorouracil. Pharmacol. Ther. 1990, 48, 381–395. [Google Scholar] [CrossRef]
- Longley, D.B.; Latif, T.; Boyer, J.; Allen, W.L.; Maxwell, P.J.; Johnston, P.G. The interaction of thymidylate synthase expression with p53-regulated signaling pathways in tumor cells. Semin. Oncol. 2003, 30, 3–9. [Google Scholar] [CrossRef]
- He, Y.F.; Wei, W.; Zhang, X.; Li, Y.H.; Li, S.; Wang, F.H.; Lin, X.B.; Li, Z.M.; Zhang, D.S.; Huang, H.Q.; Hu, B.; Jiang, W.Q. Analysis of the DPYD gene implicated in 5-fluorouracil catabolism in Chinese cancer patients. J. Clin. Pharm. Ther. 2008, 33, 307–314. [Google Scholar] [CrossRef]
- Bruni, P.; Minopoli, G.; Brancaccio, T.; Napolitano, M.; Faraonio, R.; Zambrano, N.; Hansen, U.; Russo, T. Fe65, a ligand of the Alzheimer's beta-amyloid precursor protein, blocks cell cycle progression by down-regulating thymidylate synthase expression. J. Biol. Chem. 2002, 277, 35481–35488. [Google Scholar] [CrossRef]
- Chernyshev, A.; Fleischmann, T.; Kohen, A. Thymidyl biosynthesis enzymes as antibiotic targets. Appl. Microbiol. Biotechnol. 2007, 74, 282–289. [Google Scholar] [CrossRef]
- Roberts, S.A.; Hyatt, D.C.; Honts, J.E.; Changchien, L.; Maley, G.F.; Maley, F.; Montfort, W.R. Structure of the Y94F mutant of Escherichia coli thymidylate synthase. Acta Crystallogr. Sect. F. Struct. Biol. Cryst Commun. 2006, 62, 840–843. [Google Scholar]
- Newby, Z.; Lee, T.T.; Morse, R.J.; Liu, Y.; Liu, L.; Venkatraman, P.; Santi, D.V.; Finer-Moore, J.S.; Stroud, R.M. The role of protein dynamics in thymidylate synthase catalysis: variants of conserved 2'-deoxyuridine 5'-monophosphate (dUMP)-binding Tyr-261. Biochemistry 2006, 45, 7415–7428. [Google Scholar] [CrossRef]
- Sotelo-Mundo, R.R.; Changchien, L.; Maley, F.; Montfort, W.R. Crystal structures of thymidylate synthase mutant R166Q: structural basis for the nearly complete loss of catalytic activity. J. Biochem. Mol. Toxicol. 2006, 20, 88–92. [Google Scholar] [CrossRef]
- Danenberg, P.V. Thymidylate synthetase - a target enzyme in cancer chemotherapy. Biochim. Biophys. Acta. 1977, 473, 73–92. [Google Scholar]
- Jarmuła, A.; Dowierciał, A.; Rode, W. A molecular modeling study of the interaction of 2'-fluoro-substituted analogues of dUMP/FdUMP with thymidylate synthase. Bioorg. Med. Chem. Lett. 2008, 18, 2701–2708. [Google Scholar] [CrossRef]
- Santi, D.V.; McHenry, C.S.; Raines, R.T.; Ivanetich, K.M. Kinetics and thermodynamics of the interaction of 5-fluoro-2'-deoxyuridylate with thymidylate synthase. Biochemistry 1987, 26, 8606–8613. [Google Scholar] [CrossRef]
- Houghton, J.A.; Tillman, D.M.; Harwood, F.G. Ratio of 2'-deoxyadenosine-5'-triphosphate/thymidine-5'-triphosphate influences the commitment of human colon carcinoma cells to thymineless death. Clin. Cancer Res. 1995, 1, 723–730. [Google Scholar]
- An, Q.; Robins, P.; Lindahl, T.; Barnes, D.E. 5-Fluorouracil incorporated into DNA is excised by the Smug1 DNA glycosylase to reduce drug cytotoxicity. Cancer Res. 2007, 67, 940–945. [Google Scholar] [CrossRef]
- Hoskins, J.; Scott Butler, J. Evidence for distinct DNA- and RNA-based mechanisms of 5-fluorouracil cytotoxicity in Saccharomyces cerevisiae. Yeast 2007, 24, 861–870. [Google Scholar] [CrossRef]
- Gustavsson, M.; Ronne, H. Evidence that tRNA modifying enzymes are important in vivo targets for 5-fluorouracil in yeast. RNA 2008, 14, 666–674. [Google Scholar] [CrossRef]
