Abstract
A simple and efficient protocol has been developed for the Michael addition of amines to α,β-unsaturated esters under microwave irradiation. Under these conditions there was a significant decrease in the reaction time, increases in the yields and increased purity of the products.
Introduction
Microwave-assisted chemistry offers new possibilities for the development of any chemical reaction that is thermally possible. It typically produces faster reactions and higher yields and minimizes the formation of by-products. In addition, there exists the possibility that the reactions that fail under conventional heating could give the desired products using microwave irradiation [1].
The number of papers on this topic has increased considerably in the last decade. The result of this has been a diverse range of chemical transformations performed with success through microwaves, for example, cycloadditions, eliminations, substitutions and additions [2,3]. Additionally, some recently reported examples include one-pot reactions [4] and particular organic reactions such as Suzuki couplings [5], Claisen rearrangements [6], Mitsunobu reactions [7] and Michael additions [8].
The Michael reaction is one of the most versatile reactions in organic synthesis, and this has been further enhanced through the use of microwaves. [8] One of the more useful applications of this process of 1,4-addition is the synthesis of β-amino acids and derivatives, which can be carried out under asymmetric conditions by means of chiral induction that the nucleophile or the Michael acceptor could exert [9,10]. Furthermore, it is possible carry out the synthesis of such compounds in racemic form and later resolves the enantiomers through some separation method such as enzymatic kinetic resolution [11].
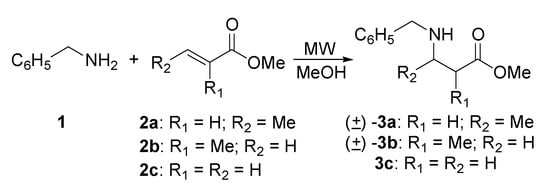
Motivated by the development of biocatalysts applied specifically to the resolution of racemic β-amino esters [12], our research group developed a procedure for the preparation of a series of β-amino esters derived from 1,4-addition of benzylamine (1) and (S)-(-)-α-methylbenzylamine (4) to methyl crotonate (2a), methyl methacrylate (2b), and methyl acrylate (2c), under microwave irradiation. The addition reactions were carried out in methanol in a monomode microwave CEM Discover apparatus, by two methods: a) sealed system, in vessels rated for high pressure; b) open system, in conventional round bottom flasks equipped with condensers. In every case the experiments were performed on the 1-3 to 45 mmol scale. We have decided to use methanol as reaction solvent in order to compare our results with those reported in the literature [10a,c, 13].
Results and Discussion
Preparation of N-benzyl-β-amino esters derived from benzylamine
The reaction between benzylamine (1) and methyl crotonate (2a) (Scheme 1), under microwave irradiation in methanol allowed the preparation of the compound (±)-3a with 83-98 % yield within a short period of time (3 h) (Table 1). Conventionally, the reaction between these substrates is carried out in 5 days under thermal conditions (65 °C, oil bath) with stirring in a sealed tube, providing a 73 % yield. The best yield for 3a was obtained when the stoichiometric ratio utilized was 1 mmol (entry 1, Table 1) compared when it was 45 mmol. The lower temperature utilized in entry 2 could be a determinant factor for the diminished yield, however, the yield is high in comparison with that obtained under conventional conditions and the shorter reaction time is notable.
Scheme 1.
The addition of benzylamine to methyl methacrylate (2b) proceeded under similar reaction conditions. Starting with 2b it is possible to form side products due to the high temperature and a long reaction time. For this reason, we decided to begin at 115 °C for two hours (entry 3, Table 1). TLC analysis revealed only starting material. The temperature was then increased at 130 °C for one hour more, providing 3b in 97 % yield. The same experiment performed with 45 mmol at 140 °C (entry 4) was complete in 6.5 hours with slightly lower yield.

Table 1.
1,4-Addition of benzylamine to α,β-unsaturated esters.
| Entry | Product | Power (Watts) | T (oC) | Pressure (psi) | Time (min) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | (±)-3a | 37 | 150 | 110 | 180 | 98 |
| 2 | (±)-3a | 55 | 140 | a | 180 | 83 |
| 3 | (±)-3b | 50 | 115-130 | 68 | 180 | 97 |
| 4 | (±)-3b | 55 | 140 | a | 390 | 80 |
| 5 | 3c:6 | 50 | 115 | 59 | 180 | 50:50b |
| 6 | 3c:6 | 50 | 65 | 5 | 10 | 70:30b |
| 7 | 3c:6 | 40 | 65 | c | 10 | 70:30b |
| 8 | 3c:6 | 40 | 65 | c | 3 | 90:10b |
anot determined.bRatio between reaction products 3c and 6 (see description results)cReaction was performed in an open system.
