A New Sesquiterpenoid Hydroquinone from the Marine Sponge Dysidea arenaria
Abstract
:Introduction
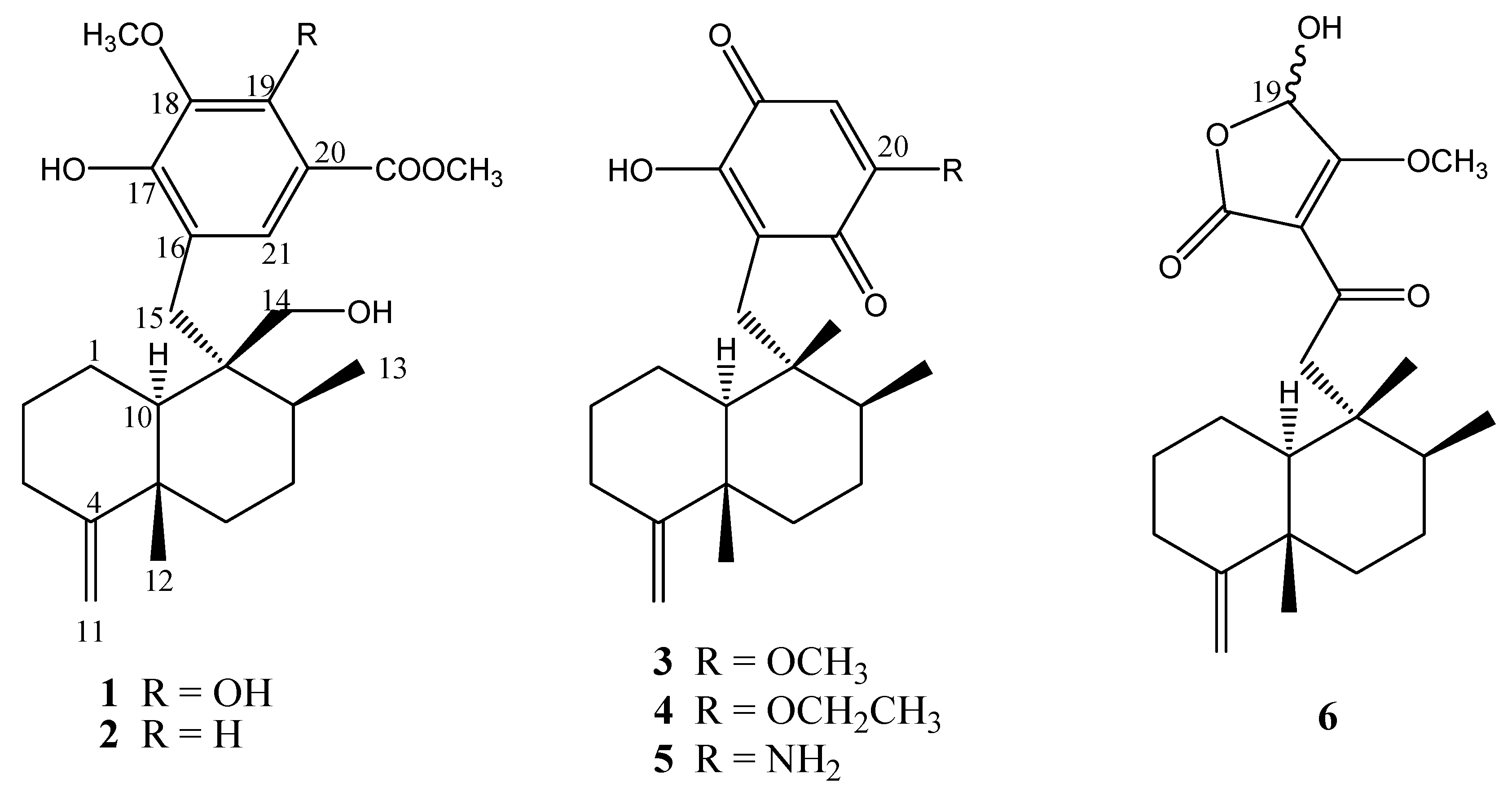
Results and Discussion
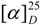 = +2.3 (c 0.12, CHCl3), was isolated as a white amorphous solid. The molecular formula C24H34O6was deduced on the basis of HRFAB-MS (m/z: 417.2277, [M-H]-, calcd. 417.2283). The hypothesis of the presence of a phenolic group was confirmed by the IR (3422, 1670, 1440, 1342, 1210, 1056, 985, 892 cm-1) and UV (224, 269, 310 nm) data. In its 1H-NMR spectrum, the two terminal-vinyl protons at δH 4.44, 4.47 (each 1H, br s), two methyl signals (δH 1.07, s; 1.11, d, J = 6.7 Hz), and an additional oxygenated methylene at δH 3.81, 3.90 (d, J = 11.7 Hz) suggested the presence of a hydroxymethyl group on a 4,9-friedodrimane-4-ene skeleton. HMBC correlations between the two protons and the carbons at δC 31.0, 37.1, 49.2 located the –CH2OH group at C-9. A comparison with the related known compound polyfibrospongol B (2) showed a molecular weight 16 a.m.u. greater, which together with the presence of only one aromatic proton at δH 7.38 (1H, s) with a singlet (δH 10.90, 1H, s) at lower fields indicated an additional hydroxyl substitution on the aromatic ring. HMBC correlations: 10.90/105.3, 133.7, 153.0; 7.38/133.7, 153.0, 154.2, 170.7; 3.99/133.7; 3.92/170.7 (Figure 2) confirmed the structure of a pentasubstituted phenolic group. It became clear that the 4,9-friedodrimane skeleton was connected to the aromatic group when the correlation between δH 7.38 and δC 31.0 in the HMBC spectrum was observed. The relative configuration was