HPLC, NMR and MALDI-TOF MS Analysis of Condensed Tannins from Lithocarpus glaber Leaves with Potent Free Radical Scavenging Activity
Abstract
Introduction
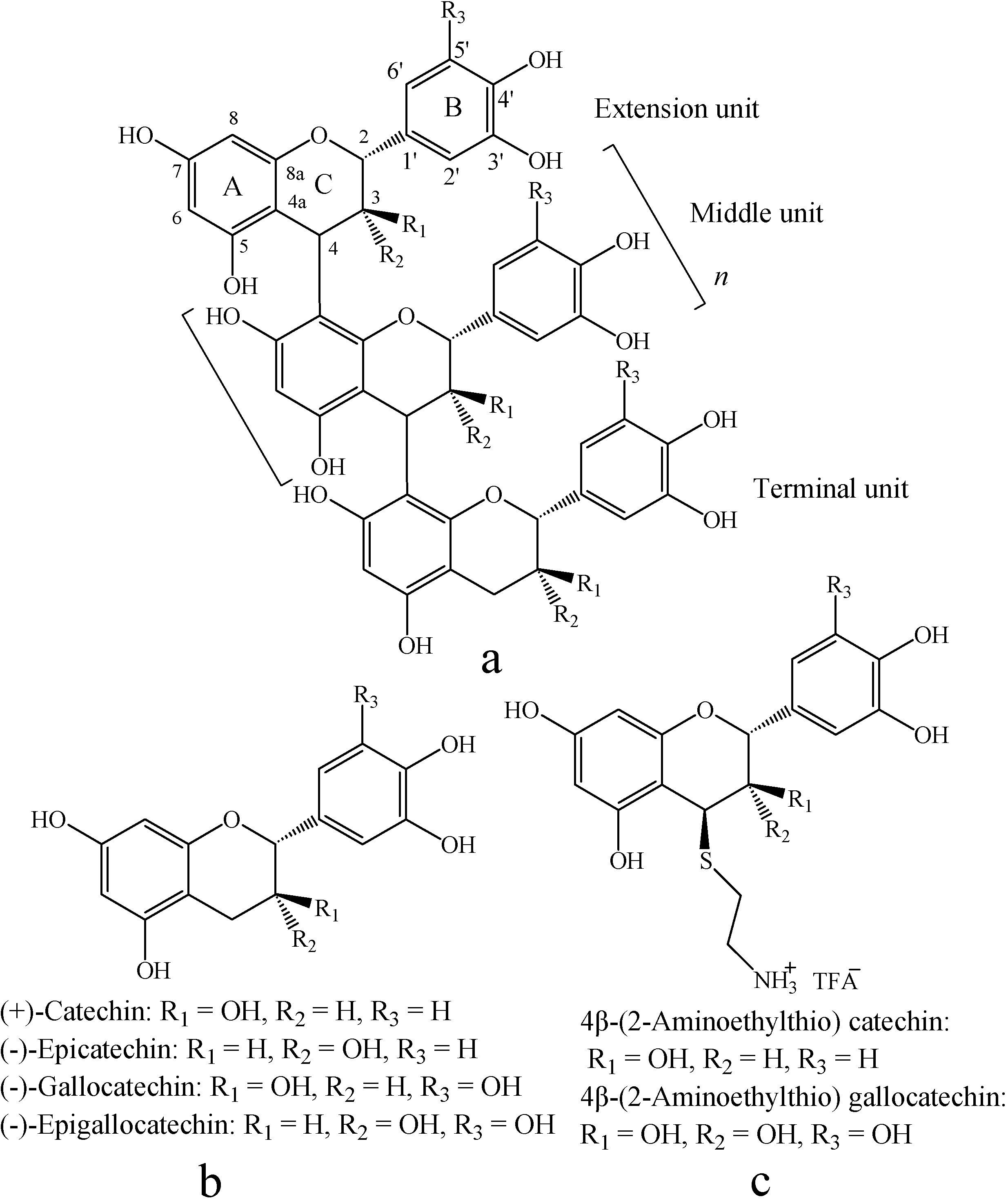
Results and Discussion
Total phenolics and condensed tannins contents
Thiolysis with cysteamine followed by reversed-phase HPLC

| Concentration (mg/g dried tannins) | |
|---|---|
| Aminoethylthio catechin | 8.38 ± 0.58 |
| Catechin | 18.53 ± 0.63 |
| Epicatechin | 222.73 ± 2.51 |
| Aminoethylthio gallocatechin | 258.72 ± 4.21 |
| Gallocatechin | 65.93 ± 2.22 |
| Epigallocatechin | 247.08 ± 7.15 |
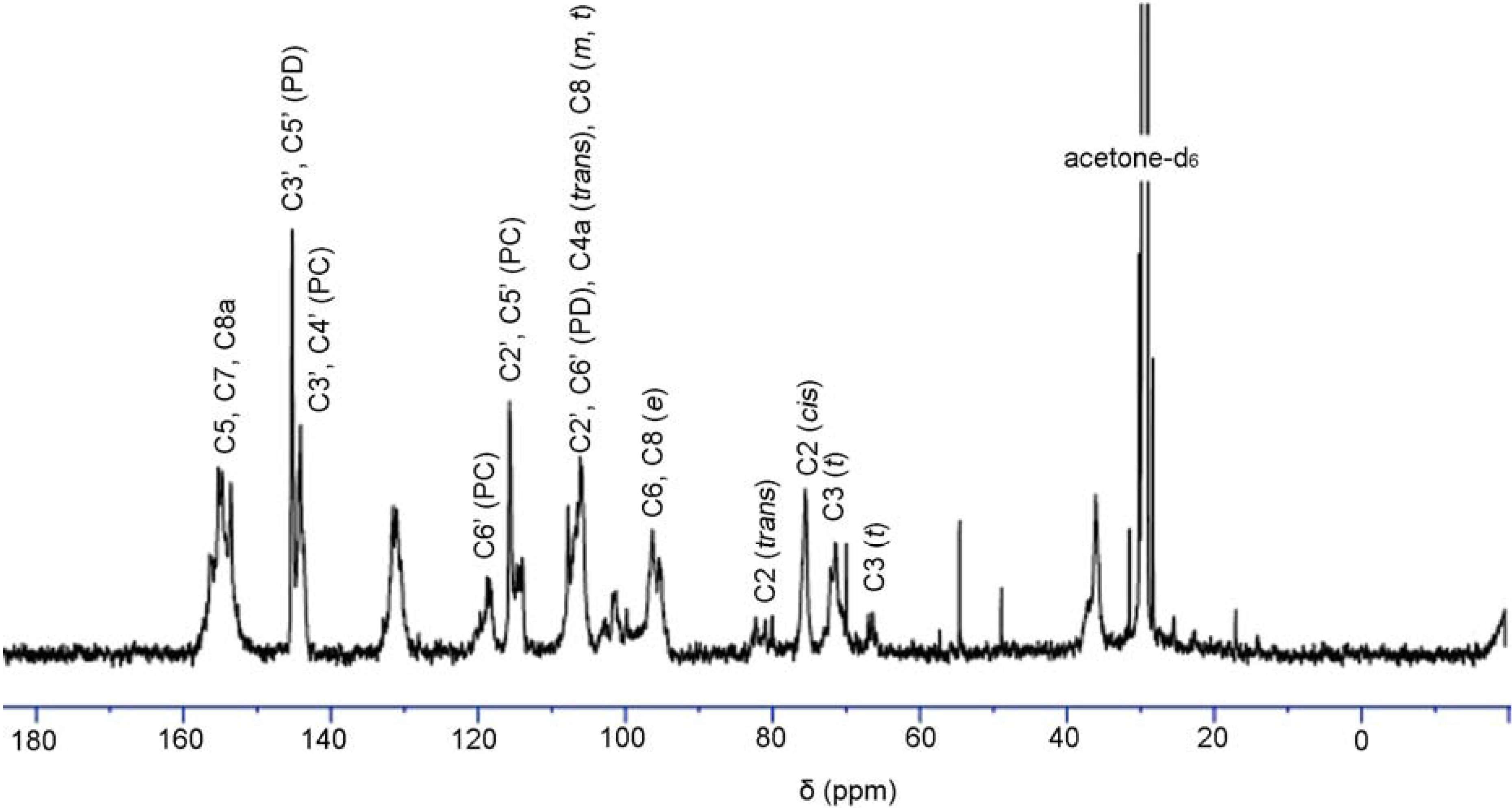
13C-NMR analysis of condensed tannins
MALDI-TOF MS analysis
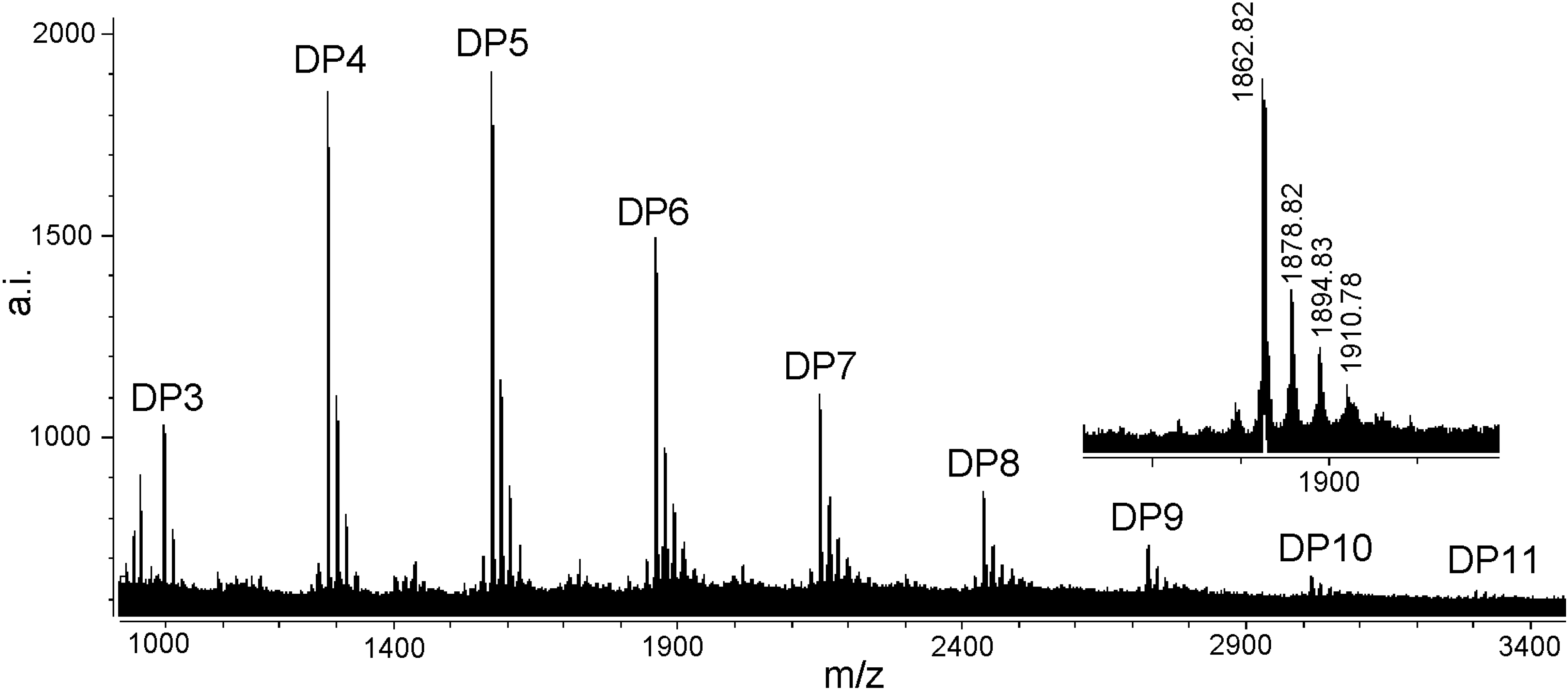
| Polymer | Number of catechin units | Number of gallocatechin units | Calculated [M + Cs]+ | Observed [M + Cs]+ |
|---|---|---|---|---|
| Trimer | 3 | 0 | 999 | 998.81 |
| 2 | 1 | 1015 | 1014.82 | |
| 1 | 2 | 1031 | 1030.88 | |
| Tetramer | 4 | 0 | 1287 | 1286.76 |
| 3 | 1 | 1303 | 1302.75 | |
| 2 | 2 | 1319 | 1318.78 | |
| 1 | 3 | 1335 | 1334.71 | |
| Pentamer | 5 | 0 | 1575 | 1574.78 |
| 4 | 1 | 1591 | 1590.84 | |
| 3 | 2 | 1607 | 1606.83 | |
| 2 | 3 | 1623 | 1622.85 | |
| Hexamer | 6 | 0 | 1863 | 1862.82 |
| 5 | 1 | 1879 | 1878.82 | |
| 4 | 2 | 1895 | 1894.83 | |
| 3 | 3 | 1911 | 1910.78 | |
| Heptamer | 7 | 0 | 2151 | 2151.83 |
| 6 | 1 | 2167 | 2167.78 | |
| 5 | 2 | 2183 | 2183.86 | |
| 4 | 3 | 2199 | 2198.87 | |
| Octamer | 8 | 0 | 2439 | 2439.84 |
| 7 | 1 | 2455 | 2455.86 | |
| 6 | 2 | 2471 | 2471.78 | |
| Nonamer | 9 | 0 | 2727 | 2728.80 |
| 8 | 1 | 2743 | 2743.85 | |
| 7 | 2 | 2759 | 2758.79 | |
| Decamer | 10 | 0 | 3015 | 3015.83 |
| 9 | 1 | 3031 | 3031.65 | |
| 8 | 2 | 3047 | 3048.18 | |
| Undecamer | 11 | 0 | 3303 | 3305.33 |
| 10 | 1 | 3319 | 3320.87 | |
| 9 | 2 | 3335 | 3336.78 |