Due to the possible polymerization of methyl acrylate (2c), we decided to use an initial temperature of 115 °C. TLC analysis of the reaction after one hour showed two products, with the less polar one as the major component. Finally, after 3 hours of reaction time the ratio was 1:1 (entry 5). After purification by column chromatography, analysis by 1H- and 13C-NMR indicated the less polar product corresponded to the 1,4-addition product 3c, whereas the more polar product 6 corresponded to a double 1,4-addition (Figure 1).
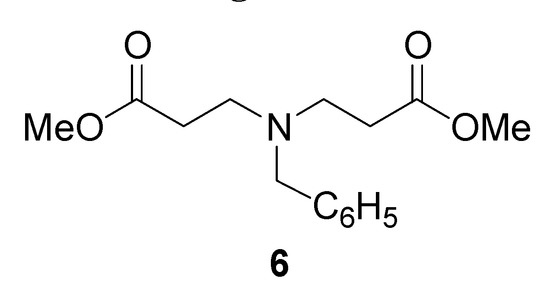
Figure 1.
In order to eliminate the by-product and to shorten the reaction time (entries 6-8), the temperature was reduced to 70 °C. After 10 minutes, the reaction provided a ratio of 70:30 for 3c:6 in comparison with entry 5. Interestingly, when we tested a (1:2c) ratio of 6.2:6.4 mmol (entry 7) at atmospheric pressure, compound 3c was obtained in a 70:30 ratio. This ratio was determined by 1H-NMR. Finally, entry 8 shows a ratio 90:10 obtained in a short time.
Preparation of β-amino esters derived from (S)-(-)-α-methylbenzylamine.
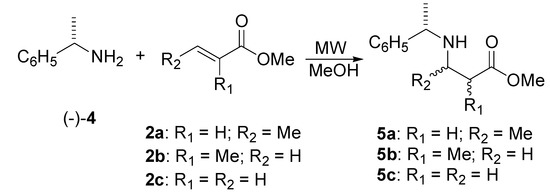
We also carried out the Michael addition of (S)-(-)-α-methylbenzylamine (4) to the α,β-unsaturated esters 2a, 2b, and 2c by microwave heating for further investigation of this methodology (Scheme 2).
Scheme 2.
The addition of (S)-α-methylbenzylamine (4) to methyl crotonate (2a) was carried out under microwave irradiation at 150 °C for 6 hours (entry 1, Table 2). This provided the β-amino ester in a moderate yield with a low diastereomeric ratio [(S,S)-5a:(S,R)-5a; 44:56]. The products can be separated by conventional chromatographic methods. In spite of the low diastereoselectivity in this case, the evidence of the microwave effect was demonstrated by comparing the results reported to those obtained conventionally [10c,13]. With traditional methods, β-amino ester 5a can be obtained in 35-74 % yield in long periods of time (9 days) [13].

Table 2.
1,4-Addition of (S)-α-methylbenzylamine to α,β-unsaturated esters.
| Entry | Product | Power (Watts) | T (oC) | Pressure (psi) | Time (min) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 5a | 26 | 150 | 71 | 360 | 74 |
| 2 | 5b | 14 | 130-150 | 100 | 540 | 70 |
| 3 | 5b | 48 | 130-150 | a | 360 | 85 |
| 4 | 5c | 50 | 80 | 18 | 10 | 95 |
| 5 | 5c | 50 | 70 | b | 20 | quantitative |
a Not determined.b Reaction was performed in an open system.
The addition reaction of (S)-α-methylbenzylamine to methyl methacrylate (2b) was started at 130 °C for 6 hours and subsequently the temperature was increased to 150 °C for three more hours in order to give in moderate yield the β-amino ester 5b (entry 2, Table 2), which was isolated as a ca.1:1 diastereomeric mixture [(S,S)-5b:(S,R)-5b]. It should be noted that the optimization of the synthesis by conventional heating required prolonged refluxing for 9 days to provide 74 % yield [14].