- Giaever, G.; Flaherty, P.; Kumm, J.; Proctor, M.; Nislow, C.; Jaramillo, D.F.; Chu, A.M.; Jordan, M.I.; Arkin, A.P.; Davis, R.W. Chemogenomic profiling: identifying the functional interactions of small molecules in yeast. Proc. Natl. Acad. Sci. U S A 2004, 101, 793–798. [Google Scholar] [CrossRef]
- Lum, P.Y.; Armour, C.D.; Stepaniants, S.B.; Cavet, G.; Wolf, M.K.; Butler, J.S.; Hinshaw, J.C.; Garnier, P.; Prestwich, G.D.; Leonardson, A.; Garrett-Engele, P.; Rush, C.M.; Bard, M.; Schimmack, G.; Phillips, J.W.; Roberts, C.J.; Shoemaker, D.D. Discovering modes of action for therapeutic compounds using a genome-wide screen of yeast heterozygotes. Cell 2004, 116, 121–137. [Google Scholar]
- Fang, F.; Hoskins, J.; Butler, J.S. 5-fluorouracil enhances exosome-dependent accumulation of polyadenylated rRNAs. Mol. Cell Biol. 2004, 24, 10766–10776. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Y.T. Incorporation of 5-fluorouracil into U2 snRNA blocks pseudouridylation and pre-mRNA splicing in vivo. Nucleic Acids Res. 2007, 35, 550–558. [Google Scholar] [CrossRef]
- Samuelsson, T. Interactions of transfer RNA pseudouridine synthases with RNAs substituted with fluorouracil. Nucleic Acids Res. 1991, 19, 6139–6144. [Google Scholar] [CrossRef]
- Hoskins, J.; Butler, J.S. RNA-Based 5-Fluorouracil Toxicity Requires the Pseudouridylation Activity of Cbf5p. Genetics 2008, 179, 323–330. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Dolnick, B.J.; Cheng, Y.C. Human thymidylate synthetase. II. Derivatives of pteroylmono- and -polyglutamates as substrates and inhibitors. J. Biol. Chem. 1978, 253, 3563–3567. [Google Scholar]
- Radparvar, S.; Houghton, P.J.; Houghton, J.A. Effect of polyglutamylation of 5,10-methylene-tetrahydrofolate on the binding of 5-fluoro-2′-deoxyuridylate to thymidylate synthase purified from a human colon adenocarcinoma xenograft. Biochem. Pharmacol. 1989, 38, 335–342. [Google Scholar] [CrossRef]
- Lu, K.; McGuire, J.J.; Slocum, H.K.; Rustum, Y.M. Mechanisms of acquired resistance to modulation of 5-fluorouracil by leucovorin in HCT-8 human ileocecal carcinoma cells. Biochem. Pharmacol. 1997, 53, 689–696. [Google Scholar] [CrossRef]
- Spears, C.P.; Gustavsson, B.G.; Berne, M.; Frösing, R.; Bernstein, L.; Hayes, A.A. Mechanisms of innate resistance to thymidylate synthase inhibition after 5-fluorouracil. Cancer Res. 1988, 48, 5894–5900. [Google Scholar]
- Omura, K. Advances in Chemotherapy against Advanced or Metastatic Colorectal Cancer. Digestion 2008, 77, 13–22. [Google Scholar] [CrossRef]
- Carbone, A.; Rodeck, U.; Mauri, F.A.; Sozzi, M.; Gaspari, F.; Smirne, C.; Prati, A.; Addeo, A.; Novarino, A.; Robecchi, A.; Bertetto, O.; Emanuelli, G.; Bellone, G. Human Pancreatic Carcinoma Cells Secrete Bioactive Interleukin-18 after Treatment with 5-Fluorouracil. Cancer Biol. Ther. 2005, 4, 231–241. [Google Scholar] [CrossRef]
- Kondo, M.; Nagano, H.; Wada, H.; Damdinsuren, B.; Yamamoto, H.; Hiraoka, N.; Eguchi, H.; Miyamoto, A.; Yamamoto, T.; Ota, H.; Nakamura, M.; Marubashi, S.; Dono, K.; Umeshita, K.; Nakamori, S.; Sakon, M.; Monden, M. Combination of IFN-A and 5-Fluorouracil Induces Apoptosis through IFN-A/B Receptor in Human Hepatocellular Carcinoma. Clin. Cancer Res. 2005, 11, 1277–1286. [Google Scholar]
