Abstract
2-(2,4-Dimethylphenyl)-2H-benzotriazole (1) has been synthesized in a three step procedure starting from 2,4-dimethyl-N-(2-nitrophenyl)benzamide via a 5-(2,4-dimethylphenyl)-1-(2-nitrophenyl)-1H-tetrazole intermediate. Its structure and those of Tinuvin® P and 2-phenyl-2H-benzotriazole (5) have been studied by multinuclear NMR (1H-, 13C- and 15N-) in solution and in the solid state. X-ray diffraction analysis of 1 and 5 allowed to us establish the molecular conformation around the single bond connecting the two aromatic systems, in agreement with the conclusions drawn from the NMR study. In the case of 1 ab initio geometry optimization was achieved at the Hartree-Fock HF/6-31G** and DFT B3LYP/6-31G** levels.
Introduction
In our search for new systems related to Ciba-Geigy's photoprotector Tinuvin® P [2-(2-hydroxy-5-methylphenyl)benzotriazole, Figure 1] [1,2], whose efficiency is strongly dependent on the conservation of planarity in the 2-aryl-2H-benzotriazole system during the proton transfer process, we successfully prepared the novel compound 2-(2,4-dimethylphenyl)-2H-benzotriazole (1, Figure 1).
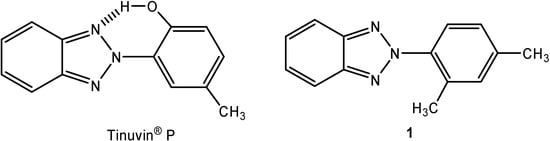
Figure 1.
The two aromatic systems (the 2H-benzotriazole and the phenyl ring) can rotate about the single bond connecting them adopting molecular conformations corresponding to the highest or lowest energy values for the molecular system in the electronic state concerned. The energy balance for the system at the minimum will reflect a compromise between delocalization of π electrons and electrostatic or steric non-bonding interactions from molecular sites, positions 2' and 6', adjacent to the connecting bond. Changes in such interactions can be expected to alter the twist angle corresponding to the equilibrium situation and hence change its molecular structure.

Scheme 1.
In the structure of 2-aryl-2H-benzotriazoles the single bond connecting the two aromatic systems retains its character throughout a torsional movement in the ground state as it is formed by an sp2 carbon atom that contributes with one π electron to the phenyl π system and an sp2 nitrogen atom that contributes with two electrons to the benzotriazole heteroaromatic ring; this precludes their conjugation without producing a charged, unstable form in the ground electronic state (see Scheme 1, above). In addition, these systems can be made sterically hindered by introducing appropriate substituents in the ortho position(s) of the phenyl ring.
Results and Discussion
Synthesis
The target of this study, 2-(2,4-dimethylphenyl)-2H-benzotriazole (1), was prepared as shown in Scheme 2, following a procedure previously reported for the preparation of 2-phenyl-2H-benzotriazole (5) [3]. The starting material, 2,4-dimethyl-N-(2-nitrophenyl)benzamide (2), was converted into the corresponding 2,4-dimethyl-N-(2-nitrophenyl)benzimidoyl chloride (3), which was not isolated but rather reacted directly with sodium azide in dry N,N-dimethylformamide to yield 5-(2,4-dimethyl-phenyl)-1-(2-nitrophenyl)-1H-tetrazole (4). Finally, thermolysis of 4 in nitrobenzene afforded compound 1 in 80% yield. The benzamide 2 in turn was readily prepared by reacting 2,4-dimethyl-benzoyl chloride with 2-nitroaniline [4]. All compounds have been completely characterized by 1H- and 13C-NMR spectroscopy (see Experimental Section).
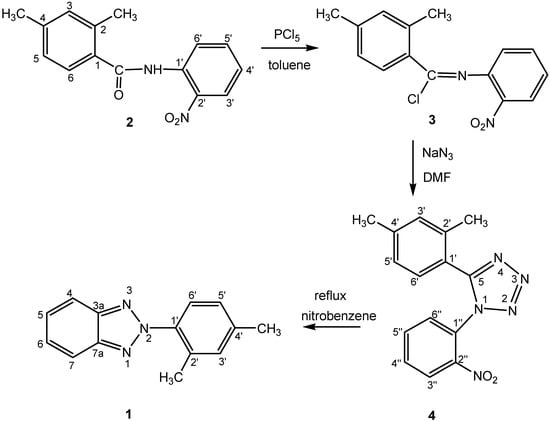
Scheme 2.
Synthesis of tetrazole 4 and benzotriazole 1.
Molecular structure and geometry optimization
Suitable crystals of compounds 1 and 5 were obtained from ethanol and N,N’-dimethylformamide solutions, respectively. Figure 2 and Figure 3 illustrate the molecular geometries of both compounds. They crystallize in the orthorhombic system, P212121 space group for 1 and Pnma space group for 5, with Z= 4 in both cases; compound 5 contains a twofold axis and there is only a half molecule in the asymmetric unit.
X-ray diffraction of 2-(2,4-dimethylphenyl)-2H-benzotriazole (1) shows that the molecule is non planar, the twisting angle between the benzotriazole ring and the phenyl group being of 48.12(6)º. Each independent moiety is almost planar: the best least-square planes of benzotriazole atoms and phenyl atoms show a rms deviation of 0.0060 and 0.0023, with a maximum deviation of 0.007(2) for C1 and 0.004(2) for C11, respectively.