Free radical scavenging activity

Conclusions
Experimental
General
Extraction of condensed tannins, characterization and determination of total phenolics and condensed tannins
Cysteamine degradation
Elution condition
MALDI-TOF MS analysis
Free radical scavenging activity
Acknowledgements
References
- Scalbert, A.; Monties, B.; Janin, G. Tannins in wood: comparison of different estimation methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Taylor, A.W. Analysis of proanthocyanidins by high-performance gel permeation chromatography. J. Chromatogr. A. 2003, 995, 99–107. [Google Scholar] [CrossRef]
- Tanner, G.J.; Francki, K.T.; Abrahams, S.; Watson, J.M.; Larkin, P.J.; Ashton, A.R. Proanthocyanidin biosynthesis in plants: purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA. J. Biol. Chem. 2003, 278, 31647–31656. [Google Scholar]
- Haslam, E. Plant Polyphenols: Vegetable Tannins Revisited. Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Svedstrom, U.; Vuorela, H.; Kostiainen, R.; Huovinen, K.; Laakso, I.; Hiltunen, R. High-performance liquid chromatographic determination of oligomeric procyanidins from dimers up to the hexamer in hawthorn. J. Chromatogr. A. 2002, 968, 53–60. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Murtijaya, J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT-Food Sci. Tech. 2007, 40, 1664–1669. [Google Scholar] [CrossRef]
- Sisti, M.; De Santi, M.; Fraternale, D.; Ninfali, P.; Scoccianti, V.; Brandi, G. Antifungal activity of Rubus ulmifolius Schott standardized in vitro culture. LWT - Food Sci. Tech. 2008, 41, 946–950. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds-nature, occurrence, dietary intake, and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Gulcin, I.; Oktay, M.; Kirecci, E.; Kufrevioglu, O.I. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003, 83, 371–382. [Google Scholar]