- Wada, H.; Nagano, H.; Yamamoto, H.; Arai, I.; Ota, H.; Nakamura, M.; Damdinsuren, B.; Noda, T.; Marubashi, S.; Miyamoto, A.; Takeda, Y.; Umeshita, K.; Doki, Y.; Dono, K.; Nakamori, S.; Sakon, M.; Monden, M. Combination therapy of interferon-alpha and 5-fluorouracil inhibits tumor angiogenesis in human hepatocellular carcinoma cells by regulating vascular endothelial growth factor and angiopoietins. Oncol. Rep. 2007, 18, 801–809. [Google Scholar]
- Chan, J.Y.; Phoo, M.S.; Clement, M.V.; Pervaiz, S; Lee, S.C. Resveratrol displays converse dose-related effects on 5-fluorouracil-evoked colon cancer cell apoptosis: the roles of caspase-6 and p53. Cancer Biol. Ther. 2008, 7. [E-pub ahead of print]. [Google Scholar]
- Hwang, P.M.; Bunz, F.; Yu, J.; Rago, C.; Chan, T.A.; Murphy, M.P.; Kelso, G.F.; Smith, R.A.; Kinzler, K.W; Vogelstein, B. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat. Med. 2001, 7, 1111–1117. [Google Scholar]
- Qin, L.; Zhang, X.; Zhang, L.; Feng, Y.; Weng, G.X.; Li, M.Z.; Kong, Q.L.; Qian, C.N.; Zeng, Y.X.; Zeng, M.S.; Liao, D.F.; Song, L.B. Downregulation of BMI-1 enhances 5-fluorouracil-induced apoptosis in nasopharyngeal carcinoma cells. Biochem. Biophys. Res. Commun. 2008, 371, 531–535. [Google Scholar] [CrossRef]
- Li, M.H.; Ito, D.; Sanada, M.; Odani, T.; Hatori, M.; Iwase, M.; Nagumo, M. Effect of 5-fluorouracil on G1 phase cell cycle regulation in oral cancer cell lines. Oral Oncol. 2004, 40, 63–70. [Google Scholar]
- Yin, X.Y.; Jiang, J.M.; Liu, J.Y.; Zhu, J.R. Effects of endogenous nitric oxide induced by 5-fluorouracil and L-Arg on liver carcinoma in nude mice. World J. Gastroenterol. 2007, 13, 6249–6253. [Google Scholar] [CrossRef]
- Fukuda, H.; Takiguchi, N.; Koda, K.; Oda, K.; Seike, K.; Miyazaki, M. Thymidylate Synthase and Dihydropyrimidine Dehydrogenase are related to histological effects of 5-Fluorouracil and cisplatin neoadjuvant chemotherapy for primary gastric cancer patients. Cancer Invest. 2006, 24, 235–241. [Google Scholar] [CrossRef]
- Ooyama, A.; Oka, T.; Zhao, H.Y.; Yamamoto, M.; Akiyama, S.I.; Fukushima, M. Anti-angiogenic effect of 5-Fluorouracil-based drugs against human colon cancer xenografts. Cancer Lett. 2008. [E-pub ahead of print]. [Google Scholar]
- Hwang, J.T.; Ha, J.; Park, O.J. Combination of 5-Fluorouracil and genistein induces apoptosis synergistically in chemo-resistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochem. Biophys. Res. Commun. 2005, 332, 433–440. [Google Scholar]
- Longley, D.B.; Johnston, P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef]
- Van Triest, B.; Pinedo, H.M.; Van Hensbergen, Y.; Smid, K.; Telleman, F.; Schoenmakers, P.S.; Van der Wilt, C.L.; Van Laar, J.A.; Noordhuis, P.; Jansen, G.; Peters, G.J. Thymidylate synthase level as the main predictive parameter for sensitivity to 5-fluorouracil, but not for folate-based thymidylate synthase inhibitors, in 13 nonselected colon cancer cell lines. Clin. Cancer Res. 1999, 5, 643–654. [Google Scholar]
- Grem, J.L. Screening for dihydropyrimidine dehydrogenase deficiency. Clin. Cancer Res. 2005, 11, 5067–5068. [Google Scholar] [CrossRef]
- Arnold, C.N.; Goel, A.; Boland, C.R. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int. J. Cancer 2003, 106, 66–73. [Google Scholar] [CrossRef]