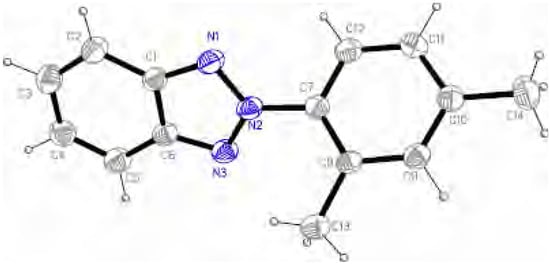
Figure 2.
ORTEP view of 1 with ellipsoids at the 40% probability level.
On the contrary, the crystals of 2-phenyl-2H-benzotriazole (5) correspond to a planar molecule (Figure 3), the twisting angle between the benzotriazole moiety and the phenyl group at the N2 being of 1.1(2)º.
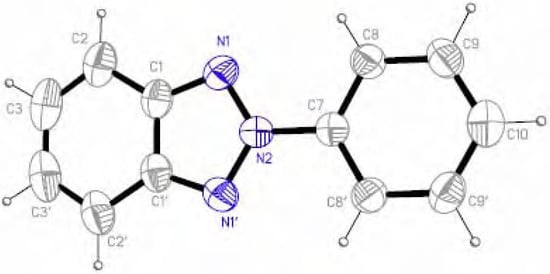
Figure 3.
ORTEP view of 5 with ellipsoids at the 40% probability level.
Selected bond lengths, angles and hydrogen bond features for both derivatives have been collected in Table 1. As expected the distances show the dienic character of the benzotriazole ring.

Table 1.
Some selected X-ray parameters, including the hydrogen bonds, for 1 and 5.
| 1 | 5 | ||
|---|---|---|---|
| Bond lengths (Å) | |||
| C1-N1 | 1.353(3) | C1-N1 | 1.348(3) |
| N1-N2 | 1.339(2) | N1-N2 | 1.331(2) |
| N2-N3 | 1.335(2) | N2-N1´ | 1.331(2) |
| N3-C6 | 1.358(3) | N1’-C1’ | 1.348(3) |
| N2-C7 | 1.440(3) | N2-C7 | 1.432(4) |
| C1-C6 | 1.409(3) | C1-C1´ | 1.395(5) |
| C1-C2 | 1.408(3) | C1-C2 | 1.415(4) |
| C2-C3 | 1.385(3) | C2-C3 | 1.361(4) |
| C3-C4 | 1.419(4) | C3-C3´ | 1.395(7) |
| C4-C5 | 1.355(3) | C3´-C2´ | 1.361(4) |
| C5-C6 | 1.409(3) | C2´-C1´ | 1.415(4) |
| C14-H142 | 0.95(4) | C9-H9 | 0.99(3) |
| C14…N1´´ | 3.679(4) | C9···N1´´´ | 3.452(4) |
| N1…H142´´ | 2.73(4) | N1···H9´´´ | 2.76(3) |
| Angles (°) | |||
| N1-N2-C7 | 120.2(2) | N1-N2-C7 | 121.4(1) |
| N1-N2-N3 | 117.2(2) | N1-N2-N1´ | 117.3(3) |
| N3-N2-C7 | 122.6(2) | N1´-N2-C7 | 121.4(1) |
| N2-N1-C1 | 102.5(2) | N2-N1-C1 | 102.4(2) |
| N1-C1-C6 | 109.1(2) | N1-C1-C1´ | 109.0(1) |
| C1-C6-N3 | 108.4(2) | C1-C1´-N1´ | 109.0(1) |
| C6-N3-N2 | 102.8(2) | C1´-N1´-N2 | 102.4(2) |
| C2-C1-C6 | 120.9(2) | C2-C1-C1´ | 121.3(2) |
| C1-C2-C3 | 116.9(2) | C1-C2-C3 | 116.0(3) |
| C3-C4-C5 | 122.4(2) | C3-C3´-C2´ | 122.7(2) |
| C4-C5-C6 | 116.7(2) | C3´-C2´-C1´ | 116.0(3) |
| C5-C6-C1 | 121.2(2) | C2´-C1´-C1 | 121.3(2) |
| C14-H142···N1´´ | 176(3) | C9-H9···N1´´´ | 127(2) |
(´) x,-y+1/2, z
(´´) –x+1, y+1/2, -z+1/2,
(´´´) -x+1/2, -y+1, z-1/2
The geometry for 2-(2,4-dimethylphenyl)-2H-benzotriazole (1) has been optimized at two different levels with the Windows Titan 1.0.5 package [5]. The twisting angle between the benzotriazole moiety and the 2,4-dimethylphenyl group (N1-N2-C7-C8) at the ab initio Hartree-Fock HF/6-31G** level is -47.91º (energy -701.061 Hartree, μ= 0.76 D) and at the B3LYP/6-31G** DFT level is -35.52º (energy -705.576 Hartree, μ= 0.96 D), the first result showing a better agreement with the experimental X-ray data. In both cases the molecules present specific interactions with the surrounding molecules as depicted in Figure 4a/Figure 4b and Figure 5, where the crystal packings are presented. Weak hydrogen bond interactions, C14-H14··· N1(-x+1,+y+1/2,-z+1/2) in 1 and C9-H9··· N1(-x+1/2, -y+1, z-1/2) in 5, give rise to independent zigzag chains parallel to the b axis. No π-π stacking interactions have been found in any of them.