- Minussi, R.C.; Rossi, M.; Bologna, L.; Cordi, L.; Rotilio, D.; Pastore, G.M.; Duran, N. Phenolic compounds and total antioxidant potential of commercial wines. Food Chem. 2003, 82, 409–416. [Google Scholar] [CrossRef]
- Noferi, M.; Masson, E.; Merlin, A.; Pizzi, A.; Deglise, X. Antioxidant characteristics of hydrolysable and polyflavonoid tannins: an ESR kinetics study. J. Appl. Polym. Sci. 1997, 63, 475–482. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavanoids and phenolic acids. Free Rad. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Tanaka, N.; Shimomura, K.; Ishimaru, K. Tannin production in callus cultures of Quercus acutissima. Phytochemistry 1995, 40, 1151–1154. [Google Scholar] [CrossRef]
- Tam, P.C.F.; Griffiths, D.A. Mycorrhizal associations in Hong Kong Fagaceae. Mycorrhiza 1993, 2, 125–131. [Google Scholar] [CrossRef]
- Hemingway, R.W. Chemistry and Significance of Condensed Tannins. Hemingway, R.W., Karchesy, J.J., Eds.; Plenum Press: New York, USA, 1989; p. 83. [Google Scholar]
- Jerez, M.; Pinelo, M.; Sineiro, J.; Nunez, M.J. Influence of extraction conditions on phenolic yields from pine bark: assessment of procyanidins polymerization degree by thiolysis. Food Chem. 2006, 94, 406–414. [Google Scholar] [CrossRef]
- Guyot, S.; Marnet, N.; Drilleau, J.F. Thiolysis-HPLC characterization of apple procyanidins covering a large range of polymerization states. J. Agric. Food Chem. 2001, 49, 14–20. [Google Scholar] [CrossRef]
- Guyot, S.; Marnet, N.; Laraba, D.; Sanoner, P.; Drilleau, J.F. Reversed-phase HPLC following thiolysis for quantitative estimation and characterization of the four main classes of phenolic compounds in different tissue zones of a French cider apple variety (Malus domestica var. Kermerrien). J. Agric. Food Chem. 1998, 46, 1698–1705. