Camptothecinoids from the seeds of Taiwanese Nothapodytes foetida
Abstract
:Introduction
Results and Discussion
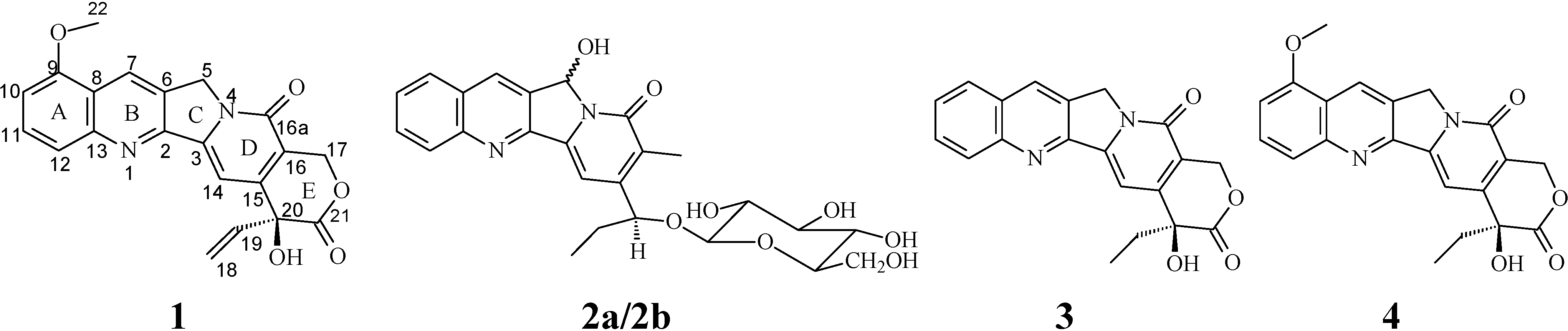
Determination of Isolated Compounds
| 1H-NMR | 13C-NMR | |||
|---|---|---|---|---|
| Position | 1 | 2a/2b | 1 | 2a/2b |
| 1 | ||||
| 2 | 152.6 | 152.9/153.0 | ||
| 3 | 146.3 | 141.9 | ||
| 4 | ||||
| 5 | 5.28 (2H, s) | 6.99/7.00 (1H, s) | 50.6 | 84.3/84.4 |
| 6 | 129.2 | 133.1 | ||
| 7 | 8.87 (1H, s) | 8.58/8.59 (1H, s) | 126.1 | 134.5 |
| 8 | 120.0 | 129.9 | ||
| 9 | 8.06 (1H, d, 7.8), | 154.9 | 130.1 | |
| 10 | 7.18 (1H, d, 7.8) | 7.85 (1H, t, 7.8) | 106.0 | 132.2 |
| 11 | 7.78 (1H, dd, 7.8, 8.4) | 7.67 (1H, t, 7.8) | 130.6 | 128.8 |
| 12 | 7.73 (1H, d, 8.4) | 8.12 (1H, d, 7.8) | 121.1 | 129.7 |
| 13 | 148.8 | 150.4 | ||
| 14 | 7.32 (1H, s) | 7.59 (1H, s) | 96.8 | 101.8/101.9 |
| 15 | 148.5 | 153.2/153.3 | ||
| 16 | 119.4 | 130.6/130.7 | ||
| 16a | 156.7 | 163.5 | ||
| 17 | 5.37 (2H, dd, 15.6, 16.2) | 2.25 (3H, s) | 65.1 | 12.2 |