The addition reaction with the (S)-α-methylbenzylamine and methyl acrylate (2c) was carried out with 2 mmol at 80 °C for 10 minutes (entry 4, Table 2). It is worth noting that no product of double addition was detected and the compound 5c could be isolated in 95 % yield. A second experiment, which was a scale-up utilizing a [(-)-4:2c 48:50 mmol] molar ratio and an open system also produced the product in a quantitative yield (entry 5, Table 2).
Conclusions
In summary, an efficient and rapid methodology for the Michael addition of benzylamines to α,β-unsaturated esters has been reported. The use of microwave irradiation afforded the addition products in a low reaction time and good yield, in comparison with traditional methods.
Experimental
General
Specific rotations were measured in a Perkin-Elmer 341 polarimeter at room temperature and λ = 589 nm. 1H and 13C-NMR spectra were obtained in CDCl3 solutions with TMS as internal standard on Varian Gemini 200 or Inova 400 spectrometers. Reactions were performed in sealed vessels or round bottom flasks equipped with condensers in a monomode microwave CEM Discover apparatus, with a power output ranging from 0 to 300 W. Methanol, benzylamine (1), methyl crotonate (2a), methyl methacrylate (2b), methyl acrylate (2c) and (S)-(-)-α-methylbenzylamine (4) were purchased from Aldrich and used without further purification.
General procedure: Synthesis of N-benzyl-β-amino esters via microwave irradiation
A solution of benzylamine (1) or (S)-(-)-α-methylbenzylamine (4) and the α,β-unsaturated ester in methanol were added to a 10-mL glass microwave reaction vessel containing a stir bar. The reaction vessel was sealed with a cap and then placed into the microwave cavity. The microwave unit was programmed to heat the reaction mixture to the desired temperature. After the reaction was completed and the vessel was cooled to below 50 oC using a flow of compressed air, the solvent was evaporated in vacuo and the crude material was purified by column chromatography to give the product.
(rac)-Methyl N-benzyl-3-aminobutanoate [(±)-3a]
Prepared from benzylamine (1, 0.10 g, 1 mmol), methyl crotonate (2a, 0.10 g, 1 mmol) and methanol (3 mL). The reaction was heated at T = 150 °C and 110 psi for 3 h. Column chromatography (hexane-ethyl acetate, 8:2) afforded 3a (98 % yield) as a colorless liquid. 1H-NMR (200 MHz) δ 1.15 (d, J = 6.2 Hz, 3H), 1.96 (br s, 1H), 2.45 (m, 2H), 3.15 (m, 1H), 3.66 (s, 3H), 3.80 (m, 2H), 7.29-7.42 (m, 5H); 13C-NMR (50 MHz) δ 20.64, 41.5, 49.8, 51.3, 51.7, 127.0, 128.2, 128.5, 140.3, 172.8.
(rac)-Methyl 3-(benzylamino)-2-methylpropanoate, [(±)-3b]
Prepared from benzylamine (1, 0.10 g, 1 mmol), methyl methacrylate (2b, 0.10 g, 1 mmol) and methanol (3 mL). The reaction was heated at Ti = 115 °C and 68 psi for 2 hr and then Tf = 130 °C for 1 h, to afford compound 3b (in 97 % yield) as a oil after purification by column chromatography on silica, eluting with hexane-ethyl acetate (8:2). 1H-NMR (200 MHz) δ 1.16 (d, J = 6.2 Hz, 3H), 1.79 (br s, 1H), 2.60-2.75 (m, 2H), 2.88 (m, 1H), 3.68 (s, 3H), 3.79 (s, 2H), 7.20-7.45 (m, 5H); 13C-NMR (50 MHz) δ 15.5, 40.1, 51.8, 52.2, 53.8, 127.0, 128.1, 128.4, 140.1, 176.3.