suggested by the NOESY correlations found between Me-12 and Me-13, and between Me-12 and H-14. The proton at δH 1.06 (1H, m, H-10) was overlapped with Me-12 and Me-13. Detailed elucidation with NOESY spectrum revealed the crosspeak between H-10 and H-15a (1H, d, J = 15 Hz), while no crosspeaks were observed between H-10 and H2-14, which indicated the same orientation of H-10 and H2-15.
= +2.3 (c 0.12, CHCl3), was isolated as a white amorphous solid. The molecular formula C24H34O6was deduced on the basis of HRFAB-MS (m/z: 417.2277, [M-H]-, calcd. 417.2283). The hypothesis of the presence of a phenolic group was confirmed by the IR (3422, 1670, 1440, 1342, 1210, 1056, 985, 892 cm-1) and UV (224, 269, 310 nm) data. In its 1H-NMR spectrum, the two terminal-vinyl protons at δH 4.44, 4.47 (each 1H, br s), two methyl signals (δH 1.07, s; 1.11, d, J = 6.7 Hz), and an additional oxygenated methylene at δH 3.81, 3.90 (d, J = 11.7 Hz) suggested the presence of a hydroxymethyl group on a 4,9-friedodrimane-4-ene skeleton. HMBC correlations between the two protons and the carbons at δC 31.0, 37.1, 49.2 located the –CH2OH group at C-9. A comparison with the related known compound polyfibrospongol B (2) showed a molecular weight 16 a.m.u. greater, which together with the presence of only one aromatic proton at δH 7.38 (1H, s) with a singlet (δH 10.90, 1H, s) at lower fields indicated an additional hydroxyl substitution on the aromatic ring. HMBC correlations: 10.90/105.3, 133.7, 153.0; 7.38/133.7, 153.0, 154.2, 170.7; 3.99/133.7; 3.92/170.7 (Figure 2) confirmed the structure of a pentasubstituted phenolic group. It became clear