| 18 | 5.33 (1H, d, 10.2) | 1.00 (3H, t) | 117.1 | 10.1 |
| 5.34 (1H, d, 17.4) | ||||
| 19 | 5.99 (1H, dd, 10.2, 17.4) | 1.89 (2H, m) | 134.2 | 29.9 |
| 20 | 5.27 (1H, t) | 73.4 | 76.6 | |
| 21 | 170.8 | |||
| -Ome | 4.05 (3H, s) | 56.2 | ||
| -OH | 7.06 (1H, s) | |||
| 20-O-β-glucoside | ||||
| 1' | 4.09/4.10 (1H, d, 7.8) | 101.7 | ||
| 2' | 3.21- 3.35 (1H, m) | 75.2 | ||
| 3' | 3.21- 3.35 (1H, m) | 77.9 | ||
| 4' | 3.21- 3.35 (1H, m) | 71.8 | ||
| 5' | 3.18 (1H, m) | 78.2 | ||
| 6'a | 3.91 (1H, m) | 62.9 | ||
| 6'b | 3.69 (1H, m) | |||
Qualitative and quantitative analysis
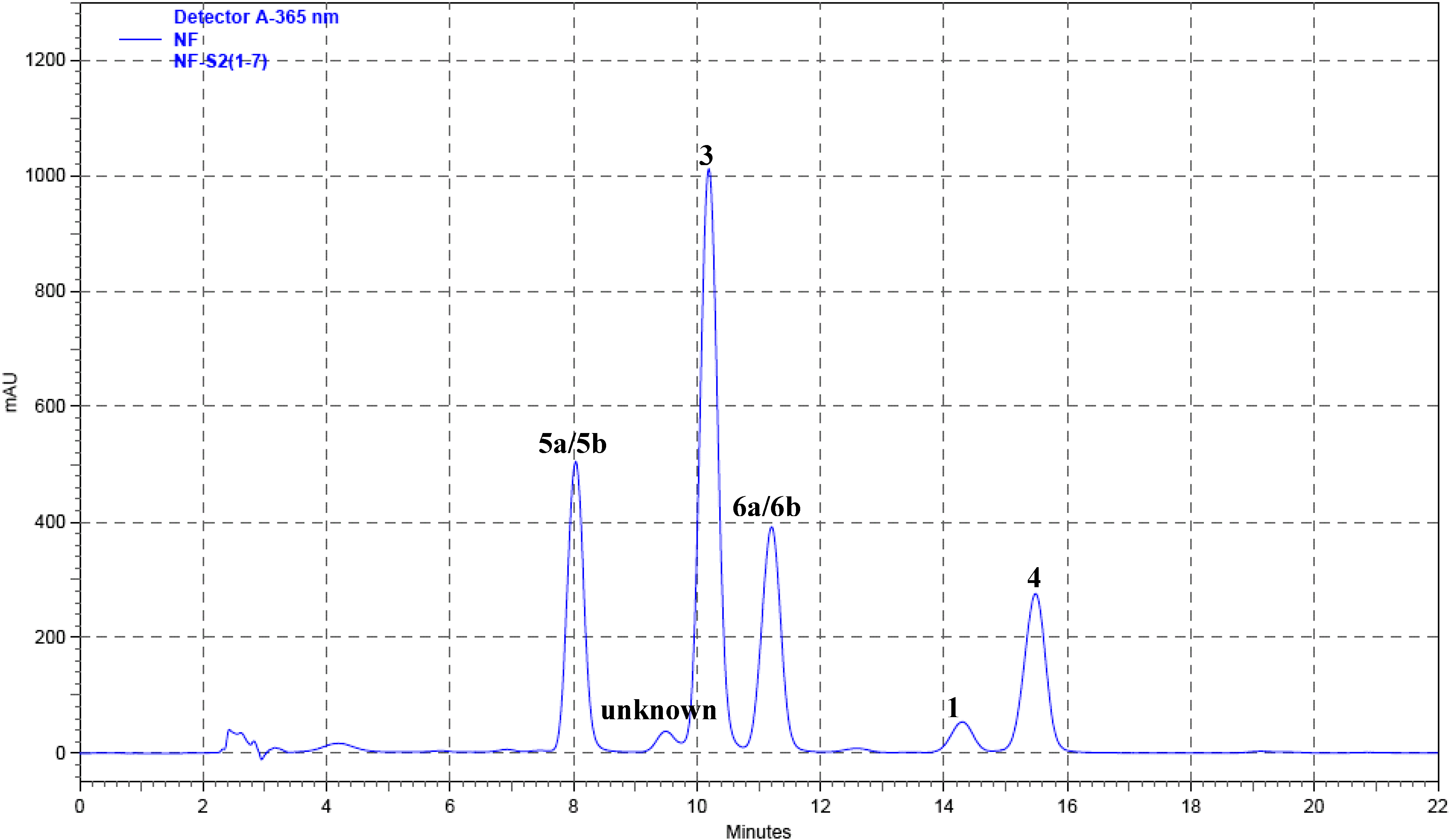
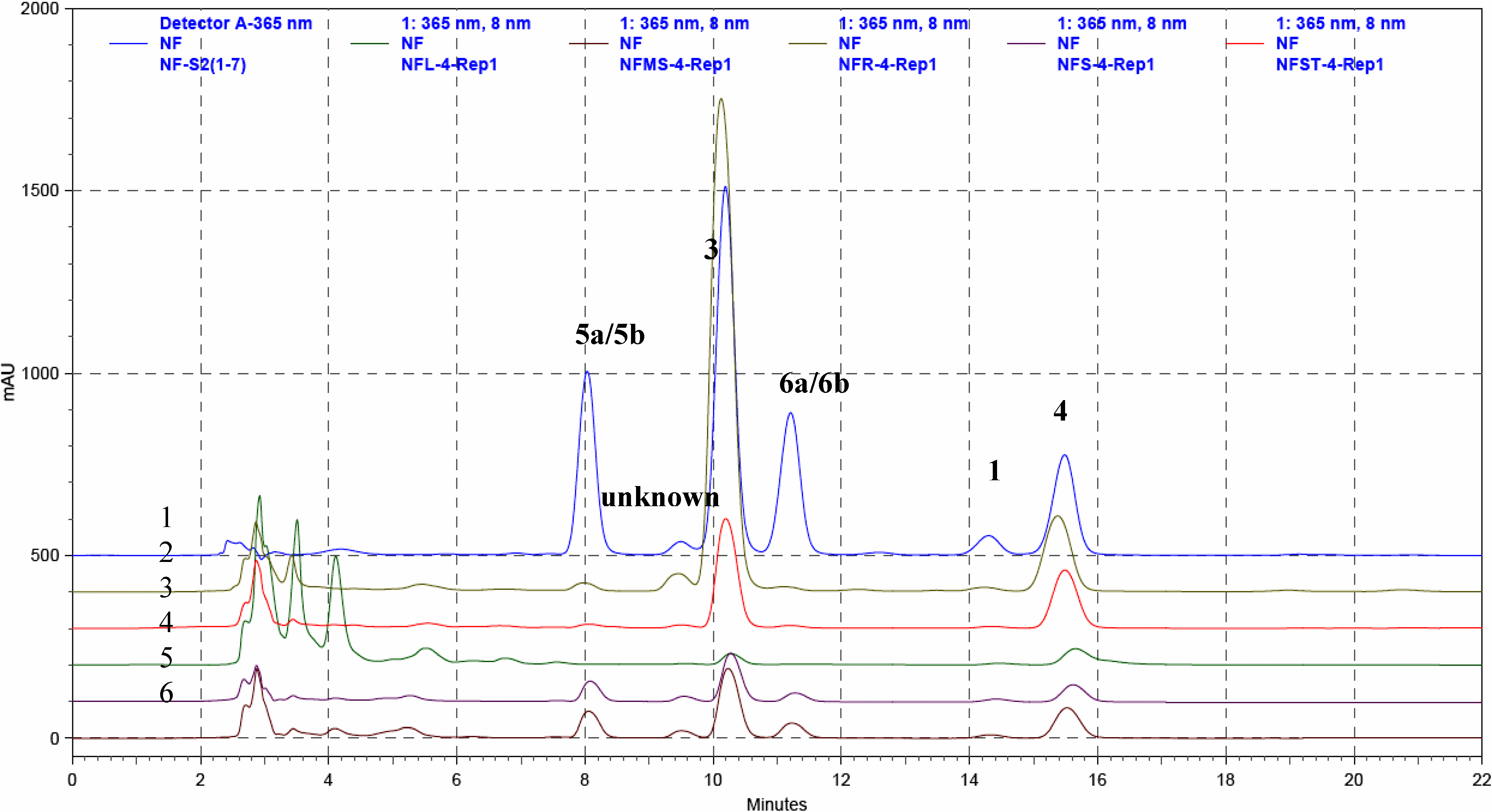
| Compound | Rt (min) | Regression equation | Linear range (mg/mL) | R2 |
|---|---|---|---|---|
| Camptothecin (3) | 10.186 | y = 4E+07x + 138346 | 0.015625-0.5 | 0.9998 |
| 9-methoxy-camptothecin (4) | 15.478 | y = 3E+07x + 134716 | 0.015625-0.5 | 0.9999 |
| Plant part | Leaves | Mature seeds | Roots | Immature seeds | Stems |
