A Standard Addition Method to Assay the Concentration of Biologically Interesting Polyphenols in Grape Berries by Reversed-Phase HPLC
Abstract
:Introduction
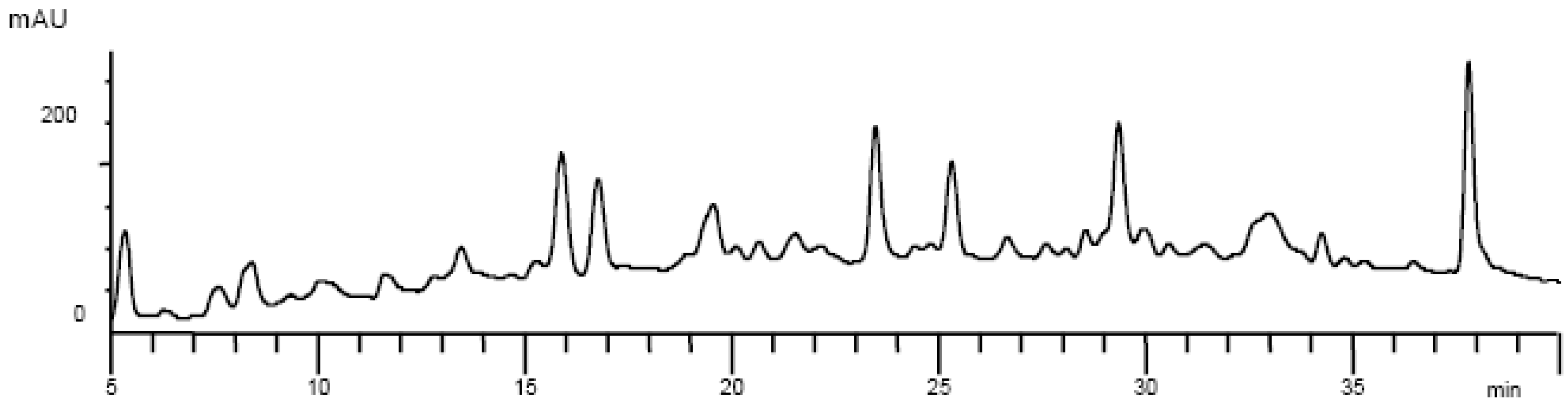
Results and Discussion
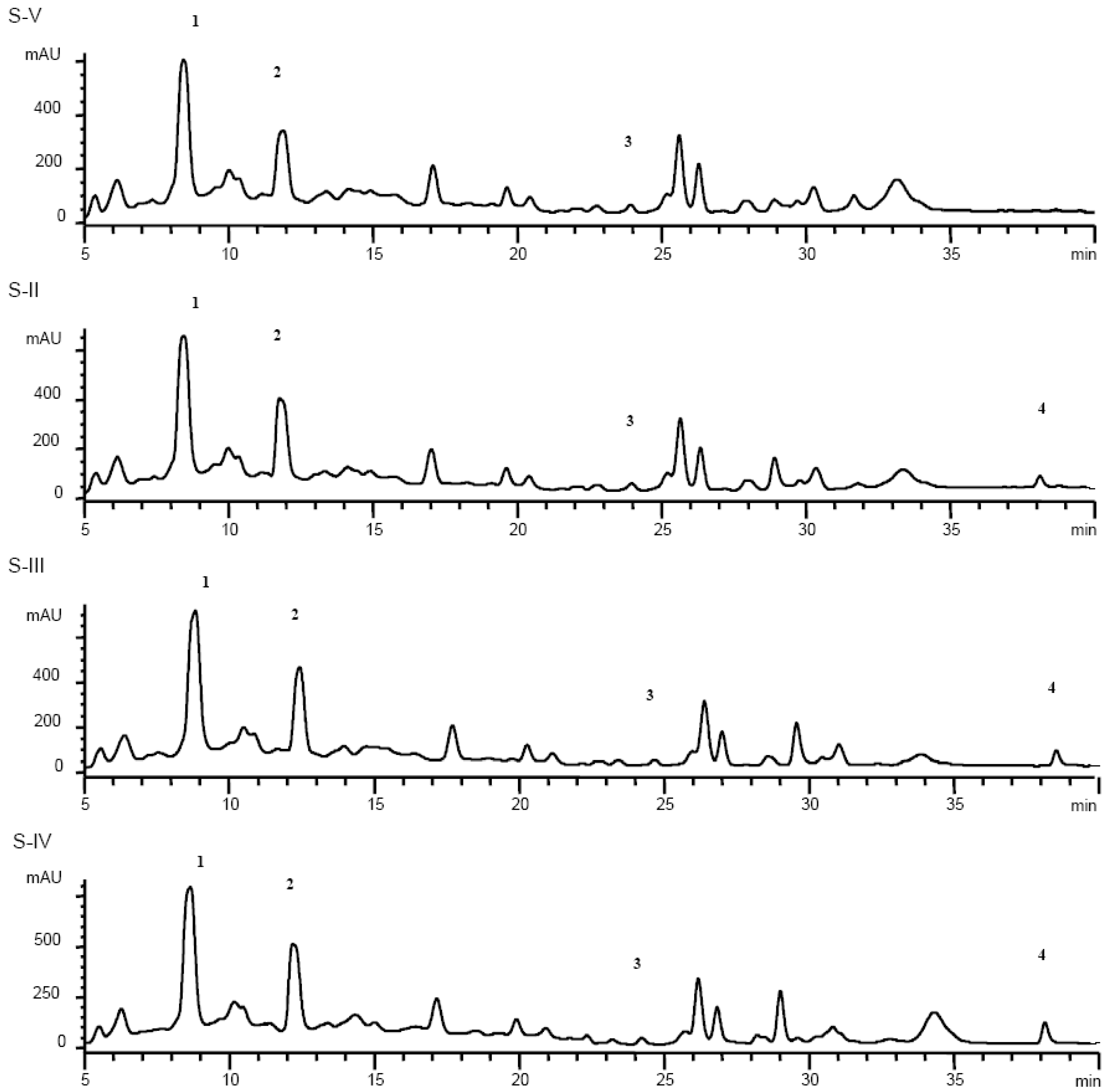
| Slope±SD (× 10-1) | Intercept±SD (×10-2) | |
| Catechin | 42.5±0.8 | 693±4 |
| Epicatechin | 48.0±0.9 | 499±2 |
| t-Resveratrol | 530±4 | 20±2 |
| Quercetin | 265±9 | 12±1 |
| Slope±SD (× 10-1) | Intercept±SD (×10-2) | |
| Catechin | 45±2 | 220±1 |
| Epicatechin | 52±2 | 145±1 |
| t-Resveratrol | 510±2 | 25±2 |
| Quercetin | 250±1 | 12.5±0.8 |
| C, mg Kg-1 | ||||
|---|---|---|---|---|
| Found ± SD (n=3) | added | recovered ± SD (n=3) | recovery, % | |
| Catechin | 49±2 | 16 | 62±3 | 95.4 |
| 28 | 75±1 | 97.4 | ||
| 40 | 83.9±0.5 | 94.3 | ||
| Mean: 95.7 | ||||
| Epicatechin | 28±1 | 10 | 35±2 | 92.1 |
| 17.5 | 43±2 | 94.5 | ||
| 25 | 52.3±0.8 | 98.7 | ||
| Mean: 95.1 | ||||
| trans-Resveratrol | 0.49±0.04 | 1.2 | 1.61±0.03 | 95.3 |