that the 4,9-friedodrimane skeleton was connected to the aromatic group when the correlation between δH 7.38 and δC 31.0 in the HMBC spectrum was observed. The relative configuration was suggested by the NOESY correlations found between Me-12 and Me-13, and between Me-12 and H-14. The proton at δH 1.06 (1H, m, H-10) was overlapped with Me-12 and Me-13. Detailed elucidation with NOESY spectrum revealed the crosspeak between H-10 and H-15a (1H, d, J = 15 Hz), while no crosspeaks were observed between H-10 and H2-14, which indicated the same orientation of H-10 and H2-15.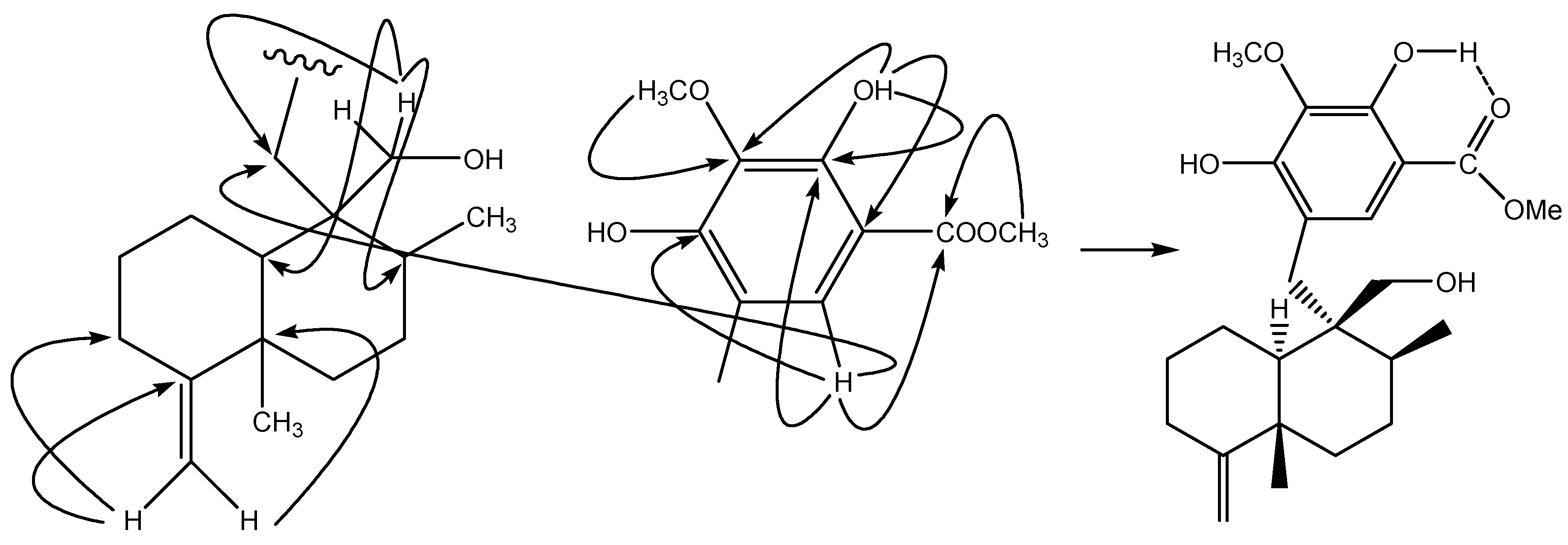
| No. | Compound 1 | No. | Compound 1 | ||
| C | H (J Hz) | C | H (J Hz) | ||
| 1 | 24.1 | 2.12, m | 13 | 18.9 | 1.11, d, (6.7) |
| 2 | 28.3 | 1.85, m | 14 | 64.5 | 3.90, d, (11.7)
3.81, d, (11.7) |
| 3 | 33.2 | 2.33, m
2.12, m | 15 | 31.0 | 3.02, d, (15.0)
2.77, d, (15.0) |
| 4 | 159.7 | 16 | 116.4 | ||
| 5 | 40.0 | 17 | 154.2 | ||
| 6 | 36.9 | 1.60, m | 18 | 133.7 | |
| 7 | 27.9 | 1.47, m | 19 | 153.0 | |
| 8 | 37.1 | 1.30, m | 20 | 105.3 | |
| 9 | 46.2 | 21 | 128.6 | 7.38, s | |
| 10 | 49.2 | 1.06, m | 18-OMe | 60.8 | 3.99, s |
| 11 | 103.3 | 4.47, 4.44, br s | 20-COOMe | 52.1 | 3.92, s |
| 12 | 20.9 | 1.08, s | 170.7 | ||
Experimental
General
Extraction and Isolation
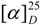 = +2.3 (c 0.12, CHCl3); UV (MeOH) λmax (log ε) 224 (4.55), 269 (4.26), 310 (3.78) nm; IR (neat) νmax 3422, 1670, 1440, 1342, 1210, 1056, 985, 892, 795 cm-1; for 1H-NMR and 13C-NMR see Table 1; ESI-TOF MS (m/z): 417 [M-H]-, 403 [M-Me]-, 386; HRFAB-MS (m/z) 417.2277, [M-H]-, (calcd. for C24H33O6, 417.2283).