|---|---|---|---|---|---|
| Camptothecin (3) | 0.58 | 0.54 | 15.59 | 0.40 | 1.78 |
| 9-methoxy-camptothecin (4) | 1.80 | 0.38 | 3.85 | 0.21 | 1.54 |
Cytotoxicity of Isolated Compounds
| Compound | HepG2 | Hep3B | MDA-MB-231 | MCF-7 | A549 | Ca9-22 |
|---|---|---|---|---|---|---|
| 1 | 3.43 | 3.80 | 6.57 | 6.22 | 2.77 | 0.24 |
| 2a/2b | - | - | - | - | - | - |
| 3 | 44.02 | 0.40 | 2.36 | 0.37 | 0.11 | 0.02 |
| 4 | 41.01 | 0.58 | 1.88 | 0.37 | 0.16 | 0.01 |
| 5a/5b | 42.06 | 8.10 | - | 35.25 | 5.44 | 8.11 |
| 6a/6b | 39.52 | 2.87 | 26.78 | 12.39 | 5.20 | 1.85 |
| Doxorubicin | 0.15 | 0.33 | 0.28 | 0.18 | 0.24 | 0.22 |
Experimental
General
Plant Materials
Extraction and Isolation
Compound characterization
 +24.5° (CHCl3; c 0.20); UV
+24.5° (CHCl3; c 0.20); UV  nm (log ε): 261 (3.66), 357 (3.49); IR (neat) νmax 3406, 2922, 1745, 1652, 1600, 1450, 1372, 1118 cm-1; 1H- and 13C-NMR (DMSO), see Table 1; HRESIMS m/z 377.1135 [M+H]+, (calcd. for C21H17N2O5, 377.1137).