- Violette, S.; Poulain, L.; Dussaulx, E.; Pepin, D.; Faussat, A.M.; Chambaz, J.; Lacorte, J.M.; Staedel, C.; Lesuffleur, T. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-X(L) in addition to Bax and p53 status. Int. J. Cancer 2002, 98, 498–504. [Google Scholar] [CrossRef]
- Liu, R.; Page, C.; Beidler, D.R.; Wicha, M.S.; Núñez, G. Overexpression of Bclx(L) promotes chemotherapy resistance of mammary tumors in a syngeneic mouse model. Am. J. Pathol. 1999, 155, 861–867. [Google Scholar]
- Shi, X.; Liu, S.; Kleeff, J.; Friess, H.; Büchler, M.W. Acquired resistance of pancreatic cancer cells towards 5-Fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes. Oncology 2002, 62, 354–362. [Google Scholar] [CrossRef]
- Miyashita, T. Tumor suppressor p53 is a regulator of Bcl-2 and Bax gene expression in vitro and in vivo. Oncogene 1994, 9, 1799–1805. [Google Scholar]
- Petak, I.; Tillman, D.M.; Houghton, J.A. p53 dependence of Fas induction and acute apoptosis in response to 5-fluorouracil-leucovorin in human colon carcinoma cell lines. Cancer Res. 2000, 6, 4432–4441. [Google Scholar]
- Yoshioka, A.; Tanaka, S.; Hiraoka, O.; Koyama, Y.; Hirota, Y.; Ayusawa, D.; Seno, T.; Garrett, C.; Wataya, Y. Deoxyribonucleoside triphosphate imbalance. 5-Fluorodeoxyuridine-induced DNA double strand breaks in mouse FM3A cells and the mechanism of cell death. J. Biol. Chem. 1987, 262, 8235–8241. [Google Scholar]
- Aherne, G.W.; Hardcastle, A.; Raynaud, F.; Jackman, A.L. Immunoreactive dUMP and TTP pools as an index of thymidylate synthase inhibition; effect of tomudex (ZD1694) and a nonpolyglutamated quinazoline antifolate (CB30900) in L1210 mouse leukaemia cells. Biochem. Pharmacol. 1996, 51, 1293–1301. [Google Scholar] [CrossRef]
- Webley, S.D.; Hardcastle, A.; Ladner, R.D.; Jackman, A.L.; Aherne, G.W. Deoxyuridine triphosphatase (dUTPase) expression and sensitivity to the thymidylate synthase (TS) inhibitor ZD9331. Br. J. Cancer 2000, 83, 792–799. [Google Scholar] [CrossRef]
- Costi, M.P.; Tondi, D.; Rinaldi, M.; Barlocco, D.; Pecorari, P.; Soragni, F.; Venturelli, A.; Stroud, R.M. Structure-based studies on species-specific inhibition of thymidylate synthase. Biochim. Biophys. Acta 2002, 1587, 206–214. [Google Scholar] [CrossRef]
- Forsthoefel, A.M.; Peña, M.M.; Xing, Y.Y.; Rafique, Z.; Berger, F.G. Structural determinants for the intracellular degradation of human thymidylate synthase. Biochemistry 2004, 43, 1972–1979. [Google Scholar] [CrossRef]
- Peña, M.M.; Xing, Y.Y.; Koli, S.; Berger, F.G. Role of N-terminal residues in the ubiquitin-independent degradation of human thymidylate synthase. Biochem. J. 2006, 394, 355–363. [Google Scholar] [CrossRef]
- Forsthoefel, A.M.; Peña, M.M.; Xing, Y.Y.; Rafique, Z.; Berger, F.G. Structural determinants for the intracellular degradation of human thymidylate synthase. Biochemistry 2004, 43, 1972–1979. [Google Scholar] [CrossRef]
- Peña, M.M.; Xing, Y.Y.; Koli, S.; Berger, F.G. Role of N-terminal residues in the ubiquitin-independent degradation of human thymidylate synthase. Biochem. J. 2006, 394, 355–363. [Google Scholar] [CrossRef]
- Houghton, J.A.; Houghton, P.J. Elucidation of pathways of 5-fluorouracil metabolism in xenografts of human colorectal adenocarcinoma. Eur. J. Cancer Clin. Oncol. 1983, 19, 807–815. [Google Scholar] [CrossRef]