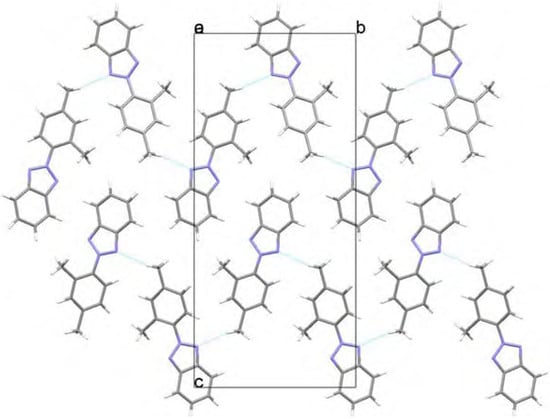
Figure 4a.
View along the a axis showing the C14-H14··· N1 interaction in 1.
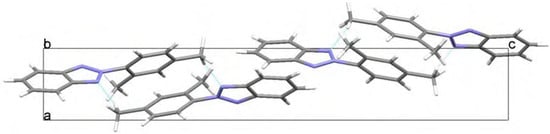
Figure 4b.
View along the b axis showing the phenyl groups alignment in 1.
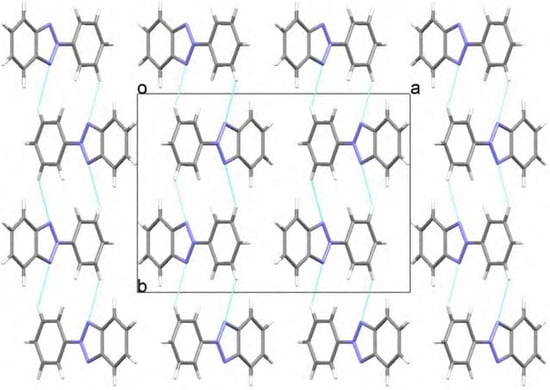
Figure 5.
View along the c axis showing the C9-H9··· N1 interaction in 5.
NMR spectroscopy
Full assignment of the 1H-, 13C- and 15N-NMR signals for benzotriazole 1 was achieved by careful analysis of the chemical shifts, coupling constants and bidimensional experiments. As a complete search through the Chemical Abstracts database did not provide any references in which full 1H-, 13C- and 15N-NMR assignments for 2-phenyl-2H-benzotriazole (5) and Tinuvin® P were described, we also undertook their study for structural comparison purposes (Figure 6 and Figure 7).
Useful conclusions regarding the conformation of compound 1 can be derived from the proton chemical shifts of the ortho protons, which appear at 7.59 ppm in CDCl3 (7.83 ppm if the effects of the methyl groups are considered) [6]. As shown in Figure 6, when such a value is compared with that of the ortho protons in the planar molecules Tinuvin® P (8.20 ppm or 8.57 ppm, if the effects of the methyl and hydroxy groups are considered) or 2-phenyl-2H-benzotriazole (5) (8.37 ppm in the same solvent), it proves that 1 is sterically hindered [7].
The deshielded signal at 11.10 ppm of Tinuvin® P corresponds to a proton involved in an intramolecular hydrogen bond (IMHB). However, it was noted that the signals of the benzotriazole ring (H-4/H-7 and H-5/H-6) are magnetically equivalent. This is a first indication that the IMHB is in a dynamic situation in solution allowing for a rapid rotation of the 2H-benzotriazole ring about the N-C single bond.
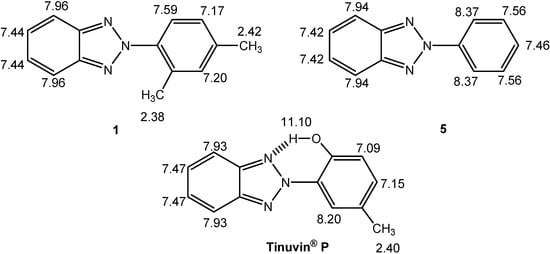
Figure 6.
1H-NMR chemical shifts (δ in ppm) in CDCl3 of 2-(2,4-dimethylphenyl)-2H-benzotriazole (1), 2-phenyl-2H-benzotriazole (5) and Tinuvin® P.
13C-NMR data in solution for 2-phenyl-2H-benzotriazole (5) appear in reference [8], but chemical shifts and coupling constants were obtained in DMSO-d6 instead of CDCl3. To our knowledge no data for Tinuvin® P have been described in the literature.
The extent of interannular conjugation in N-phenylazoles can also be studied by using 13C-NMR spectroscopy [8,9]. In compound 1, both the 13C chemical shifts for the ortho-phenyl carbon δ C-2’ (or δ C-6’) and the chemical shift difference between the ortho- and meta- carbons (Table 2 and Table 3) reflect that the bond is twisted and confirms the conformation of the system in qualitative terms. In the unhindered molecules Tinuvin® P and 2-phenyl-2H-benzotriazole (5), the difference between ortho- and meta- carbons is around 9 ppm [9].

