Two New Constituents from Artemisia capillaris Thunb.
Abstract
:Introduction
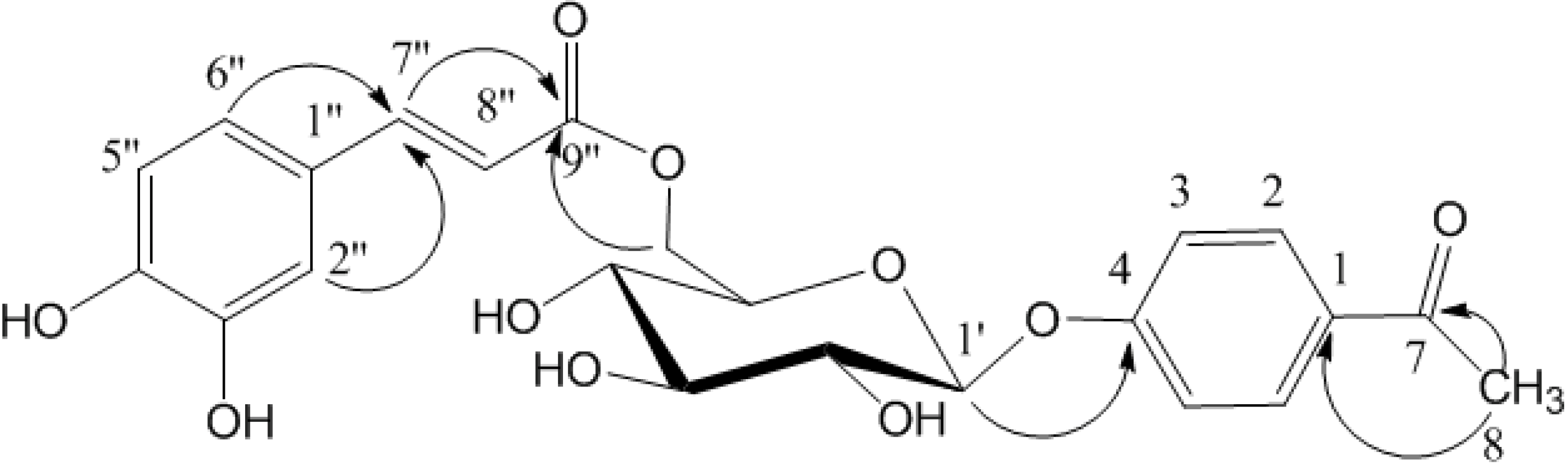
Results and Discussion

Experimental
General
Plant material
Extraction, isolation and product characterization
| Position | 1 | 2 | |||
|---|---|---|---|---|---|
| H | C | H | C | ||
| 1 | 130.9 | ||||
| 2 | 7.84 (d, J=8.7 Hz) | 130.2 | 8.11 (s) | 152.4 | |
| 3 | 7.10 (d, J=8.7 Hz) | 116.0 | |||
| 4 | 160.9 | 149.2 | |||
| 5 | 7.10 (d, J=8.7 Hz) | 116.0 | 118.9 | ||
| 6 | 7.84 (d, J=8.7Hz) | 130.2 | 156.0 | ||
| 7 | 196.4 | ||||
| 8 | 2.38 (s) | 26.3 | 8.27 (s) | 139.2 | |
| 1′ | 5.07 (d, J=9.0 Hz) | 99.6 | 132.5 | ||
| 2′ | 3.30 (m) | 73.2 | 6.69 (s) | 114.0 | |
| 3′ | 3.32 (m) | 76.4 | 145.2 | ||
| 4′ | 3.20 (m) | 70.1 | 149.2 | ||
| 5′ | 3.70 (m) | 74.0 | 6.67 (d, J=8.4 Hz) | 115.3 | |
| 6′ | 4.41(d, J=11 Hz), 4.17 (dd, J=11, 7.3 Hz) | 63.3 | 6.64 (d, J=8.4 Hz) | 117.0 | |
| 7′ | 5.64 (q, J=7.2 Hz) | 52.0 | |||
| 8′ | 1.85 (d, J=7.2 Hz) | 20.6 | |||
| 1″ | 125.0 | ||||
| 2″ | 7.05 (s) | 114.1 | |||
| 3″ | 145.2 | ||||
| 4″ | 148.8 | ||||
| 5″ | 6.76 (d, J=8.1 Hz) | 115.2 | |||
| 6″ | 6.96 (d, J=8.1 Hz) | 121.0 | |||
| 7″ | 7.46 (d, J=15.8 Hz) | 145.8 | |||
| 8″ | 6.25 (d, J=15.8 Hz) | 113.0 | |||
| 9″ | 166.4 | ||||
Acid Hydrolysis of 1 [12]
Acknowledgements
References
- Tang, W.; Eisenbrand, G. Chinese Drugs of Plant Origin, Chemistry, Phamacology and use in traditional and modern medicine; Springer Verlag: New York, 1992; p. 179. [Google Scholar]
- Han, K.-H.; Jeon, Y.-J.; Athukorala, Y.; Choi, K.-D.; Kim, C.-J.; Cho, J.-K.; Sekikawa, M.; Lee, C.-H. A water extract of Artemisia capillaris prevents 2,2'-azobis(2-amidinopropane) dihydro-chloride-induced liver damage in rats. J. Med. Food 2006, 9, 342–347. [Google Scholar] [CrossRef]
- Han, J.; Zhao, Y.-L.; Shan, Li-M.; Huang, F.-J.; Xiao, X.-H. An experiment on standardized cell culture assay in assessing the activities of Composite Artemisia Capillaris Tablets against hepatitis B virus replication in vitro. Chin. J. Integr. Med. 2005, 11, 54–56. [Google Scholar] [CrossRef]
- Jang, S.; Kim, Y.-J.; Lee, W.-Y.; Kwak, K. C.; Baek, S. H.; Kwak, G. B.; Yun, Y.-G.; Chai, K.-Y. Scoparone from Artemisia capillaris inhibits the release of inflammatory mediators in RAW 264.7 cells upon stimulation cells by interferon-gamma Plus LPS. Arch. Pharm. Res. 2005, 28, 203–208. [Google Scholar] [CrossRef]
- Hong, S. H.; Seo, S. H.; Lee, J. H.; Choi, B. T. The aqueous extract from Artemisia capillaris Thunb. inhibits lipopolysaccharide-induced inflammatory response through preventing NF-kappaB activation in human hepatoma cell line and rat liver. Int. J. Mol. Med. 2004, 13, 717–720. [Google Scholar]