Methyl 3-(benzylamino)propanoate (3c) and bis [Methyl 3-(benzylamino)]propanoate (6)
Prepared from benzylamine (1, 0.10 g, 1 mmol), methyl acrylate (2c, 0.09 g, 1 mmol), and methanol (3 mL). The reaction was heated at 115 °C, 59 psi for 3 hr, to afford a 1:1 mixture of the compounds 3c-6, which were purified by column chromatography on silica, eluting with hexane-ethyl acetate (8:2). Compound 3c: 1H-NMR (200 MHz) δ 1.82 (br s, 1H), 2.54 (t, J = 6.6 Hz, 2H), 2.89 (t, J = 6.4 Hz, 2H), 3.67 (s, 3H), 3.80 (s, 2H), 7.29-7.42 (m, 5H); 13C-NMR (50 MHz) δ 34.7, 44.6, 51.8, 53.9, 127.0, 128.1, 128.5, 140.0, 173.2. Compound 6: 1H-NMR (200 MHz) δ 2.47 (t, J = 7.0 Hz, 4H), 2.80 (t, J = 7.2 Hz, 4H), 3.58 (s, 2H), 3.64 (s, 6H), 7.20-7.35 (m, 5H); 13C-NMR (50 MHz) δ 32.7, 48.8, 49.3, 49.7, 51.5, 51.9, 58.4, 128.0, 128.2, 128.5, 129.0, 139.9, 172.9.
Methyl N-α-Methylbenzylaminobutanoate (5a)
(S)-(-)-α-methylbenzylamine (4, 0.12 g, 1 mmol), methyl crotonate (2a, 0.10 g, 1 mmol) and methanol (3 mL) were heated at 150 °C and 71 psi for and 6 hr, according to the general procedure and then concentrated on a rotatory evaporator, providing 5a (0.16 g, 74 % yield) as a ca. 1:1 mixture of diastereomers (according to the 1H-NMR spectrum in CDCl3). Separation of the mixture was accomplished by gradient flash chromatography (eluent: hexane-ethyl acetate, 19:2 to 1:1) to yield pure fractions of (αS,3S)-5a and (αS,3R)-5a. (S,3S)-5a: [α]D = -45.5 (c = 1.1, CHCl3) [lit.[13] [α]D = -37.6 (c = 0.98, CHCl3)]; 1H-NMR (400 MHz) δ 1.05 (d, J = 12.4 Hz, 3H), 1.32 (d, J = 13.2 Hz, 3H), 1.65 (br s, 1H), 2.41 (t, J = 6.6 Hz, 2H), 2.96 (m, 1H), 3.65 (s, 3H), 3.87 (q, J = 12.4 Hz, 1H), 7.19-7.4 (m, 5H); 13C-NMR (100 MHz) δ 21.7, 24.8, 40.8, 47.9, 51.6, 55.43, 126.6, 127.0, 128.5, 146.0, 172.7; (S,3R)-5a: [α]D = -49.5 (c =1.2, CHCl3) [lit. [13] [α]D = -41.0 (c = 1.31, CHCl3)]; 1H-NMR (400 MHz) δ 1.03 (d, J = 6.4 Hz, 3H), 1.30 (d, J = 6.4 Hz, 3H), 2.2 (br s, 1H), 2.30 (m, 2H), 2.83 (m, 1H), 3.60 (s, 3H), 3.87 (q, J = 6.8 Hz, 1H), 7.2-7.4 (m, 5H); 13C-NMR (100 MHz) δ 20.0, 25.3, 42.3, 47.2, 51.6, 55.1, 126.7, 127.0, 128.5, 145.3, 172.8.
Methyl N-α-methylbenzyl-3-amino-2-methylpropionate (5b)
(S)-(-)-α-Methylbenzylamine (4, 0.12 g, 1 mmol), methyl methacrylate (2b, 0.10 g, 1 mmol) and methanol (3 mL) were heated at Ti = 130 °C, 100 psi for 6 hr, and then Tf = 150 °C during 3 h. Concentration of the mixture on a rotatory evaporator gave 5b (0.15 g, 70 % yield) as a ca. 44:56, mixture of diasteromers (according to the 1H-NMR spectrum in CDCl3) that was separated by gradient flash chromatography (eluent: hexane-ethyl acetate, 90:10 to 50:50) to yield pure fractions of (αS,2S)-5b and (αS,2R)-5b. (αS,2S)-5b [α]D = -22.6 (c =2.3, CHCl3). [lit.[14] [α]D = -24.7 (c = 2.3, CHCl3)]; 1H-NMR (200 MHz) δ 1.13 (d, J = 6.6 Hz, 3H), 1.32 (d, J = 6.6 Hz, 3H), 1.77 (br s, 1H), 2.45 (dd, J = 11.1 Hz, J = 5.2 Hz, 1H), 2.59 (m, 1H), 2.77 (dd, J = 11.1 Hz, J = 7.6 Hz, 1H), 3.70 (s, 3H), 3.77 (m, 1H), 7.19-7.35 (m, 5H); 13C-NMR (50 MHz) δ 15.5, 24.7, 40.4, 50.8, 51.8, 58.4, 126.7, 126.9, 128.5, 145.7, 176.4. (αS,2R)-5b. [α]D = -54.0 (c = 2.1, CHCl3) [lit. [14] [α]D = -56.1 (c = 2, CHCl3); 1H-NMR (200 MHz) δ 1.11 (d, J = 6.6 Hz, 3H), 1.32 (d, J = 6.6 Hz, 3H), 2.4 (br s, 1H), 2.43-2.72 (m, 3H), 3.7 (s, 3H), 3.77 (q, J = 6.6 Hz, 1H), 7.19-7.40 (m, 5H); 13C-NMR (50 MHz) δ 15.6, 24.7, 40.3, 50.6, 51.9, 58.1, 58.3, 126.7, 126.8, 128.5, 145.4, 176.3.