Table 2.
13C-NMR chemical shifts of 2H-benzotriazoles in CDCl3 with the methyl-and hydroxy-induced chemical shifts correction on the C-ortho and C-meta atoms (CH3: Z1 9.2, Z2 0.7, Z3 -0.1, Z4 -3.0; OH: Z1 28.8, Z2 –12.8, Z3 1.4, Z4 –7.4 ) [6].
| Comp. | 1 | 5 | Tinuvin® P |
| δC-ortho | 125.8 + 0.1 + 0.1 = 126.0 | 120.6 | 147.5 –28.8 + 3.0 = 121.7 |
| 132.9 – 9.2 + 0.1 = 123.8 | 121.1 –1.4 –0.7 = 119.0 | ||
| δC-ortho (average) | 124.9 | 120.6 | 120.3 |
| δC-meta | 127.2 + 3.0 – 0.7 = 129.5 | 129.4 | 118.7 +12.8 + 0.1 = 131.6 |
| 132.3 – 0.7 – 0.7 = 130.9 | 129.6 +7.4 – 9.2 = 127.8 | ||
| δC-meta (average) | 130.2 | 124.9 | 129.7 |
| δC-meta-δC-ortho | 5.3 | 8.8 | 9.4 |
| Degree of Interannular Conjugation | Hindered | Extensive | Extensive |
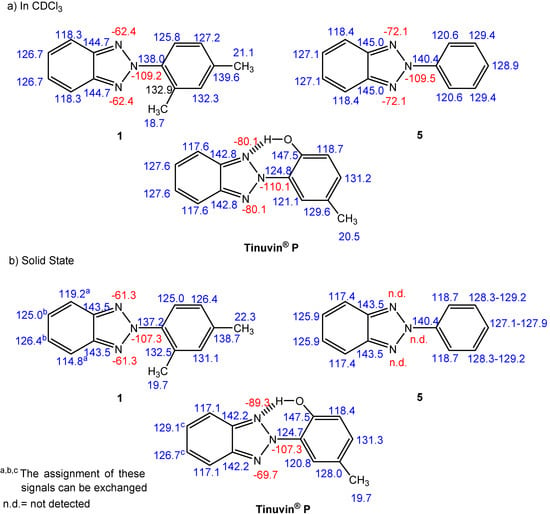
Figure 7.
13C-NMR (blue) and 15N-NMR (red) chemical shifts (δ in ppm) in CDCl3 and in the solid state of 2-(2,4-dimethylphenyl)-2H-benzotriazole (1), 2-phenyl-2H-benzo-triazole (5) and Tinuvin® P.
As seen in the data shown in Figure 7, no significant changes in the 13C-NMR chemical shifts were observed on going from solution to the solid state, meaning that in the three derivatives the solid state conformation is very similar to the one in solution.
Finally the 15N-NMR chemical shift of N-2 in compounds 1, 5 and Tinuvin® P ranged from –107.3 ppm in the solid state to a mean value of –109.6 ppm in solution (between –109.2 to –110.1 ppm), typical of a pyrrole-like nitrogen atom [10], which is not sensitive to the torsional angle between the two aromatic moieties.

Table 3.
13C-NMR chemical shifts of 2H-benzotriazoles in solid state with the methyl-and hydroxy-induced chemical shifts correction on the C-ortho and C-meta atoms (CH3: Z1 9.2, Z2 0.7, Z3 -0.1, Z4 -3.0; OH: Z1 28.8, Z2 –12.8, Z3 1.4, Z4 –7.4 ) [6].
| Comp. | 1 | 5 | Tinuvin® P |
|---|---|---|---|
| δC-ortho | 125.0 + 0.1 + 0.1 = 125.2 | 118.7 | 147.5 –28.8 + 3.0 = 121.7 |
| 132.5 – 9.2 + 0.1 = 123.4 | 120.8 –1.4 –0.7 = 118.7 | ||
| δC-ortho (average) | 124.3 | 118.7 | 120.2 |
| δC-meta | 126.4 + 3.0 – 0.7 = 128.7 | 128.7 | 118.4 +12.8 + 0.1 = 131.3 |
| 131.1 – 0.7 – 0.7 = 129.7 | 128.0 +7.4 – 9.2 = 126.2 | ||
| δC-meta (average) | 129.2 | 128.7 | 128.7 |
| δC-meta-δC-ortho | 4.9 | 10.0 | 8.5 |
| Degree of Interannular Conjugation | Hindered | Extensive | Extensive |
In solution the pyridine-like nitrogen atoms N-1 and N-3 show a unique chemical shift for the three derivatives, –62.4 ppm in 1, –72.1 ppm in 5 and –80.1 ppm in Tinuvin® P (Figure 8).
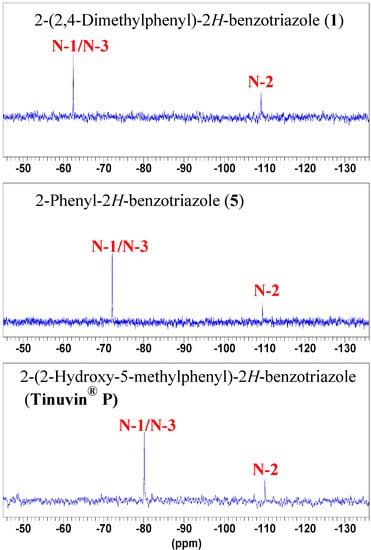
Figure 8.