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Composition of grape skin proanthocyanidins at different stages of berry development. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Torres, J.L.; Lozano, C. Chromatographic characterization of proanthocyanidins after thiolysis with cysteamine. Chromatographia 2001, 54, 523–526. [Google Scholar] [CrossRef]
- Czochanska, Z.; Foo, L.Y.; Newman, R.H.; Porter, L.J. Polymeric proanthocyanidins: stereochemistry, structural units and molecular weight. J. Chem. Soc. Perkin Trans. 1. 1980, 2278–2286. [Google Scholar] [CrossRef]
- Pasch, H.; Schrepp, W. MALDI-TOF Mass Spectrometry of Synthetic Polymers. Springer: Berlin Heidelberg, New York, 2003. [Google Scholar]
- Xiang, P.; Lin, Y.M.; Lin, P.; Xiang, C. Effects of adduct ions on matrix-assisted laser desorption/ionization time of flight mass spectrometry of condensed tannins: a prerequisite knowledge. Chin. J. Anal. Chem. 2006, 34, 1019–1022. [Google Scholar] [CrossRef]
- Xiang, P.; Lin, Y.M.; Lin, P.; Xiang, C.; Yang, Z.W.; Lu, Z.M. Effect of cationization reagents on the matrix-assisted laser desorption/ionization time-of-flight mass spectrum of Chinese gallotannins. J. Appl. Polym. Sci. 2007, 105, 859–864. [Google Scholar] [CrossRef]
- Zhang, L.L.; Lin, Y.M. Tannins from Canarium album with potent antioxidant activity. J. Zhejiang Univ. Sci. B. 2008, 9, 407–415. [Google Scholar] [CrossRef]
- Krueger, C.G.; Dopke, N.C.; Treichel, P.M.; Folts, J.; Reed, J.D. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of polygalloyl polyfavan-3-ols in grape seed extract. J. Agric. Food Chem. 2000, 48, 1663–1667. [Google Scholar] [CrossRef]
- Soares, J.R.; Dins, T.C.P.; Cunha, A.P.; Ameida, L.M. Antioxidant activity of some extracts of Thymus zygis. Free Rad. Res. 1997, 26, 469–478. [Google Scholar] [CrossRef]