- Peters, G.J.; Backus, H.H.; Freemantle, S.; van Triest, B.; Codacci-Pisanelli, G.; van der Wilt, C.L.; Smid, K.; Lunec, J.; Calvert, A.H.; Marsh, S.; McLeod, H.L.; Bloemena, E.; Meijer, S.; Jansen, G.; van Groeningen, C.J.; Pinedo, H.M. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim. Biophys. Acta 2002, 1587, 194–205. [Google Scholar] [CrossRef]
- Priest, D.G.; Ledford, B.E.; Doig, M.T. Increased thymidylate synthetase in 5-fluorodeoxyuridine resistant cultured hepatoma cells. Biochem. Pharmacol. 1980, 29, 1549–1553. [Google Scholar] [CrossRef]
- Berger, S.H.; Barbour, K.W.; Berger, F.G. A naturally occurring variation in thymidylate synthase structure is associated with a reduced response to 5-fluoro-2'-deoxyuridine in a human colon tumor cell line. Mol. Pharmacol. 1988, 34, 480–484. [Google Scholar]
- Jenh, C.H.; Geyer, P.K.; Baskin, F.; Johnson, L.F. Thymidylate synthase gene amplification in fluorodeoxyuridine-resistant mouse cell lines. Mol. Pharmacol. 1985, 28, 80–85. [Google Scholar]
- Grem, J.L.; Fischer, P.H. Enhancement of 5-fluorouracil's anticancer activity by dipyridamole. Pharmacol Ther. 1989, 40, 349–371. [Google Scholar] [CrossRef]
- Chu, E.; Grem, J.L.; Johnston, P.G.; Allegra, C.J. New concepts for the development and use of antifolates. Stem Cells 1996, 14, 41–46. [Google Scholar] [CrossRef]
- Wang, W.; McLeod, H.L.; Cassidy, J.; Collie-Duguid, E.S. Mechanisms of acquired chemoresistance to 5-fluorouracil and tomudex: thymidylate synthase dependent and independent networks. Cancer Chemother. Pharmacol. 2007, 59, 839–845. [Google Scholar] [CrossRef]
- Tajima, A.; Hess, M.T.; Cabrera, B.L.; Kolodner, R.D.; Carethers, J.M. The mismatch repair complex hMutS alpha recognizes 5-fluorouracil-modified DNA: implications for chemosensitivity and resistance. Gastroenterology 2004, 127, 1678–1684. [Google Scholar] [CrossRef]
- Sasaki, S.; Watanabe, T.; Kobunai, T.; Konishi, T.; Nagase, H.; Sugimoto, Y.; Oka, T.; Nagawa, H. hRFI overexpressed in HCT116 cells modulates Bcl-2 family proteins when treated with 5-fluorouracil. Oncol. Rep. 2006, 15, 1293–1298. [Google Scholar] [Green Version]
- Konishi, T.; Sasaki, S.; Watanabe, T.; Kitayama, J.; Nagawa, H. Overexpression of hRFI inhibits 5-fluorouracil-induced apoptosis in colorectal cancer cells via activation of NF-kappaB and upregulation of BCL-2 and BCL-XL. Oncogene 2006, 25, 3160–3169. [Google Scholar] [CrossRef]
- Tseng, Y.S.; Tzeng, C.C.; Chiu, A.W.; Lin, C.H.; Won, S.J.; Wu, I.C.; Liu, H.S. Exp. Cell Res. 2003, 288, 403–414. [CrossRef]
- Brenes, O.; Arce, F.; Gätjens-Boniche, O.; Díaz, C. Characterization of cell death events induced by anti-neoplastic drugs cisplatin, paclitaxel and 5-fluorouracil on human hepatoma cell lines: Possible mechanisms of cell resistance. Biomed. Pharmacother. 2007, 61, 347–355. [Google Scholar] [CrossRef]
- Guo, X.; Goessl, E.; Jin, G.; Collie-Duguid, E.S.; Cassidy, J.; Wang, W.; O'Brien, V. Cell cycle perturbation and acquired 5-fluorouracil chemoresistance. Anticancer Res. 2008, 28, 9–14. [Google Scholar]
- Lechner, M.; Lirk, P.; Rieder, J. Inducible nitric oxide synthase (iNOS) in tumor biology: the two sides of the same coin. Semin. Cancer Bio. 2005, 15, 277–289. [Google Scholar]