| 2.1 | 2.55±0.05 | 98.5 | ||
| 3.0 | 3.52±0.04 | 100.9 | ||
| Mean: 98.2 | ||||
| Quercetin | 0.50±0.03 | 1.2 | 1.67±0.04 | 98.2 |
| 2.1 | 2.61±0.01 | 100.4 | ||
| 3.0 | 3.41±0.01 | 97.4 | ||
| Mean: 98.7 | ||||
| Variety | C ± SD (n=3), mg Kg-1 | ||||
|---|---|---|---|---|---|
| (+)-catechin | (–)-epicatechin | trans-resveratrol | quercetin | quercetin glucosides | |
| Red grapes | |||||
| Agiorgitiko | (14±2)×10 | 70±6 | 0.55±0.09 | 0.34±0.08 | 1.4±0.4 |
| Cab. Franc | (17±2) ×10 | 55±4 | 0.33±0.02 | 0.18±0.04 | 1.1±0.3 |
| Cab.Sauvignon | 163±3 | 104±2 | 0.38±0.04 | 0.45±0.04 | 5.3±0.9 |
| Kotsifali | 77.3±0.9 | 33±1 | 3.39±0.09 | 0.75±0.03 | 1.49±0.09 |
| Limnio | (54±1) ×10 | (17±1) ×10 | 2.74±0.04 | 0.41±0.01 | # |
| Merlot | 70±6 | 89±3 | 0.35±0.04 | 0.29±0.07 | 10±2 |
| Refosko | (11±1)×10 | 36±3 | 0.34±0.07 | 0.7±0.2 | 6±2 |
| Romeiko | 53±2 | 46±3 | 0.08±0.03 | # | # |
| Sefka | 151±7 | 68±2 | 0.17±0.03 | 0.25±0.03 | 3.1±0.4 |