15N-NMR spectra of compounds 1, 5 and Tinuvin® P in CDCl3.
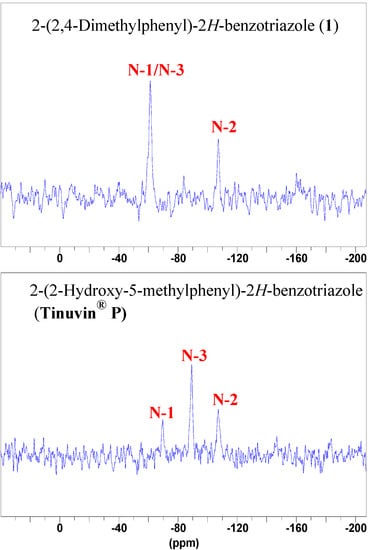
Figure 9.
Solid state 15N-CPMAS-NMR spectra of compounds 1 and Tinuvin® P.
The nitrogen chemical shift difference of 9.7 ppm between 1 (twisted, dihedral angle 48.12º) and 5 (planar, dihedral angle 0.1(2)º) results from their different conformations. The fact that the signals of N-1 and N-3 appear both at –80.1 ppm in Tinuvin® P proves that in solution there is a rapid rotation about the N-C single bond although an IMHB is present. This is confirmed by the spectrum of Tinuvin® P in the solid state (Figure 9), where two signals are observed, one at –69.7 ppm (N-1), and the other at –89.3 ppm (N-3), more intense because of the IMHB and the proximity of an H atom in the Cross Polarization (CP) experiments. The average of these signals is –79.5 ppm, close to the value in solution (–80.1 ppm). No signals for the nitrogen atoms of compound 5 in solid state could be detected using the same experimental acquisition conditions.
Conclusions
Solution and solid state multinuclear NMR studies, in combination with X-ray diffraction analysis, have proven to be a valuable tool to establish the molecular conformation of 2-aryl-2H-benzotriazole systems related to Tinuvin® P. The twisted 2-(2,4-dimethylphenyl)-2H-benzotriazole molecule (1) is a very promising candidate for photophysics and photochemistry studies, affording new insights to understand the deactivation processes that confer photostability.
Experimental Section
General
Tinuvin® P was supplied by Ciba-Geigy and recrystallized from heptane. 2-Phenyl-2H-benzo-triazole (5) was prepared as described in the literature [3]. Melting points were determined with a ThermoGalen hot stage microscope and are given uncorrected. Elemental analyses for carbon, hydrogen and nitrogen were performed by the Microanalytical Service of the Universidad Complutense of Madrid, using a Perkin-Elmer 240 analyzer. Column chromatography was conducted on silica gel (Merck 60, 70-230 mesh). Rf values were measured on aluminum-backed TLC plates of silica gel 60 F254 (Merck, 0.2 mm) with the indicated eluent.
NMR Spectroscopy
Solution. NMR spectra were recorded on a Bruker DRX 400 spectrometer (9.4 Tesla, operating at 400.13 MHz for 1H, 100.62 MHz for 13C and 40.56 MHz for 15N, respectively) with a 5-mm inverse-detection H-X probe equipped with a z-gradient coil, at 300 K. Chemical shifts (δ, in ppm) are given from internal solvent CDCl3 (7.26 for 1H and 77.0 for 13C) and DMSO-d6 (2.49 for 1H and 39.5 for 13C), and for 15N, nitromethane (0.00) was used as external reference. 2D gs-COSY (1H-1H) and 2D inverse proton detected heteronuclear shift correlation spectra [gs-HMQC (1H-13C), gs-HMBC (1H-13C) and gs-HMBC (1H-15N)] were acquired and processed using standard Bruker NMR software and in non-phase-sensitive mode [11]. 1D 15N-NMR was acquired with inverse gated decoupling.
Solid state. 13C (100.73 MHz) and 15N (40.60 MHz) CPMAS NMR spectra were obtained on a Bruker WB 400 spectrometer at 300 K using a 4 mm DVT probehead. Samples were carefully packed in a 4-mm diameter cylindrical zirconia rotor with Kel-F end-caps. Operating conditions involved 3.2 µs 90° 1H pulses and decoupling field strength of 78.1 kHz by TPPM sequence. 13C spectra were originally referenced to a glycine sample and then the chemical shifts were recalculated to the Me4Si (for the carbonyl atom δ (glycine) = 176.1 ppm) and 15N spectra to 15NH4Cl and then converted to nitromethane scale using the relationship: δ15N(nitromethane) = δ 15N(ammonium chloride) – 338.1 ppm. The typical acquisition parameters for 13C-CPMAS were: spectral width, 40 kHz; recycle delay, 25 s for 1, 70 s for 5 and 60 s for Tinuvin® P; acquisition time, 30 ms; contact time, 2 ms; and spin rate, 12 kHz. In order to distinguish protonated and unprotonated carbon atoms, the NQS (Non-Quaternary Suppression) experiment by conventional cross-polarization was recorded; before the acquisition the decoupler is switched off for a very short time of 25 μs. For 15N-CPMAS they were: spectral width, 40 kHz; recycle delay, 25 s for 1, 70 s for 5 and 60 s for Tinuvin® P; acquisition time, 35 ms; contact time, 9 ms; and spin rate, 6 kHz.