- Romashkova, J.A.; Makarov, S.S. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature 1999, 401, 86–90. [Google Scholar] [CrossRef]
- Islam, S.; Hassan, F.; Tumurkhuu, G.; Ito, H.; Koide, N.; Mori, I.; Yoshida, T.; Yokochi, T. 5-Fluorouracil prevents lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage cells by inhibiting Akt-dependent nuclear factor-kappaB activation. Cancer Chemother. Pharmacol. 2007, 59, 227–233. [Google Scholar]
- Shin, Y.K.; Yoo, B.C.; Chang, H.J.; Jeon, E.; Hong, S.H.; Jung, M.S.; Lim, S.J.; Park, J.G. Down-regulation of Mitochondrial F1F0-ATP Synthase in Human Colon Cancer Cells with Induced 5-Fluorouracil Resistance. Cancer Res. 2005, 65, 3162–3170. [Google Scholar]
- Hwang, I.T.; Chung, Y.M.; Kim, J.J.; Chung, J.S.; Kim, B.S.; Kim, H.J.; Kim, J.S.; Yoo, Y.D. Drug resistance to 5-FU linked to reactive oxygen species modulator. Biochem. Biophys. Res. Commun. 2007, 359, 304–310. [Google Scholar] [CrossRef]
- Chu, E.; Zinn, S.; Boarman, D.; Allegra, C.J. Interaction of γ interferon and 5-Fluorouracil in the H630 human colon carcinoma cell line. Cancer Res. 1990, 50, 5834–5840. [Google Scholar]
- Swain, S.M.; Boarman, D.; Allegra, C.J. Fluorouracil and high-dose leucovorin in previously treated patients with metastatic breast cancer. J. Clin. Oncol. 1989, 7, 890–899. [Google Scholar]
- Ishii, T.; Marumo, K. Biochemical modulation of 5-fluorouracil with interferonα/βandγon murine renal cell carcinoma. Int. J. Urol. 2004, 11, 993–1000. [Google Scholar] [CrossRef]
- Kang, H.C.; Kim, I.J.; Park, H.W.; Jang, S.G.; Ahn, S.A.; Yoon, S.N.; Chang, H.J.; Yoo, B.C.; Park, J.G. Regulation of MDK expression in human cancer cells modulates sensitivities to various anticancer drugs: MDK overexpression confers to a multi-drug resistance. Cancer Lett. 2007, 247, 40–47. [Google Scholar] [CrossRef]
- Yoo, B.C.; Jeon, E.; Hong, S.H.; Shin, Y.K.; Chang, H.J.; Park, J.G. Metabotropic glutamate receptor 4-mediated 5-Fluorouracil resistance in a human colon cancer cell line. Clin. Cancer Res. 2004, 15, 4176–4184. [Google Scholar]
- Katakura, K.; Fujise, H.; Takeda, K.; Kaneko, O.; Torii, M.; Suzuki, M.; Chang, K.P.; Hashiguchi, Y. Overexpression of LaMDR2, a novel multidrug resistance ATP-binding cassette transporter, causes 5-fluorouracil resistance in Leishmania amazonensis. FEBS Lett. 2004, 561, 207–212. [Google Scholar] [CrossRef]
- Pratt, S.; Shepard, R.L.; Kandasamy, R.A.; Johnston, P.A.; Perry, W., 3rd.; Dantzig, A.H. The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol. Cancer Ther. 2005, 4, 855–863. [Google Scholar]
- Fanciullino, R.; Giacometti, S.; Mercier, C.; Aubert, C.; Blanquicett, C.; Piccerelle, P.; Ciccolini, J. In vitro and in vivo reversal of resistance to 5-fluorouracil in colorectal cancer cells with a novel stealth double-liposomal formulation. Br. J. Cancer. 2007, 97, 919–926. [Google Scholar]
- Tsujie, M.; Nakamori, S.; Nakahira, S.; Takahashi, Y.; Hayashi, N.; Okami, J.; Nagano, H.; Dono, K.; Umeshita, K.; Sakon, M.; Monden, M. Human equilibrative nucleoside transporter 1, as a predictor of 5-fluorouracil resistance in human pancreatic cancer. Anticancer Res. 2007, 27, 2241–2249. [Google Scholar]
- Park, J.S.; Young, Yoon. S; Kim, J.M.; Yeom, Y.I.; Kim, Y.S.; Kim, N.S. Identification of novel genes associated with the response to 5-FU treatment in gastric cancer cell lines using a cDNA microarray. Cancer Lett. 2004, 214, 19–33. [Google Scholar]