Methyl N-α-Methylbenzylaminopropanoate (5c)
(S)-(-)-α-methylbenzylamine (4, 0.24 g, 2 mmol), methyl acrylate (2c, 0.18 g, 2 mmol) and methanol (3 mL) were heated at 80 °C and 18 psi for 10 minutes, according to the general procedure and then concentrated on a rotatory evaporator, providing 5c in 95 % yield after purification by column chromatography (eluent: hexane-ethyl acetate, 9:1): [α]D = -31.5 (c=1.47, CHCl3); 1H-NMR (200 MHz) δ 1.35 (d, J = 6.6 Hz, 3H), 1.93 (br s, 1H), 2.47 (t, J = 6.2 Hz, 2H), 2.70 (m, 2H), 3.66 (s, 3H), 7.20-7.35 (m, 5H,); 13C-NMR (50 MHz) δ 24.6, 34.8, 43.0, 51.7, 58.3, 126.6, 127.0, 128.5, 145.4, 173.3.
Acknowledgements
We are grateful to José A. González-Espinoza and Berenice Sánchez-García for technical support, to the CONACYT for financial support (Project No. 48356-Q), Instrumentos y Equipos Falcon, and Grace Vanier for many important observations. Carrillo-Morales thanks CONACyT for a scholarship.
References and Notes
- Hayes, B. L. Microwave Synthesis. Chemistry at the Speed of Light; CEM Publishing: Mattehews, NC, USA, 2002. [Google Scholar]
- For reviews see: Kappe, C. O. Controlled Microwave Heating in Modern Organic Synthesis. Angew. Chem., Int. Ed. 2004, 43, 6250–6284. [Google Scholar] Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis- a review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] Loupy, A.; Petit, A.; Hamelin, J.; Texier-Boullet, F.; Jacquault, P.; Mathé, D. New Solvent-Free Organic Synthesis Using Focused Microwaves. Synthesis 1998, 1213–1234. [Google Scholar] Caddick, S. Microwave Assisted Organic Reactions. Tetrahedron 1995, 51, 10403–10432. [Google Scholar]
- For other books see: Loupy, A. (Ed.) Microwaves in Organic Synthesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2006. Tierney, J.P.; Lidström, P. (Eds.) Microwave Assisted Organic Synthesis; Blackwell Publishing: Oxford, UK, 2005. Kappe, C. O.; Stadler, A. Microwaves in Organic and Medicinal Chemistry; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Santra, S.; Andreana, R. A. One-Pot, Microwave-Influenced Synthesis of Diverse Small Molecules by Multicomponent Reaction Cascades. Org. Lett. 2007, 9, 5035–5038. [Google Scholar] [CrossRef]
- Sharma, A. K.; Gowdahalli, K.; Krzeminski, J.; Amin, S. Microwave-Assisted Suzuki Cross-Coupling Reaction, a Key Step in the Synthesis of Polycyclic Aromatic Hydrocarbons and Their Metabolites. J. Org. Chem. 2007, 72, 8987–8989. [Google Scholar] [CrossRef]
- Baxendale, I. R.; Lee, A.-I.; Ley, S. V. A Concise Synthesis of Carpanone Using Solid-Supported Reagents and Scavengers. J. Chem. Soc., Perkin Trans. 1 2002, 1850–1857. [Google Scholar] [CrossRef]
- Steinreiber, A.; Stadler, A.; Mayer, S. F.; Faber, K.; Kappe, C. O. High-Speed Microwave-Promoted Mitsunobu Inversions. Application Toward the Deracemization of Sulcatol. Tetrahedron Lett. 2001, 42, 6283–6286. [Google Scholar] [CrossRef]
- Amore, K. M.; Leadbeater, N. E.; Millar, T. A.; Schmink, J.R. Fast, easy, solvent-free, microwave-promoted Michael addition of anilines to α,β-unsaturated alkenes: synthesis of N-aryl functionalized β-amino esters and acids. Tetrahedron Lett 2006, 47, 8583–8586. [Google Scholar] Moghaddam, F. M.; Mohammadi, M.; Hosseinnia, A. Water promoted Michael Addition of Secondary Amines to α,β-unsaturated Carbonyl Compounds under Microwave Irradiation. Hosseini, M. Synth. Commun. 2000, 30, 643–650. [Google Scholar]
- Juaristi, E.; Soloshonok, V. A. (Eds.) Enantioselective Synthesis of β-Amino Acids, 2nd ed.; Wiley-VCH: New York, USA, 2005.