2-Phenyl-2H-benzotriazole (5)
1H-NMR (CDCl3): δ (ppm) 8.37 (m, 2H, H-2’, H-6’), 7.94 (m, 2H, H-4, H-7, 3J4,5= 8.7, 5J4,7 = 0.9), 7.56 (m, 2H, H-3’, H-5’), 7.46 (m, 1H, H-4’), 7.42 (m, 2H, H-5, H-6, 3J5,6= 6.7, 4J5,7 = 1.0); 13C-NMR (CDCl3): δ (ppm) 145.0 (C-3a, C-7a, 3J = 3J = 9.4, 2J = 4.1), 140.4 (C-1’), 129.4 (C-3’, C-5’, 1J = 162.5, 3JH5’ = 8.2), 128.9 (C-4’, 1J = 162.4, 3J = 3J = 7.7), 127.1 (C-5, C-6, 1J = 160.8, 3J = 8.2, 2J = 1.8), 120.6 (C-2’, C-6’, 1J = 166.0), 118.4 (C-4, C-7, 1J = 165.4).
Tinuvin® P
1H-NMR (CDCl3): δ (ppm) 11.10 (broad s, 1H, OH), 8.20 (dddddd, 1H, H-6’, 4J6’,4’= 2.1, 4J6’,Me= 5J6’,3’= 5J6’,OH= 0.5), 7.93 (m, 2H, H-4, H-7, 3J4,5= 8.7, 5J4,7 = 1.0), 7.47 (m, 2H, H-5, H-6, 3J5,6= 6.8, 4J5,7 = 1.0), 7.15 (dddddd, 1H, H-4’, 3J4’,3’ = 8.4, 4J4’,Me = 4J4’,OH= 0.5), 7.09 (broad d, 1H, H-3’), 2.40 (broad s, 3H, CH3); 13C-NMR (CDCl3): δ (ppm) 147.5 (C-2’), 142.8 (C-3a, C-7a, 3J = 3J = 9.2, 2J = 4.6), 131.2 (C-4’, 1J = 159.5, 3JH6’ = 7.7, 3JMe = 4.6), 129.6 (C-5’, 3JH2’ = 6.5, 2JMe = 6.5), 127.6 (C-5, C-6, 1J = 161.8, 3J = 8.4), 124.8 (C-1’), 121.1 (C-6’, 1J = 162.6, 3JH4’ = 6.1, 3JMe = 6.1), 118.7 (C-3’, 1J = 161.5, 3JOH = 7.7), 117.6 (C-4, C-7, 1J = 167.2), 20.5 (CH3, 1J = 127.3, 3J = 4.6).
Synthesis
2,4-dimethyl-N-(2-nitrophenyl)benzamide (2). 2,4-Dimethylbenzoyl chloride (2.86 g, 17.01 mmol) was added to o-nitroaniline (2.35 g, 17.01 mmol) in pyridine (14 mL). After 1 h at room temperature water was added and the precipitate filtered off, washed with water and dried in a vacuum oven to obtain 4.1 g (89%) of 2. M.p. 122–123 °C (crystallized from ethanol). 1H-NMR (CDCl3): δ (ppm) 10.76 (s, 1H, NH), 8.97 (dd, 1H, H-3’, 3J3’,4’= 8.5, 4J3’,5’ = 1.5), 8.26 (dd, 1H, H-6’, 3J6’,5’= 8.5, 4J6’,4’ = 1.5). 7.70 (ddd, 1H, H-4’, 3J4’,5’= 7.3), 7.53 (m, 1H, H-6), 7.21 (ddd, 1H, H-5’), 7.10-7.14 (broad m, 2H, H-3, H-4), 2.54 (s, 3H, CH3-2), 2.38 (s, 3H, CH3-4); 13C-NMR (CDCl3): δ (ppm) 168.2 (CO), 141.5 (C-4), 137.6 (C-2), 136.5 (C-1’), 136.0 (C-4′), 135.3 (C-2′), 132.6 (C-3), 132.4 (C-1), 127.1 (C-6), 126.9 (C-5), 125.8 (C-6′), 123.2 (C-5′), 122.1 (C-3′), 21.3 (CH3-4), 20.3 (CH3-2′); 15N-NMR (CDCl3): δ (ppm) –255.9 (NH, 1JNH = 90.0 Hz).