| Syrah | 34.6±0.7 | 35.5±0.6 | 2.6±0.1 | # | # |
| Vertzami | 117±4 | 43±2 | 0.8±0.1 | 0.16±0.01 | 7.1±0.4 |
| Xinomavro | 117±5 | 71±6 | 0.63±0.03 | 0.07±0.01 | 5.9±0.9 |
| White grapes | |||||
| Asyrtiko | (33±1) ×10 | 38±1 | 0.154±0.001 | 0.133±0.006 | 2.6±0.1 |
| Moshofilero1 | 29±3 | 74±5 | 2.73±0.09 | 0.35±0.02 | 2.1±0.1 |
| Moshofilero2 | 65±4 | 56±3 | 0.31±0.04 | 0.22±0.02 | 0.27±0.04 |
| Moshofilero3 | 96±3 | 94±6 | 0.82±0.05 | 0.24±0.05 | 1.6±0.4 |
| Moshofilero4 | 53±1 | 36±2 | 1.12±0.03 | 0.16±0.02 | 0.27±0.05 |
| Savatiano | 49± 2 | 28±1 | 0.49±0.04 | 0.50±0.03 | 1.01±0.09 |
Conclusions
Experimental
Materials
Plant material.
Sample preparation
HPLC analysis procedure
References
- Iriti, M.; Faoro, F. Grape phytochemicals: A bouquet of old and new nutraceuticals for human health. Med. Hypoth. 2006, 67, 833–838. [Google Scholar]; Yilmaz, Y.; Toledo, R.T. Health aspects of functional grape seed constituents. Trends Food Sci. Technol. 2004, 15, 422–433. [Google Scholar].