nm (log ε): 261 (3.66), 357 (3.49); IR (neat) νmax 3406, 2922, 1745, 1652, 1600, 1450, 1372, 1118 cm-1; 1H- and 13C-NMR (DMSO), see Table 1; HRESIMS m/z 377.1135 [M+H]+, (calcd. for C21H17N2O5, 377.1137). -39.2° (CHCl3; c0.20); UV
-39.2° (CHCl3; c0.20); UV  nm (log ε): 223 (3.96), 255 (3.82), 357 (3.63); IR (neat) νmax 3397, 2929, 1670, 1596, 1457, 1340, 1195, 1113 cm-1; 1H- and 13C-NMR (CD3OD) see Table 1; HRESIMS m/z 485.1925 [M+H]+, (calcd. for C25H29N2O8, 485.1924).
nm (log ε): 223 (3.96), 255 (3.82), 357 (3.63); IR (neat) νmax 3397, 2929, 1670, 1596, 1457, 1340, 1195, 1113 cm-1; 1H- and 13C-NMR (CD3OD) see Table 1; HRESIMS m/z 485.1925 [M+H]+, (calcd. for C25H29N2O8, 485.1924).Crude samples prepared from different parts of N. foetida for qualitative and quantitative analysis
Preparation of reference samples
Analytical HPLC
Calibration
Cytotoxicity assay
Acknowledgements
References
- Wall, M.E.; Wani, M.C.; Cook, C.E.; Palmer, K.H.; McPhail, A.T.; Sim, G.A. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Viswanathan, N. Alkaloids of Mappia foetida. Phytochemistry 1972, 11, 3529–3531. [Google Scholar] [CrossRef]
- Arisawa, M.; Gunasekera, S.P.; Cordell, G.A.; Farnsworth, N.R. Plant anticancer agents XXI. Constituents of Merrilliodendron megacarpum. Planta Med. 1981, 43, 404–407. [Google Scholar] [CrossRef]
- Zhou, B.N.; Hoch, J.M.; Johnson, R.K.; Mattern, M.R.; Eng, W.K.; Ma, J.; Hecht, S.M.; Newman, D.J.; Kingston, D.G. Use of COMPARE analysis to discover new natural product drugs: isolation of camptothecin and 9-methoxycamptothecin from a new source. J. Nat. Prod. 2000, 63, 1273–1276. [Google Scholar] [CrossRef]
- Tafur, S.; Nelson, J.D.; DeLong, D.C.; Svoboda, G.H. Antiviral components of Ophiorrhiza mungos isolation of camptothecin and 10-methoxycamptothecin. Lloydia 1976, 39, 261–262. [Google Scholar]
- Saito, K.; Sudo, H.; Yamazaki, M.; Koseki-Nakamura, M.; Kitajima, M.; Takayama, H.; Aimi, N. Feasible production of camptothecin by hairy root culture of Ophiorrhiza pumila. Plant Cell Rep. 2001, 20, 267–271. [Google Scholar] [CrossRef]
- Klausmeyer, P.; McCloud, T.G.; Melillo, G.; Scudiero, D.A.; Cardellina, J.H.II.; Shoemaker, R.H. Identification of a new natural camptothecin analogue in targeted screening for HIF-1α inhibitors. Planta Med. 2007, 73, 49–52. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Badawi, M.M.; Cordell, G.A.; Farnsworth, N.R.; Chitnis, M. Plant anticancer agents X. Isolation of camptothecin and 9-methoxycamptothecin from Ervatamia heyneana. J. Nat. Prod. 1979, 42, 475–477. [Google Scholar] [CrossRef]
- Dai, J.R.; Cardellina, J.H.; Boyd, M.R. 20-Oβ-Glucopyranosyl camptothecin from Mostuea brunonis: a potential camptothecin pro-drug with improved solubility. J. Nat. Prod. 1999, 62, 1427–1429. [Google Scholar] [CrossRef]
- Fulzele, D.P.; Satdive, R.K.; Pol, B.B. Untransformed root cultures of Nothapodytes foetida and production of camptothecin. Plant Cell, Tissue and Organ Culture 2002, 69, 285–288. [Google Scholar] [CrossRef]