- Hu, Y. Q.; Tan, R. X.; Chu, M. Y.; Zhou, J. Apoptosis in human hepatoma cell line SMMC-7721 induced by water-soluble macromolecular components of Artemisia capillaris Thunberg. Jap. J. Cancer Res.: Gann 2000, 91, 113–117. [Google Scholar] [CrossRef]
- Yamahara, J.; Kobayashi, G.; Matsuda, H.; Katayama, T.; Fujimura, H. The effect of scoparone, a coumarin derivative isolated from the Chinese crude drug Artemisiae capillaris flos, on the heart. Chem. Pharm. Bull. 1989, 37, 1279–1299. [Google Scholar] [CrossRef]
- Fakeya, K.; Yoshitomo, N.; Haruji, O. Studies on ‘Inchinko’ II. Studies on the compounds related to capillarisin and flavonoids. Yakugaku Zasshi 1976, 96, 855–862. [Google Scholar]
- Yang, Z. G.; Li, H. R.; Wang, L. Y.; Li, Y. H.; Lu, S. G.; Wen, X. F.; Wang, J.; Akihiro, D.; Susumu, K. Triterpenoids from Hippophae rhamnoides L. and Their Nitric OxideProduction-Inhibitory and DPPH Radical-Scavenging Activities. Chem. Pharm. Bull. 2007, 55, 15–18. [Google Scholar] [CrossRef]
- Logendra, S.; Ribnicky, D. M.; Yang, H.; Poulev, A.; Ma, J.; Kennelly, E. J.; Raskin, I. Bioassay-guided isolation of aldose reductase inhibitors from Artemisia dracunculus. Phytochemistry 2006, 67, 1539–1546. [Google Scholar] [CrossRef]
- Shao, Y.; Li, Y. L; Zhou, B. N. Structural elucidation and synthesis of asterbatanoside A from Aster batanfensis. Chin. Chem. Lett. 1994, 5, 675–678. [Google Scholar]
- Sun, J.-M.; Yang, J.-S.; Zhang, H. Two New Flavanone Glycosides of Jasminum lanceolarium and Their Anti-oxidant Activities. Chem. Pharm. Bull. 2007, 55, 474–476. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors.
© 2008 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ma, H.-Y.; Sun, Y.; Zhou, Y.-Z.; Hong, M.; Pei, Y.-H. Two New Constituents from Artemisia capillaris Thunb. Molecules 2008, 13, 267-271. https://doi.org/10.3390/molecules13020267
Ma H-Y, Sun Y, Zhou Y-Z, Hong M, Pei Y-H. Two New Constituents from Artemisia capillaris Thunb. Molecules. 2008; 13(2):267-271. https://doi.org/10.3390/molecules13020267
Chicago/Turabian StyleMa, Hong-Yu, Yi Sun, Yu-Zhi Zhou, Min Hong, and Yue-Hu Pei. 2008. "Two New Constituents from Artemisia capillaris Thunb." Molecules 13, no. 2: 267-271. https://doi.org/10.3390/molecules13020267
APA StyleMa, H.-Y., Sun, Y., Zhou, Y.-Z., Hong, M., & Pei, Y.-H. (2008). Two New Constituents from Artemisia capillaris Thunb. Molecules, 13(2), 267-271. https://doi.org/10.3390/molecules13020267