- Kim, H.K.; Choi, I.J.; Kim, H.S.; Kim, J.H.; Kim, E.; Park, I.S.; Chun, J.H.; Kim, I.H.; Kim, I.J.; Kang, H.C.; Park, J.H.; Bae, J.M.; Lee, J.S.; Park, J.G. DNA microarray analysis of the correlation between gene expression patterns and acquired resistance to 5-FU/cisplatin in gastric cancer. Biochem Biophys Res. Commun. 2004, 316, 781–789. [Google Scholar] [CrossRef]
- De Angelis, P.M.; Svendsrud, D.H.; Kravik, K.L.; Stokke, T. Cellular response to 5-fluorouracil (5-FU) in 5-FU-resistant colon cancer cell lines during treatment and recovery. Mol. Cancer 2006, 5, 20. [Google Scholar]
- Wang, W.; Cassidy, J.; O'Brien, V.; Ryan, K.M.; Collie-Duguid, E. Mechanistic and predictive profiling of 5-Fluorouracil resistance in human cancer cells. Cancer Res. 2004, 64, 8167–8176. [Google Scholar] [CrossRef]
- de Angelis, P.M.; Fjell, B.; Kravik, K.L.; Haug, T.; Tunheim, S.H.; Reichelt, W.; Beigi, M.; Clausen, O.P.; Galteland, E.; Stokke, T. Molecular characterizations of derivatives of HCT116 colorectal cancer cells that are resistant to the chemotherapeutic agent 5-fluorouracil. Int. J. Oncol. 2004, 24, 1279–1288. [Google Scholar]
- Nordgard, S.H.; Alnaes, G.I.; Hihn, B.; Lingjaerde, O.C.; Liestøl, K.; Tsalenko, A.; Sørlie, T.; Lønning, P.E.; Børresen-Dale, A.L.; Kristensen, V.N. Pathway based analysis of SNPs with relevance to 5-FU therapy: relation to intratumoral mRNA expression and survival. Int. J. Cancer. 2008, 123, 577–585. [Google Scholar] [CrossRef]
- Ooyama, A.; Okayama, Y.; Takechi, T.; Sugimoto, Y.; Oka, T.; Fukushima, M. Genome-wide screening of loci associated with drug resistance to 5-fluorouracil-based drugs. Cancer Sci. 2007, 98, 577–583. [Google Scholar] [CrossRef]
- Schmidt, W.M.; Kalipciyan, M.; Dornstauder, E.; Rizovski, B.; Steger, G.G; Sedivy, R; Mueller, M.W; Mader, R.M. Dissecting progressive stages of 5-fluorouracil resistance in vitro using RNA expression profiling. Int. J. Cancer 2004, 112, 200–212. [Google Scholar] [CrossRef]
- Szöke, D.; Györffy, A.; Surowiak, P.; Tulassay, Z.; Dietel, M.; Györffy, B. Identification of consensus genes and key regulatory elements in 5-fluorouracil resistance in gastric and colon cancer. Onkologie 2007, 30, 421–426. [Google Scholar] [CrossRef]
- Zhang, F.M.; Yao, X.J.; Tian, X.; Tu, Y.Q. Synthesis and biological evaluation of new 4beta-5-Fu-substituted 4'-demethylepipodophyllotoxin derivatives. Molecules 2006, 11, 849–857. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Du, G.J.; Xie, S.Q.; Zhao, J.; Gao, W.Y.; Wang, C.J. Synthesis and bioevaluation of 5-fluorouracil derivatives. Molecules 2007, 12, 2450–2457. [Google Scholar] [CrossRef]
- Etienne, M.C.; Cheradame, S.; Fischel, J.L.; Formento, P.; Dassonville, O.; Renée, N.; Schneider, M.; Thyss, A.; Demard, F.; Milano, G. Response to fluorouracil therapy in cancer patients: the role of tumoral dihydropyrimidine dehydrogenase activity. J. Clin. Oncol. 1995, 13, 1663–1670. [Google Scholar]
- Johnston, P.G.; Lenz, H.J.; Leichman, C.G.; Danenberg, K.D.; Allegra, C.J.; Danenberg, P.V.; Leichman, L. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res. 1995, 55, 1407–1412. [Google Scholar]
