Electrochemical Synthesis and Structural Characterization of a Novel Mixed-valence Copper (I)-copper (II) Complex: {[Bis(ethylenediamine) Copper (II)] Bis[diiodocuprate (I)]}
Abstract
:Introduction
Results and Discussion
Electrosynthesis
| Cathode: | ½ I2 +e → I |
| Anode: | Cu → Cu+ + e |
| 2Cu+ → Cu2+ + Cu | |
| Cu+ + 2I- → CuI2– | |
| Cu+2 + 2en + 2 CuI2 – → [Cu(en)2][ CuI2]2 |
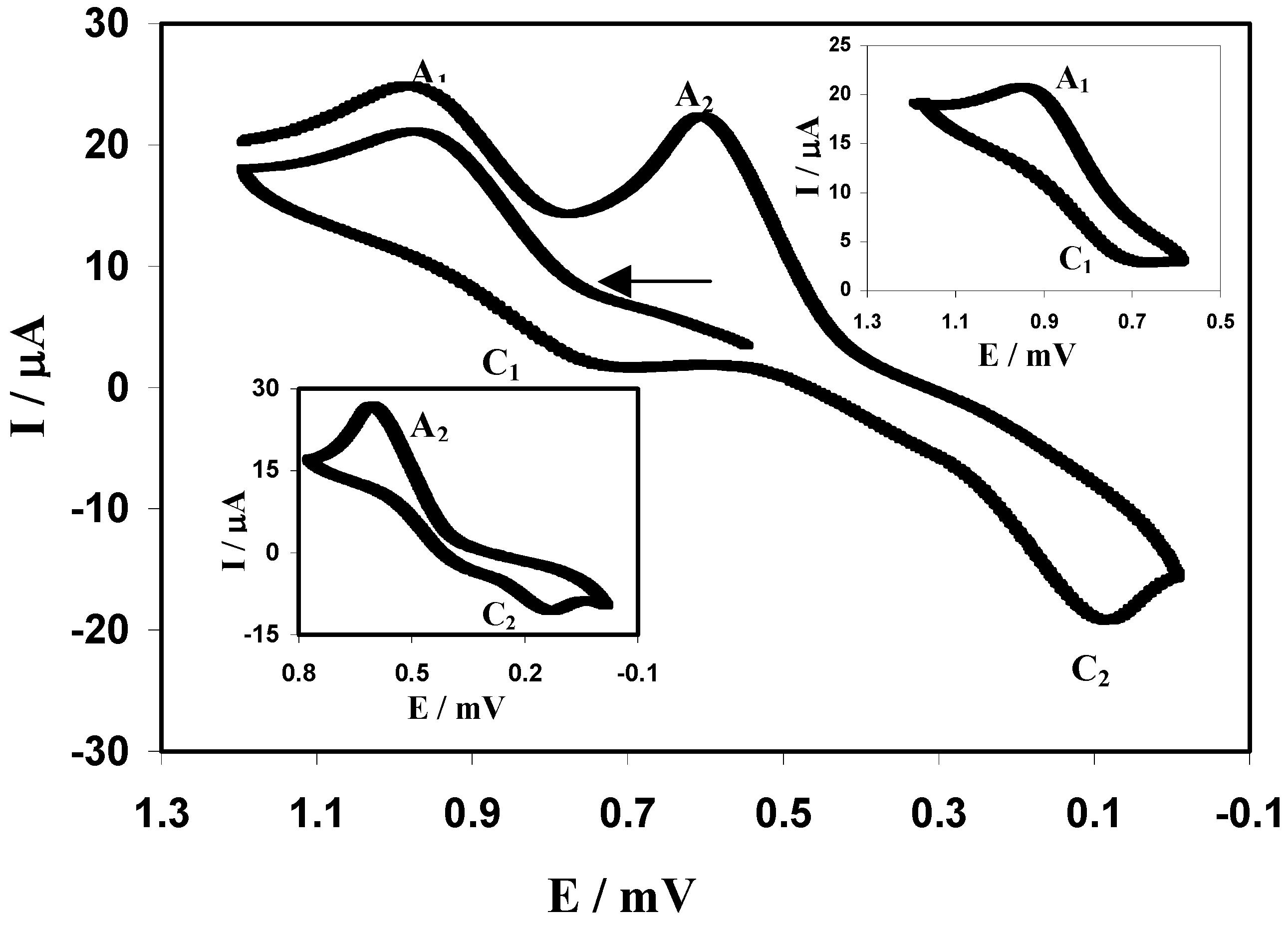
Electrochemistry
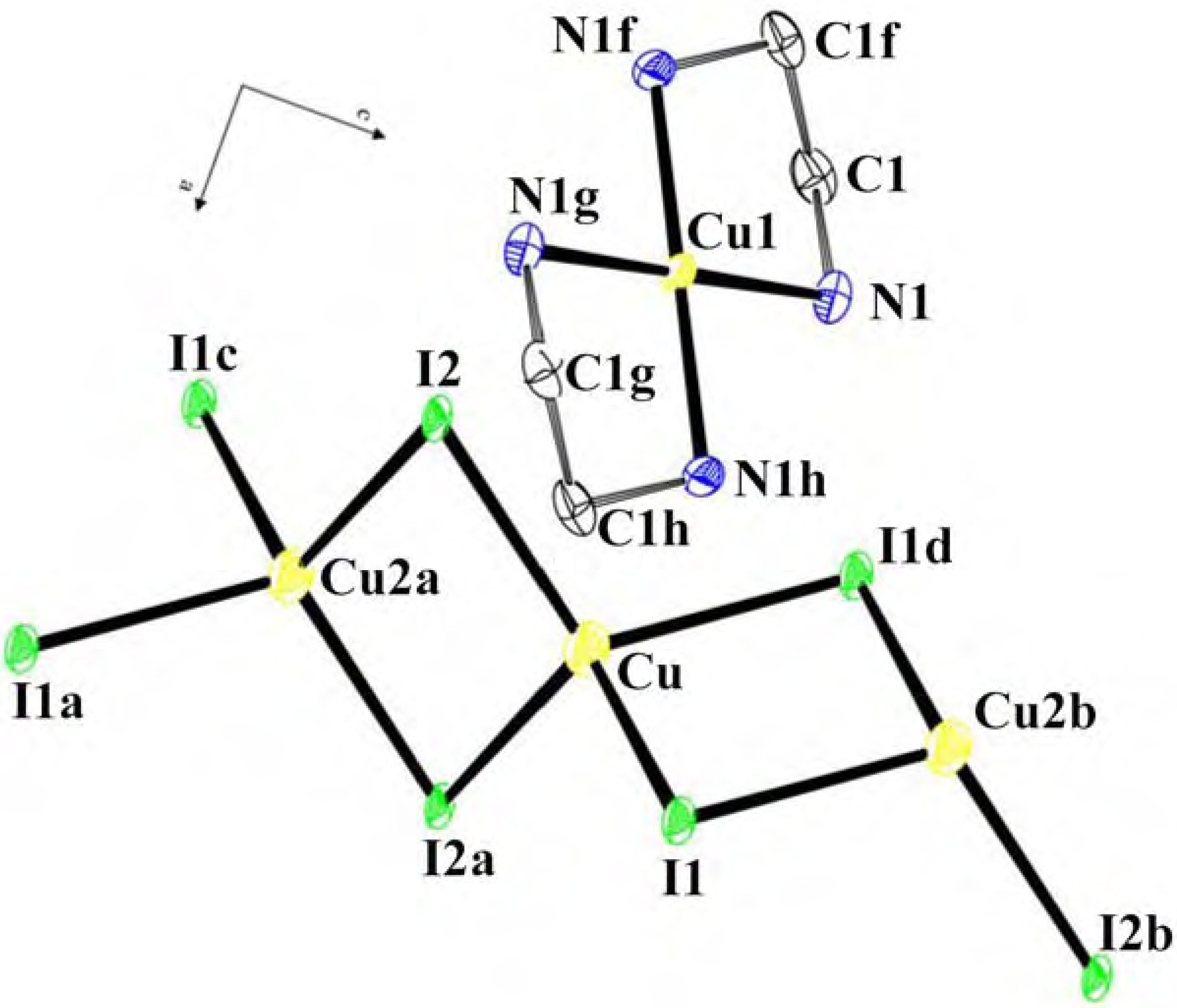
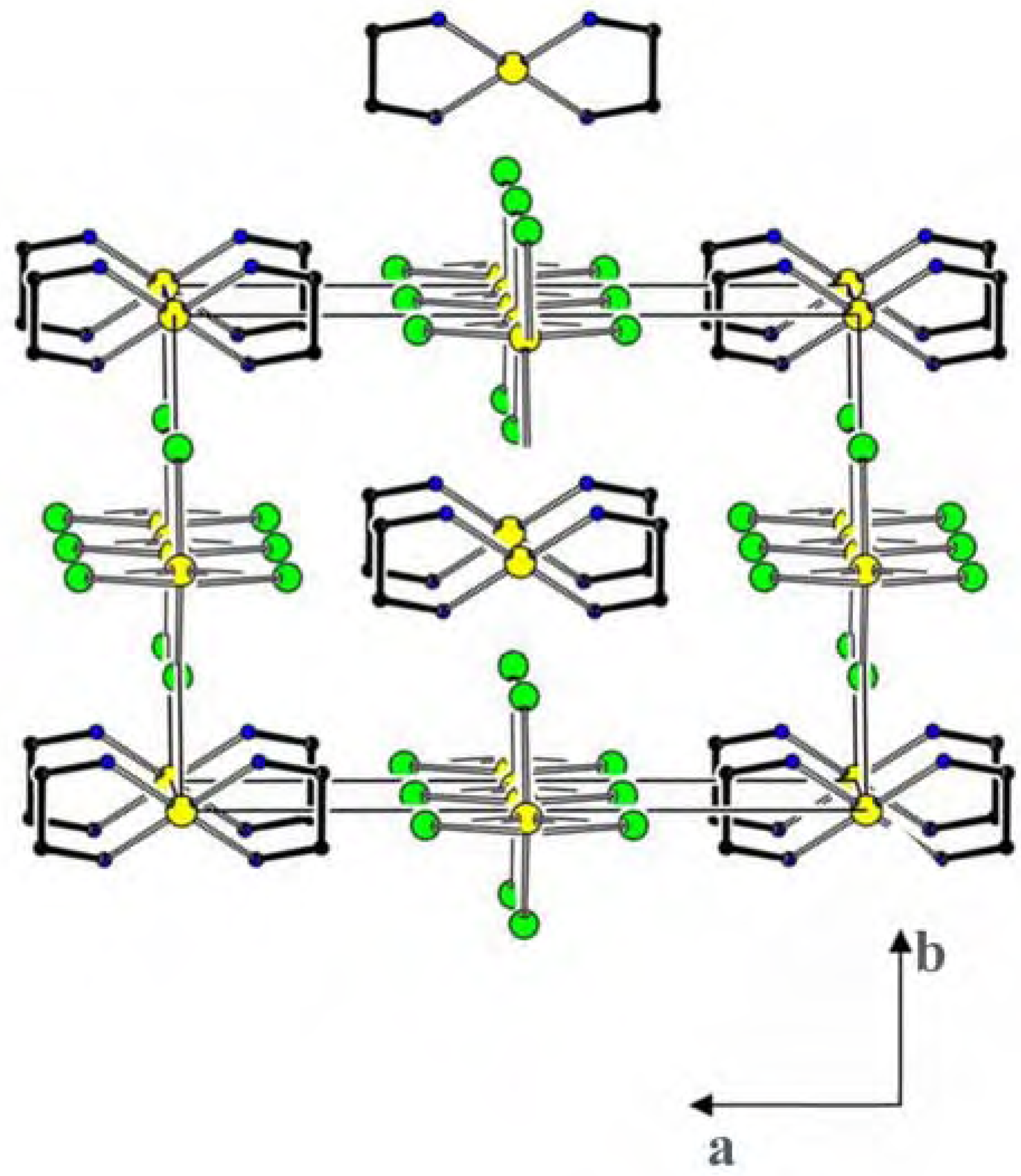
Structural studies
| 1 | |
|---|---|
| T/K | 293(2) |
| Crystal System | Monoclinic |
| Space group | C 2/m |
| a/Å | 10.3632(11) |
| b/Å | 13.2843(13) |