= +2.3 (c 0.12, CHCl3); UV (MeOH) λmax (log ε) 224 (4.55), 269 (4.26), 310 (3.78) nm; IR (neat) νmax 3422, 1670, 1440, 1342, 1210, 1056, 985, 892, 795 cm-1; for 1H-NMR and 13C-NMR see Table 1; ESI-TOF MS (m/z): 417 [M-H]-, 403 [M-Me]-, 386; HRFAB-MS (m/z) 417.2277, [M-H]-, (calcd. for C24H33O6, 417.2283).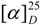 = +1.7 (c 0.26, CHCl3); UV (MeOH) λmax (log ε) 227 (4.57), 270 (4.21), 301 (3.82) nm; IR (neat) νmax 3420, 2940, 1710, 1645, 1440, 1310, 1220, 1025 cm-1; ESI-TOF MS- (m/z): 401 [M-H]-, 386[M-H-Me]-; 1H-NMR (CDCl3): δH 2.15 (2H, dd, J = 13.5, 2.0 Hz, H-1), 1.90, 1.35 (each 1H, m, H-2), 2.30, 2.10 (each 1H, m, H-3), 1.60, 1.57 (each 1H, m, H-6), 1.48 (2H, m, H-7), 1.40 (1H, m, H-8), 1.12 (1H, m, H-10), 4.46, 4.42 (each 1H, br s, H-11), 1.08 (3H, s, H-12), 1.13 (1H, d, J = 6.5 Hz, H-13), 3.92, 3.82 (each 1H, d, J = 11.7 Hz, H-14), 3.14, 2.86 (each 1H, d, J =14.5 Hz, H-15), 7.42 (1H, d, J = 1.7 Hz, H-19), 7.52 (1H, d, J = 1.7 Hz, H-21), 3.96 (3H, s, 18-OMe), 3.90 (3H, s, 20-COOMe).