- Shirasaka, T.; Nakano, K.; Takechi, T.; Satake, H.; Uchida, J.; Fujioka, A.; Saito, H.; Okabe, H.; Oyama, K.; Takeda, S.; Unemi, N.; Fukushima, M. Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res. 1996, 56, 2602–2606. [Google Scholar]
- Schöffski, P. The modulated oral fluoropyrimidine prodrug S-1, and its use in gastrointestinal cancer and other solid tumors. Anticancer Drugs 2004, 15, 85–106. [Google Scholar] [CrossRef]
- Sakurai, Y.; Sakamoto, K.; Sugimoto, Y.; Yoshida, I.; Masui, T.; Tonomura, S.; Inaba, K.; Shoji, M.; Nakamura, Y.; Uyama, I.; Komori, Y.; Ochiai, M.; Matsuura, S.; Tanaka, H.; Oka, T.; Fukushima, M. Blackwell Publishing Asia Orotate phosphoribosyltransferase levels measured by a newly established enzyme-linked immunosorbent assay in gastric carcinoma. Cancer Sci. 2006, 97, 492–498. [Google Scholar] [CrossRef]
- Shi, L.X.; Ma, R.; Lu, R.; Xu, Q.; Zhu, Z.F.; Wang, L.; Zhou, C.L.; Li, X.L.; Zhang, H.L.; Yao, Z. Reversal effect of tyroservatide (YSV) tripeptide on multi-drug resistance in resistant human hepatocellular carcinoma cell line BEL-7402/5-FU. Cancer Lett. 2008. [E-pub ahead of print]. [Google Scholar]
- Yu, Z.W.; Zhao, P.; Liu, M.; Dong, X.S.; Tao, J.; Yao, X.Q.; Yin, X.H.; Li, Y.; Fu, S.B. Reversal of 5-flouroucial resistance by adenovirus-mediated transfer of wild-type p53 gene in multidrug-resistant human colon carcinoma LoVo/5-FU cells. World J. Gastroenterol. 2004, 10, 1979–1983. [Google Scholar]
- Zhu, H.; Guo, W.; Zhang, L.; Davis, J.J.; Teraishi, F.; Wu, S.; Cao, X.; Daniel, J.; Smythe, W.R.; Fang, B. Bcl-XL small interfering RNA suppresses the proliferation of 5-fluorouracil-resistant human colon cancer cells. Mol. Cancer Ther. 2005, 4, 451–456. [Google Scholar]
- Zhu, H.; Zhang, L.; Huang, X.; Davis, J.J.; Jacob, D.A.; Teraishi, F.; Chiao, P.; Fang, B. Overcoming acquired resistance to TRAIL by chemotherapeutic agents and calpain inhibitor I through distinct mechanisms. Mol. Ther. 2004, 9, 666–673. [Google Scholar] [CrossRef]
- Mikano, G.; Etienne, M.C.; Pierrefite, V.; Barberi-Heyob, M.; Deporte-Fety, R.; Renée, N. Dihydropyrimidine dehydrogenase deficiency and fluorouracil-related toxicity. Br. J. Cancer 1999, 79, 627–630. [Google Scholar] [CrossRef]
- Raida, M.; Schwabe, W.; Häusler, P.; Van Kuilenburg, A.B.; Van Gennip, A.H.; Behnke, D.; Höffken, K. Prevalence of a common point mutation in the dihydropyrimidine dehydrogenase (DPD) gene within the 5’-splice donor site of intron 14 in patients with severe 5-fluorouracil (5-FU)-related toxicity compared with controls. Clin. Cancer Res. 2001, 7, 2832–2839. [Google Scholar]
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, N.; Yin, Y.; Xu, S.-J.; Chen, W.-S. 5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies. Molecules 2008, 13, 1551-1569. https://doi.org/10.3390/molecules13081551
Zhang N, Yin Y, Xu S-J, Chen W-S. 5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies. Molecules. 2008; 13(8):1551-1569. https://doi.org/10.3390/molecules13081551
Chicago/Turabian StyleZhang, Ning, Ying Yin, Sheng-Jie Xu, and Wei-Shan Chen. 2008. "5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies" Molecules 13, no. 8: 1551-1569. https://doi.org/10.3390/molecules13081551
APA StyleZhang, N., Yin, Y., Xu, S.-J., & Chen, W.-S. (2008). 5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies. Molecules, 13(8), 1551-1569. https://doi.org/10.3390/molecules13081551