5-(2,4-Dimethylphenyl)-1-(2-nitrophenyl)tetrazole (4). A mixture of 2,4-dimethyl-N-(2-nitrophenyl)-benzamide (2, 1.70 g, 6.29 mmol) and phosphorus pentachloride (1.31 g, 6.29 mmol) in toluene (20 mL) was heated under reflux for 1 h, after which the hot solution was filtered and the solvent removed under reduced pressure. The resulting 2,4-dimethyl-N-(2-nitrophenyl)benzimidoyl chloride (3) oil was dissolved in dry DMF (20 mL) and added dropwise to a vigorously stirred mixture of sodium azide (0.82 g, 12.58 mmol) and dry DMF (10 mL) over a period of 45 min, the temperature being kept below 25 ºC throughout. The suspension thus obtained was heated at 80 ºC for 1 h and then cooled. Next, enough water to dissolve any inorganic salts present and then cause turbidity was added and the mixture stored refrigerated at 5 ºC for one day. The crystals thus formed were filtered off and washed with water to obtain 1.37 g (74%) of 4. M.p. 111–112 °C (crystallized from ethanol:water); 1H-NMR (DMSO-d6): δ (ppm) 8.22 (dd, 1H, H-3′′, J3′′,4′′ = 8.2, J3′′,5′′ = 1.2), 7.97 (ddd, 1H, H-5′′, J5′′,6′′ = 7.3, J5′′,4′′ = 6.9), 7.94 (dd, 1H, H-6′′, J4′′,6′′ = 2.1), 7.87 (ddd, 1H, H-4′′), 7.20 (s, 1H, H-3′), 7.05 (d, 1H, H-6′, J6′,5′ = 7.9), 6.98 (d, 1H, H-5′), 2.26 (s, 3H, CH3-4′), 2.24 (s, 3H, CH3-2′); 13C-NMR (DMSO-d6): δ (ppm) 154.3 (C-5), 143.8 (C-2′′), 141.3 (C-4′), 137.9 (C-2′), 135.3 (C-5′′), 132.7 (C-4′′), 131.7 (C-3′), 129.9 (C-6′′), 129.7 (C-6′), 126.7 (C-5′), 126.3 (C-1′′), 126.2 (C-3′′), 118.6 (C-1′), 20.8 (CH3-4′), 19.2 (CH3-2′).
2-(2,4-Dimethylphenyl)-2H-benzotriazole (1). 5-(2,4-Dimethylphenyl)-1-(2-nitrophenyl)tetrazole (4, 1.25 g, 4.23 mmol) and freshly distilled nitrobenzene (5.5 mL) were heated together under vigorous reflux for 1 h. After the reaction mixture was cooled, the nitrobenzene was removed by vacuum distillation and the residue purified by chromatography on silica gel Rf = 0.27 (chloroform/hexane 6:4). Compound 1 was obtained in 80% yield (0.75 g); M.p. 72-73 °C (crystallized from ethanol); Anal. Calcd for C14H13N3: C, 75.31; H, 5.87; N, 18.82%. Found: C, 75.35; H, 5.95; N, 18.73%; 1H-NMR (CDCl3): δ (ppm) 7.96 (m, 2H, H-4, H7, 3J4,5= 8.7, 5J4,7 = 0.9), 7.59 (d, 1H, H-6’, 3J6’,5’ = 8.1), 7.44 (m, 2H, H-5, H-6, 3J5,6= 6.8, 4J5,7 = 1.0), 7.20 (broad s, 1H, H-3’), 7.17 (broad d, 1H, H-5′), 2.42 (s, 3H, CH3-4′), 2.38 (s, 3H, CH3-2′); 13C- NMR (CDCl3): δ (ppm) 144.7 (C-3a, C-7a, 3J = 3J = 9.2, 2J = 4.6), 139.6 (C-4’, 3J = 6.1, 3JMe = 6.1), 138.0 (C-1’), 132.9 (C-2’), 132.3 (C-3’, 1J = 158.0, 3JH5’ = 6.1, 3JMe = 4.6), 127.2 (C-5’, 1J = 161.0, 3JH3’ = 7.6, 3JMe = 4.6), 126.7 (C-5, C-6, 1J = 160.2, 3J = 8.4), 125.8 (C-6’, 1J = 162.5), 118.3 (C-4, C-7, 1J = 164.1), 21.1 (CH3-4′, 1J = 127.3, 3J = 4.6), 18.7 (CH3-2′, 1J = 128.8, 3J = 4.6).
X-Ray data collection and structure refinement
A summary of the fundamental crystal and refinement data for compounds 1 and 5 is given in Table 4. Colorless prismatic crystals were successfully grown from ethanol and N,N’-dimethyl-formamide and used to collect data on a Bruker Smart CCD diffractometer using graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å) operating at 50 Kv and 20 mA. The data were collected over a hemisphere of the reciprocal space by combination of three exposure sets, each exposure was of 20 s covered 0.3° in ω. The cell parameters were determined and refined by a least-squares fit of all reflections collected. The first 100 frames were recollected at the end of the data collection to monitor crystal decay after X-ray exposition and no important variation was observed. The structure was solved by direct methods and difference Fourier techniques and refined by full-matrix least-squares on F2 (SHELXL-97) [12].

Table 4.