- Naissides, M.; Mamo, J.C.L.; James, A,P.; Pal, S. The effect of chronic consumption of red wine on cardiovascular disease risk factors in postmenopausal women. Atherosclerosis 2006, 185, 438–445. [Google Scholar] [CrossRef]
- Morre, D.M.; Morre, D.J. Anticancer activity of grape and grape skin extracts alone and combined with green tea infusions. Cancer Lett. 2006, 238, 202–209. [Google Scholar]
- Kim, H.; Deshane, J.; Barnes, S.; Meleth, S. Proteomics analysis of the actions of grape seed extract in rat brain: Technological and biological implications for the study of the actions of psychoactive compounds. Life Sci. 2006, 78, 2060–2065. [Google Scholar]
- Nikfardjam, M.S.P.; Mark, L.; Avar, P.; Figler, M.; Ohmacht, R. Polyphenols, anthocyanins, and trans-resveratrol in red wines from the Hungarian Villany region. Food Chem. 2006, 98, 453–462. [Google Scholar]
- Baydar, N.G.; Sagdic, O.; Ozkan, G.; Cetin, S. Determination of antibacterial effects and total phenolic contents of grape (Vitis vinifera L.) seed extracts. Int. J. Food Sci. Technol. 2006, 41, 799–804. [Google Scholar] [CrossRef]
- Lapidot, T.; Harel, S.; Akiri, B.; Granit, R.; Kanner, J. pH-dependent forms of red wine anthocyanins as antioxidants. J. Agr. Food Chem. 1999, 47, 67–70. [Google Scholar]; Yamaguchi, F.; Yoshimura, Y.; Nakazawa, H.; Ariga, T. Free radical scavenging activity of grape seed extract and antioxidants by electron spin resonance spectrometry in an H2O2/NaOH/DMSO system. J. Agr. Food Chem. 1999, 47, 2544–2548. [Google Scholar].
- Ghiselli, A.; Nardini, M.; Baldi, A.; Scarrini, C. Antioxidant activity of different phenolic fractions separated from an Italian red wine. J. Agr. Food Chem. 1998, 46, 361–367. [Google Scholar]
- Majem, L.S.; Alvarez, A.; de la Cruz, J.N. Mediterranean diet characteristics and health benefits. Arch. Latin Amer. Nutrit. 2004, 54 Suppl.1, 44–51. [Google Scholar]; Lekakis, J.; Rallidis, L.; Andreadou, I.; Vamvakou, G.; Kazantzoglou, G.; Magiatis, P.; Skaltsounis, A.L.; Kremastinos, D. Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease. Eur. J. Cardiov. Prev. R. 2005, 12, 596–600. [Google Scholar].
- Rimm, E.B.; Williams, P.; Fosher, K.; Criqui, M; Stampfer, M.J. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effect on lipids and haemostatic factors. BMJ 1999, 319, 1523–1528. [Google Scholar]
- Heinonen, I.M.; Meyer, A.S.; Frankel, E.N. Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation. J. Agr. Food Chem. 1998, 46, 4107–4112. [Google Scholar]
- Frankel, E.N.; Waterhouse, A.L.; Teissedre, P.L. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. J. Agr. Food Chem. 1995, 43, 890–894. [Google Scholar] [CrossRef]
- Fumagalli, F.; Rossoni, M.; Iriti, M. From field to health: A simple way to increase the nutraceutical content of grape as shown by NO-dependent vascular relaxation. J. Agr. Food Chem. 2006, 54, 5344–5349. [Google Scholar] [CrossRef]
- Vitseva, O.; Varghese, S.; Chakrabarti, S.; Folts, J.D.; Freedman, J.E. Grape seed and skin extracts inhibit platelet function and release of reactive oxygen intermediates. J. Cardiovasc. Pharm. 2005, 46, 445–451. [Google Scholar] [CrossRef]
- Pace-Asiak, C.R,; Hahn, S.E.; Diamandis, E.P.; Soleas, G.; Goldberg, D.M. The red wine phenolics trans-resveratrol and quercetin block human platelet-aggregation and eicosanoid synthesis - implications for protection against coronary heart-disease. Clin. Chim. Acta 1995, 235, 207–219. [Google Scholar] [CrossRef]
- Jayantilake, G.S.; Jayasuriya, H.; Lee, E.S. Kinase inhibitors from polygonum-cuspidatum. J. Nat. Prod. 1993, 56, 1805–1810. [Google Scholar] [CrossRef]
- Avila, M.A.; Velasco, J.A.; Cansado, J.; Notario, V. Quercetin mediates the down-regulation of mutant p53 in the human breast-cancer cell-line mda-mb468. Cancer Res. 1994, 54, 2424–2428. [Google Scholar]
- Giles, T.D.; Sander, G.E. Alcohol – A cardiovascular drug? Am. J. Geriat. Cardiol. 2005, 14, 154–158. [Google Scholar]
- Vian, M.A.; Tomao, V.; Gallet, S.; Coulomb, P.O.; Lacombe, J.M. Simple and rapid method for cis- and trans-resveratrol and piceid isomers determination in wine by high-performance liquid chromatography using Chromolith columns. J. Chromatogr. A 2005, 1085, 224–229. [Google Scholar]. Jeandet, P.; Chaudruc, D.; Robillard, B. Determination of the trans-resveratrol content of Champagne wines by reversed-phase HPLC. J. Int. Des Sci. Vigne Vin 2006, 40, 117–119. [Google Scholar].