= +1.7 (c 0.26, CHCl3); UV (MeOH) λmax (log ε) 227 (4.57), 270 (4.21), 301 (3.82) nm; IR (neat) νmax 3420, 2940, 1710, 1645, 1440, 1310, 1220, 1025 cm-1; ESI-TOF MS- (m/z): 401 [M-H]-, 386[M-H-Me]-; 1H-NMR (CDCl3): δH 2.15 (2H, dd, J = 13.5, 2.0 Hz, H-1), 1.90, 1.35 (each 1H, m, H-2), 2.30, 2.10 (each 1H, m, H-3), 1.60, 1.57 (each 1H, m, H-6), 1.48 (2H, m, H-7), 1.40 (1H, m, H-8), 1.12 (1H, m, H-10), 4.46, 4.42 (each 1H, br s, H-11), 1.08 (3H, s, H-12), 1.13 (1H, d, J = 6.5 Hz, H-13), 3.92, 3.82 (each 1H, d, J = 11.7 Hz, H-14), 3.14, 2.86 (each 1H, d, J =14.5 Hz, H-15), 7.42 (1H, d, J = 1.7 Hz, H-19), 7.52 (1H, d, J = 1.7 Hz, H-21), 3.96 (3H, s, 18-OMe), 3.90 (3H, s, 20-COOMe).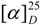 = -23.2 (c 1.23, CHCl3); UV (MeOH) λmax (log ε) 285 (4.36), 420 (3.12) nm; IR (neat) νmax 3340, 1642, 1607, 1205 cm-1; ESI-TOF MS- (m/z): 357 [M-H]-; 1H-NMR (CDCl3): δH 2.10, 1.44 (each 1H, m, H-1), 1.86, 1.18 (each 1H, m, H-2), 2.32, 2.08 (each 1H, ddd, J = 13.7, 8.6, 5.4 Hz, H-3), 1.51, 1.34 (each 1H, m, H-6), 1.39 (2H, m, H-7), 1.16 (1H, m, H-8), 0.76 (1H, dd, J = 12.0, 2.0 Hz, H-10), 4.33, 4.34 (each 1H, br s, H-11), 1.04 (3H, s, H-12), 0.98 (3H, d, J = 6.5 Hz, H-13), 0.84 (3H, s, H-14), 2.53, 2.47 (each 1H, d, J = 13.7 Hz, H-15), 5.86 (1H, s, H-19), 3.86 (3H, s, 20-OMe).
= -23.2 (c 1.23, CHCl3); UV (MeOH) λmax (log ε) 285 (4.36), 420 (3.12) nm; IR (neat) νmax 3340, 1642, 1607, 1205 cm-1; ESI-TOF MS- (m/z): 357 [M-H]-; 1H-NMR (CDCl3): δH 2.10, 1.44 (each 1H, m, H-1), 1.86, 1.18 (each 1H, m, H-2), 2.32, 2.08 (each 1H, ddd, J = 13.7, 8.6, 5.4 Hz, H-3), 1.51, 1.34 (each 1H, m, H-6), 1.39 (2H, m, H-7), 1.16 (1H, m, H-8), 0.76 (1H, dd, J = 12.0, 2.0 Hz, H-10), 4.33, 4.34 (each 1H, br s, H-11), 1.04 (3H, s, H-12), 0.98 (3H, d, J = 6.5 Hz, H-13), 0.84 (3H, s, H-14), 2.53, 2.47 (each 1H, d, J = 13.7 Hz, H-15), 5.86 (1H, s, H-19), 3.86 (3H, s, 20-OMe).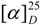 = -20.4 (c 0.82, CHCl3); UV (MeOH) λmax (log ε) 277 (4.38), 422 (3.17) nm; IR (neat) νmax 3338, 2924, 2856, 1645, 1609, 1382, 1234, 1220 cm-1; HRFAB-MS- (m/z): 371.2224 [M-1]-; 1H-NMR (CDCl3): δH 2.10, 1.47 (each 1H, m, H-1), 1.88, 1.19 (each 1H, m, H-2), 2.35, 2.09 (each 1H, ddd, J = 13.8, 8.5, 5.5 Hz, H-3), 1.55, 1.36 (each 1H, m, H-6), 1.41 (2H, m, H-7), 1.17 (1H, m, H-8), 0.80 (1H, dd, J = 12.0, 1.7 Hz, H-10), 4.46, 4.47 (each 1H, br s, H-11), 1.07 (3H, s, H-12), 1.00 (3H, d, J = 6.0 Hz, H-13), 0.87 (3H, s, H-14), 2.54, 2.49 (each 1H, d, J = 13.5 Hz, H-15), 5.85 (1H, s, H-19), 4.07 (2H, q, J = 7.0 Hz, 20-OCH2CH3), 1.52 (3H, t, J = 7.0 Hz, 20-OCH2CH3).