Crystal data and structure refinement for 1 and 5.
| Crystal Data | 1 | 5 |
| Identification code | 651191 | 659842 |
| Empirical formula | C14 H13 N3 | C12H9N3 |
| Formula weight | 223.27 | 195.22 |
| Wavelength (Å) | 0.71073 | 0.71073 |
| Crystal system | Orthorhombic | Orthorhombic |
| Space group | P212121 | Pnma |
| Unit cell dimensions | ||
| a (Å) | 3.946(2) | 15.655(2) |
| b (Å) | 11.586(7) | 11.433(2) |
| c (Å) | 25.44(1) | 5.551(1) |
| Volume (Å3) | 1163(1) | 993.6(3) |
| Z | 4 | 4 |
| Density (calculated) (Mg/m3) | 1.275 | 1.305 |
| Absorption coefficient (mm-1) | 0.078 | 0.081 |
| F(000) | 472 | 408 |
| Scan technique | phi and omega | phi and omega |
| Theta range (°) for data collection | 1.60 to 27.00 | 2.60 to 26.00 |
| Index ranges | -4<=h<=4, | -19<=h<=17, |
| -14<=k<=12, | -14<=k<=14, | |
| -32<=l<=32 | -6<=l<=6 | |
| Reflections collected | 10351 | 7629 |
| Independent reflections | 2501 | 1028 |
| [R(int) = 0.0862] | [R(int) = 0.0741] | |
| Completeness to theta max. | 99.4 % | 100% |
| Data / restraints / parameters | 2501 / 0 / 194 | 1028 / 0 / 88 |
| Goodness-of-fit on F2 | 0.938 | 1.079 |
| Final R* indices [F2>2sigma(F2)] | 0.0420 | 0.0457 |
| (1448 reflns. observed) | (569 reflns. observed) | |
| wR** (F2) (all data) | 0.1024 | 0.1573 |
| Largest diff. peak and hole (e.Å–3) | 0.133 and -0.133 | 0.136 and 0.207 |
(*) ∑||Fo|-|Fc||/∑|Fo|
(**) {∑[w(Fo2-Fc2)2]/∑[w(Fo2)2]}1/2
In both cases, anisotropic thermal parameters were used in the last cycles of refinement for all non-hydrogen atoms. The positions of the hydrogen atoms were found in difference density maps and freely refined with a common isotropic displacement parameter. Crystallographic data are deposited with the Cambridge Crystallographic Data Center (CCDC-651191 and CCDC-659842). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Acknowledgments
Thanks are given to MEC of Spain for financial support (project number CTQ2006-02586).
References
- Catalán, J.; Pérez, P.; Fabero, F.; Wilshire, J. F. K.; Claramunt, R. M.; Elguero, J. Photophysical properties of some 2-(2’-hydroxyaryl)benzotriazoles: dramatic effect of an ortho-located bulky tert-butyl group. J. Am. Chem. Soc. 1992, 114, 964–966. [Google Scholar] [CrossRef]
- Catalán, J.; de Paz, J. L. G.; Torres, M. R.; Tornero, J. D. Molecular structure of a unique UV stabilizer: Tinuvin P. J. Chem. Soc. Faraday Trans 1997, 93, 1691–1696, and references therein. [Google Scholar] [CrossRef]
- Houghton, P. G.; Pipe, D. F.; Rees, C. W. Intramolecular reaction between nitro and carbodi-imide groups; a new synthesis of 2-arylbenzotriazoles. J. Chem. Soc. Perkin Trans I 1985, 1471–1479. [Google Scholar] [CrossRef]
- Vickers, S.; Triggle, D. J.; Garrison, D. R. The preparation and diazotisation of some o- and p-aminophenyl benzoates and benzamides. J. Chem. Soc. C 1968, 632–634. [Google Scholar] [CrossRef]
- Windows Titan 1.05, Wavefunction Inc.: Irvine, CA.
- Pretsch, E.; Bühlmann, P.; Affolter, C. Structure Determination of Organic Compounds: Tables of Spectral Data; Springer: New York, 2000. [Google Scholar]
- Catalán, J.; Fabero, F.; Claramunt, R. M.; Santa María, M. D.; Foces-Foces, C.; Cano, F. H.; Martínez-Ripoll, M.; Elguero, J.; Sastre, R. New ultraviolet stabilizers: 3- and 5-(2´-hydroxy-phenyl)pyrazoles. J. Am. Chem. Soc. 1992, 114, 5039–5048. [Google Scholar] [CrossRef]
- Begtrup, M.; Elguero, J.; Faure, R.; Camps, P.; Estopa, C.; Ilavsky, D.; Fruchier, A.; Marzin, C.; de Mendoza, J. Effects of N-substituents on the 13C NMR parameters of azoles. Magn. Reson. Chem. 1988, 26, 134–151. [Google Scholar] [CrossRef]
- Begtrup, M. 13C NMR spectra of phenyl-substituted azoles: a conformational study. Acta Chem. Scand. 1973, 27, 3101–3110. [Google Scholar] [CrossRef]
- Claramunt, R. M.; Sanz, D.; López, C.; Jiménez, J. A.; Jimeno, M. L.; Elguero, J.; Fruchier, A. Susbtituent effects on the 15N NMR parameters of azoles. Magn. Reson. Chem. 1997, 35, 35–75. [Google Scholar] [CrossRef]
- Berger, S.; Braun, S. 200 and More NMR Experiments; Wiley-VCH: Weinheim, 2004. [Google Scholar]
- Sheldrick, G. M. SHELX97, Programs for Crystal Structure Analysis, release 97-2; University of Göttingen: Göttingen, Germany, 1998. [Google Scholar]
- Sample Availability: Samples of the compounds 1 and 5 are available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.