- Sakkiadi, A.-V.; Stavrakakis, M.N.; Haroutounian, S.A. Direct HPLC assay of five biologically interesting phenolic antioxidants in varietal Greek red wines. Food Sci. Technol. 2000, 34, 410–413. [Google Scholar]
- De Limai, M.T.R.; Kelly, M.T.; Cabanis, M.T.; Blaise, A. Levels of phenolic acids, catechin and epicatechin in wines of Portugal and the Azores produced from different varieties and vintages. J. Int. Des Sci. Vigne Vin 2006, 40, 47–56. [Google Scholar]
- Makris, D.P.; Kallithraka, S.; Kefalas, P. Flavonols in grapes, grape products and wines: Burden, profile and influential parameters. J. Food Comp. Anal. 2006, 19, 396–404. [Google Scholar] [CrossRef]
- Serrano-Megias, M.; Nunez-Delicado, E.; Perez-Lopez, A.J.; Lopez-Nicolas, J.M. Study of the effect of ripening stages and climatic conditions on the physicochemical and sensorial parameters of two varieties of Vitis vinifera L. by principal component analysis: influence on enzymatic browning. J. Sci. Food and Agric. 2006, 86, 592–599. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Tsang, E.; Karumanchiri, A. Method to assay the concentrations of phenolic constituents of biological interest in wines. Anal. Chem. 1996, 68, 1688–1694. [Google Scholar]
- Goldberg, D.M.; Ng, E.; Karumanchiri, A.; Diamandis, E.P.; Soleas, G.J. Resveratrol Glucosidases are Important Components of Commercial Wines. Am. J. Enol. Vitic. 1996, 47, 415–420. [Google Scholar]
- Wang, R.; Zhou, W.B.; Wen, R.A.H. Kinetic study of the thermal stability of tea catechins in aqueous systems using a microwave reactor. J. Agr. Food Chem. 2006, 54, 5924–5932. [Google Scholar]
- Sample availability: Available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Sakkiadi, A.-V.; Georgiou, C.A.; Haroutounian, S.A. A Standard Addition Method to Assay the Concentration of Biologically Interesting Polyphenols in Grape Berries by Reversed-Phase HPLC. Molecules 2007, 12, 2259-2269. https://doi.org/10.3390/12092259
Sakkiadi A-V, Georgiou CA, Haroutounian SA. A Standard Addition Method to Assay the Concentration of Biologically Interesting Polyphenols in Grape Berries by Reversed-Phase HPLC. Molecules. 2007; 12(9):2259-2269. https://doi.org/10.3390/12092259
Chicago/Turabian StyleSakkiadi, Aphrodite-Victoria, Constantino A. Georgiou, and Serkos A. Haroutounian. 2007. "A Standard Addition Method to Assay the Concentration of Biologically Interesting Polyphenols in Grape Berries by Reversed-Phase HPLC" Molecules 12, no. 9: 2259-2269. https://doi.org/10.3390/12092259
APA StyleSakkiadi, A.-V., Georgiou, C. A., & Haroutounian, S. A. (2007). A Standard Addition Method to Assay the Concentration of Biologically Interesting Polyphenols in Grape Berries by Reversed-Phase HPLC. Molecules, 12(9), 2259-2269. https://doi.org/10.3390/12092259