- Hsiang, Y.H.; Hertzberg, R.; Hecht, S.; Liu, L.F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Biol. Chem. 1985, 260, 14873–14878. [Google Scholar]
- Lorence, A.; Nessler, C.L. Camptothecin, over four decades of surprising findings. Phytochemistry 2004, 65, 2735–2749. [Google Scholar] [CrossRef]
- Wu, T.S.; Chan, Y.Y.; Leu, Y.L.; Chern, C.Y.; Chen, C.F. Nothapodytes A and B from Nothapodytes foetida. Phytochemistry 1996, 42, 907–908. [Google Scholar] [CrossRef]
- Wu, T.S.; Leu, Y.L.; Hsu, H.C.; Ou, L.F.; Chen, C.C.; Chen, C.F.; Ou, J.C.; Wu, Y.C. Constituents and cytotoxic principles of Nothapodytes foetida. Phytochemistry 1995, 39, 383–385. [Google Scholar]
- Li, C.Y.; Lin, C.H.; Wu, T.S. Quantitative analysis of camptothecin derivatives in Nothapodytes foetida using 1H-NMR method. Chem Pharm Bull (Tokyo). 2005, 53, 347–349. [Google Scholar] [CrossRef]
- Aiyama, R.; Nagai, H.; Nokata, K.; Shinohara, C.; Sawada, S. A camptothecin derivative from Nothapodytes foetida. Phytochemistry 1988, 27, 3663–3664. [Google Scholar] [CrossRef]
- Subrahmanyam, D.; Sarma, V.M.; Venkateswarlu, A.; Sastry, T.V.; Kulakarni, A.P.; Rao, D.S.; Reddy, K.V. In vitro cytotoxicity of 5-aminosubstituted 20(S)-camptothecins. Part 1. Bioorg Med Chem. 1999, 7, 2013–2020. [Google Scholar] [CrossRef]
- Pirillo, A.; Verotta, L.; Gariboldi, P.; Torregiani, E.; Bombardelli, E. Constituents of Nothapodytes foetida. J. Chem. Soc. Perkin Trans. I 1995, 5, 583–587. [Google Scholar]
- Akita, H.; Kawahar, E.; Kishida, M.; Kato, K. Synthesis of naturally occurring β-D-glucopyranoside based on enzymatic β-glycosidation. J. Mol. Catal.; B Enzym. 2006, 40, 8–15. [Google Scholar] [CrossRef]
- Fiorentino, A.; DellaGreca, M.; D'Abrosca, B.; Golino, A.; Pacifico, S.; Izzo, A.; Monaco, P. Unusual sesquiterpene glucosides from Amaranthus retroflexus. Tetrahedron 2006, 62, 8952–8958. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application toproliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, S.-F.; Hsieh, P.-W.; Wu, C.-C.; Lee, C.-L.; Chen, S.-L.; Lu, C.-Y.; Wu, T.-S.; Chang, F.-R.; Wu, Y.-C. Camptothecinoids from the seeds of Taiwanese Nothapodytes foetida. Molecules 2008, 13, 1361-1371. https://doi.org/10.3390/molecules13061361
Wu S-F, Hsieh P-W, Wu C-C, Lee C-L, Chen S-L, Lu C-Y, Wu T-S, Chang F-R, Wu Y-C. Camptothecinoids from the seeds of Taiwanese Nothapodytes foetida. Molecules. 2008; 13(6):1361-1371. https://doi.org/10.3390/molecules13061361
Chicago/Turabian StyleWu, Shou-Fang, Pei-Wen Hsieh, Chin-Chung Wu, Chia-Lin Lee, Shu-Li Chen, Chi-Yu Lu, Tian-Shung Wu, Fang-Rong Chang, and Yang-Chang Wu. 2008. "Camptothecinoids from the seeds of Taiwanese Nothapodytes foetida" Molecules 13, no. 6: 1361-1371. https://doi.org/10.3390/molecules13061361
APA StyleWu, S.-F., Hsieh, P.-W., Wu, C.-C., Lee, C.-L., Chen, S.-L., Lu, C.-Y., Wu, T.-S., Chang, F.-R., & Wu, Y.-C. (2008). Camptothecinoids from the seeds of Taiwanese Nothapodytes foetida. Molecules, 13(6), 1361-1371. https://doi.org/10.3390/molecules13061361