= -20.4 (c 0.82, CHCl3); UV (MeOH) λmax (log ε) 277 (4.38), 422 (3.17) nm; IR (neat) νmax 3338, 2924, 2856, 1645, 1609, 1382, 1234, 1220 cm-1; HRFAB-MS- (m/z): 371.2224 [M-1]-; 1H-NMR (CDCl3): δH 2.10, 1.47 (each 1H, m, H-1), 1.88, 1.19 (each 1H, m, H-2), 2.35, 2.09 (each 1H, ddd, J = 13.8, 8.5, 5.5 Hz, H-3), 1.55, 1.36 (each 1H, m, H-6), 1.41 (2H, m, H-7), 1.17 (1H, m, H-8), 0.80 (1H, dd, J = 12.0, 1.7 Hz, H-10), 4.46, 4.47 (each 1H, br s, H-11), 1.07 (3H, s, H-12), 1.00 (3H, d, J = 6.0 Hz, H-13), 0.87 (3H, s, H-14), 2.54, 2.49 (each 1H, d, J = 13.5 Hz, H-15), 5.85 (1H, s, H-19), 4.07 (2H, q, J = 7.0 Hz, 20-OCH2CH3), 1.52 (3H, t, J = 7.0 Hz, 20-OCH2CH3).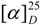 = -17.6 (c 0.82, MeOH); UV (MeOH) λmax (log ε) 206 (4.14), 317 (3.52) nm; IR (neat) νmax 3477, 3280, 2921, 2858, 1568, 1375, 1333, 1203 cm-1; ESI-TOF MS- (m/z): 342 [M-H]-; 1H-NMR (CDCl3): δH 2.05, 1.47 (each 1H, m, H-1), 1.89, 1.26 (each 1H, m, H-2), 2.53, 2.34 (each 1H, m, H-3), 1.56, 1.32 (each 1H, m, H-6), 1.40 (2H, m, H-7), 1.17 (1H, m, H-8), 1.33 (1H, m, H-10), 4.47 (2H, br s, H-11), 1.06 (3H, s, H-12), 1.23 (3H, d, J = 6.3 Hz, H-13), 0.94 (3H, s, H-14), 2.81, 2.72 (each 1H, d, J = 13.6 Hz, H-15), 5.86 (1H, s, H-19).
= -17.6 (c 0.82, MeOH); UV (MeOH) λmax (log ε) 206 (4.14), 317 (3.52) nm; IR (neat) νmax 3477, 3280, 2921, 2858, 1568, 1375, 1333, 1203 cm-1; ESI-TOF MS- (m/z): 342 [M-H]-; 1H-NMR (CDCl3): δH 2.05, 1.47 (each 1H, m, H-1), 1.89, 1.26 (each 1H, m, H-2), 2.53, 2.34 (each 1H, m, H-3), 1.56, 1.32 (each 1H, m, H-6), 1.40 (2H, m, H-7), 1.17 (1H, m, H-8), 1.33 (1H, m, H-10), 4.47 (2H, br s, H-11), 1.06 (3H, s, H-12), 1.23 (3H, d, J = 6.3 Hz, H-13), 0.94 (3H, s, H-14), 2.81, 2.72 (each 1H, d, J = 13.6 Hz, H-15), 5.86 (1H, s, H-19).Acknowledgements
References
- Rodríguez, J.; Quiñoá, E.; Riguera, R.; Peters, B. M.; Abrell, L. M.; Crewsetc, P. The structures and stereochemistry of cytotoxic sesquiterpene quinines from Dactylospongia elegans. Tetrahedron 1992, 48, 6667–6680. [Google Scholar] [CrossRef]
- Kondracki, M. L.; Guyot, M. Smenospongine: A cytotoxic and antimicrobial aminoquinone isolated from Smenospongia sp. Tetrahedron Lett. 1987, 28, 5815–5818. [Google Scholar] [CrossRef]
- Kushlan, D. M.; Faulkner, D. J.; Parkanyi, L.; Clardy, J. Metabolite of the palauan sponge Dactylospongia sp. Tetrahedron 1989, 45, 3307–3312. [Google Scholar] [CrossRef]
- Kondracki, M. L.; Debitus, C.; Guyot, M. A cytotoxic new red dimer from a new caledonian marine sponge Hyrtios sp. Tetrahedron Lett. 1996, 37, 3861–3864. [Google Scholar] [CrossRef]
- Salmoun, M.; Devijver, C.; Daloze, D.; Braekman, J. C.; Gomez, R.; Kluijver, M.; Soest, R. W. M. V. New sesquiterpene/quinones from two sponges of the genes Hyrtios. J. Nat. Prod. 2000, 63, 452–456. [Google Scholar]