- Duh, P.D.; Tu, Y.Y.; Yen, G.C. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat). LWT - Food Sci. Tech. 1999, 32, 269–277. [Google Scholar] [CrossRef]
- Lin, Y.M.; Liu, J.W.; Xiang, P.; Lin, P.; Ye, G.F.; Sternberg, L.da.S.L. Tannin dynamics of propagules and leaves of Kandelia candel and Bruguiera gymnorrhiza in the Jiulong River Estuary, Fujian, China. Biogeochemistry 2006, 78, 343–359. [Google Scholar] [CrossRef]
- Graham, H.D. Stabilization of the Prussian blue color in the determination of polyphenols. J. Agric. Food Chem. 1992, 40, 801–805. [Google Scholar] [CrossRef]
- Terrill, T.H.; Rowan, A.M.; Douglas, G.B.; Barry, T.N. Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals and cereal grains. J. Sci. Food Agric. 1992, 58, 321–329. [Google Scholar] [CrossRef]
- Braca, A.; Tommasi, N.D.; Bari, L.D.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia terapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, L.L.; Lin, Y.M. HPLC, NMR and MALDI-TOF MS Analysis of Condensed Tannins from Lithocarpus glaber Leaves with Potent Free Radical Scavenging Activity. Molecules 2008, 13, 2986-2997. https://doi.org/10.3390/molecules13122986
Zhang LL, Lin YM. HPLC, NMR and MALDI-TOF MS Analysis of Condensed Tannins from Lithocarpus glaber Leaves with Potent Free Radical Scavenging Activity. Molecules. 2008; 13(12):2986-2997. https://doi.org/10.3390/molecules13122986
Chicago/Turabian StyleZhang, Liang Liang, and Yi Ming Lin. 2008. "HPLC, NMR and MALDI-TOF MS Analysis of Condensed Tannins from Lithocarpus glaber Leaves with Potent Free Radical Scavenging Activity" Molecules 13, no. 12: 2986-2997. https://doi.org/10.3390/molecules13122986
APA StyleZhang, L. L., & Lin, Y. M. (2008). HPLC, NMR and MALDI-TOF MS Analysis of Condensed Tannins from Lithocarpus glaber Leaves with Potent Free Radical Scavenging Activity. Molecules, 13(12), 2986-2997. https://doi.org/10.3390/molecules13122986