- Davies, S.G.; Garrido, N. M.; Kruchinin, D.; Ichihara, O.; Kotchie, L. J.; Price, P. D.; Price Mortimer, A. J.; Rusell, A. J.; Smith, A. D. Homochiral Lithium Amides for the Asymmetric Synthesis of β-amino Acids. Tetrahedron: Asymmetry 2006, 17, 1793–1811. [Google Scholar] Doi, H.; Sakai, T.; Iguchi, M.; Yamada, K.-I.; Tomioka, K. Chiral Ligand-Controlled Asymmetric Conjugate Addition of Lithium Amides to Enoates. J. Am. Chem. Soc. 2003, 125, 2886–2887. [Google Scholar] Davies, S. G.; Ichihara, O. Asymmetric Synthesis of R-β-Amino Butanoic Acid and S-β-tyrosine: Homochiral Lithium Amide Equivalents for Michael Additions to α,β-unsaturated Esters. Tetrahedron: Asymmetry 1991, 2, 183–186. [Google Scholar] Juaristi, E.; Quintana, D.; Escalante, J. Enantioselective Synthesis of β-Amino Acids. Aldrichim. Acta 1994, 27, 3–11. [Google Scholar]
- Gedey, S.; Liljeblad, A.; Fülöp, F.; Kanerva, L. T. Sequential resolution of ethyl 3-aminobutyrate with carboxylic acid esters by Candida antarctica lipase B. Tetrahedron: Asymmetry 1999, 10, 2573–2581. [Google Scholar] Gedey, S.; Liljeblad, A.; Lázár, L.; Fülöp, F.; Kanerva, L. T. Structural effects on chemo- and enantioselectivity of Candida antarctica lipase B - Resolution of β-amino esters. Can. J. Chem. 2002, 80, 565–570. [Google Scholar] Solymár, M.; Liljeblad, A.; Lázar, L.; Fülöp, F.; Kanerva, L. T. Lipase-catalysed kinetic resolution in organic solvents: an approach to enantiopure α-methyl-β-alanine esters. Tetrahedron: Asymmetry 2002, 13, 1923–1928. [Google Scholar]
- Flores-Sánchez, P.; Escalante, J.; Castillo, E. Enzymatic resolution of N-protected-β3-amino methyl esters, using lipase B from Candida Antarctica. Tetrahedron: Asymmetry 2005, 16, 629–634. [Google Scholar] [CrossRef]
- Seebach, D.; Estermann, H. Diastereoselektive alkylierung von 3-aminobutansäure in der 2-stellung. Helv. Chim. Acta 1988, 71, 1824–1839. [Google Scholar] Furukawa, M.; Okawara, T.; Terawaki, Y. Asymmetric Syntheses of b-amino acids by the addition of chiral amines to C=C double bond. Chem. Pharm. Bull. 1977, 25, 1319–1325. [Google Scholar]
- Shustov, G. V.; Rauk, A. 3-Methylazetidin-2-one and its precursors: Optical resolution and Absolute Configurations. Tetrahedron: Asymmetry 1996, 7, 699–708. [Google Scholar] [CrossRef]
- Sample Availability: Small samples (a few milligrams) of the compounds mentioned in this paper are available from the authors.
© 2008 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.