- Giannini, C.; Debitus, C.; Posadas, I.; Payá, M.; D’Auria, M. V. Dysidotronic acid, a new and selective human phospholipase A2 inhibitor from the sponge Dysidea sp. Tetrahedron Lett. 2000, 41, 3257–3260. [Google Scholar]
- Talpir, R.; Rudi, A.; Kashman, Y.; Loya, Y.; Hizi, A. Three new sesquiterpene hydroquinones from marine origin. Tetrahedron 1994, 50, 4179–4184. [Google Scholar] [CrossRef]
- Piggott, A. M.; Karuso, P. 9-Hydroxyfurodysinin-O-ethyl lactone: a new sesquiterpene isolated from the tropical marine sponge Dysidea arenaria. Molecules 2005, 10, 1292–1297. [Google Scholar] [CrossRef]
- Unson, M. D.; Rose, C. B.; Faulkner, D. J.; Brinen, L. S.; Steiner, J. R.; Clardy, J. New polychlorinated amino acid derivatives from the marine sponge Dysidea herbacea. J. Org. Chem. 1993, 58, 6336–6343. [Google Scholar] [CrossRef]
- Handayani, D.; Edrada, R. A.; Proksch, P.; Wray, V.; Witte, L.; Van Soest, R. W. M.; Kunzmann, A.; Soedarsono. Four new bioactive polybrominated diphenyl ethers of the sponge Dysidea herbacea from west sumatra, Indonesia. J. Nat. Prod. 1997, 60, 1313–1316. [Google Scholar] [CrossRef]
- Shen, Y. C.; Hsieh, P. W. New sesquiterpene hydroquinones from a taiwanese marine sponge Polyfibrospongia australis. J. Nat. Prod. 1997, 60, 93–97. [Google Scholar] [CrossRef]
- Kondracki, M. L.; Guyot, M. Biologically active quinine and hydroquinone sesquiterpenoids from the sponge Smenospongia sp. Tetrahedron 1989, 45, 1995–2004. [Google Scholar] [CrossRef]
- Luibrand, R. T.; Erdman, T. R.; Vollmer, J. J.; Scheuer, P. J.; Finer, J.; Clardy, J. Ilimaquinone, a sesquiterpenoid quinone from a marine sponge. Tetrahedron 1979, 35, 609–612. [Google Scholar] [CrossRef]
- Kondracki, M. L.; Guyot, M. A. New sesquiterpene tetronic acid derivative from the marine sponge Smenospongia sp. Tetrahedron Lett. 1999, 40, 3149–3150. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the corresponding author.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qiu, Y.; Wang, X.M. A New Sesquiterpenoid Hydroquinone from the Marine Sponge Dysidea arenaria. Molecules 2008, 13, 1275-1281. https://doi.org/10.3390/molecules13061275
Qiu Y, Wang XM. A New Sesquiterpenoid Hydroquinone from the Marine Sponge Dysidea arenaria. Molecules. 2008; 13(6):1275-1281. https://doi.org/10.3390/molecules13061275
Chicago/Turabian StyleQiu, Yan, and Xiu Min Wang. 2008. "A New Sesquiterpenoid Hydroquinone from the Marine Sponge Dysidea arenaria" Molecules 13, no. 6: 1275-1281. https://doi.org/10.3390/molecules13061275
APA StyleQiu, Y., & Wang, X. M. (2008). A New Sesquiterpenoid Hydroquinone from the Marine Sponge Dysidea arenaria. Molecules, 13(6), 1275-1281. https://doi.org/10.3390/molecules13061275