| c/Å | 6.5115(7) |
| β/o | 117.339(11) |
| V/Å3 | 796.30(16) |
| Z, Dcalc./Mg.m-3 | 2, 3.346 |
| μ/mm-1 | 11.700 |
| F(000) | 702.0 |
| Crystal size/mm3 | |
| θ/o | 2.69 to 28.050 |
| h/k/l | -12,13/-17,17/-8, 8 |
| Refl. unique | 996 |
| Refl. Observed [I>2σ(I)] | 915 |
| R(int) | 0.040 |
| Data /restraints/parameters | 996/ 0 /40 |
| Goodness-of-fit on F2 | 1.280 |
| Final R indices [I>2σ(I)] | R1 =0.0614, wR2 = 0.1632 |
| R indices for all data | R1 = 0.0649, wR2 = 0.1661 |
| Largest difference Peak and hole (e. Å–3) | 0.799 and -0.740 |
| I1—Cu2i | 2.696(1) | Cu1—N1iv | 2.003(10) |
| I1—Cu2 | 2.696(1) | Cu1—N1 | 2.003(10) |
| I2—Cu2 | 2.642(24) | Cu1—N1iii | 2.003(10) |
| I2—Cu2ii | 2.671(3) | Cu1—N1v | 2.003(10) |
| Cu2—I2ii | 2.671(3) | N1—C1 | 1.477(11) |
| Cu2—Cu2ii | 2.930(2) | C1—C1v | 1.506(16) |
| Cu2i—I1—Cu2 | 83.28(0) | N1iii—Cu1—N1 | 179.99(27) |
| Cu2—I2—Cu2ii | 66.93(5) | N1iii—Cu1—N1iv | 85.51(27) |
| I2—Cu2—I1 | 112.25(0) | N1—Cu1—N1iv | 94.49(27) |
| I2ii—Cu2—I1 | 110.73(0) | N1iii—Cu1—N1v | 94.49(27) |
| I1i—Cu2—I1 | 96.72(0) | N1—Cu1—N1v | 85.51(27) |
| I2—Cu2—Cu2ii | 57.01(4) | N1iv—Cu1—N1v | 179.99(27) |
| I2ii—Cu2—Cu2ii | 56.06(4) | C1—N1—Cu1 | 107.34(49) |
| I1i—Cu2—Cu2ii | 131.61(0) | N1—C1—C1v | 107.20(69) |
| I2—Cu2—I2ii | 113.07(6) |
| x | y | z | Uiso*/Ueq | |
| I1 | 0.0000 | 0.34835 (5) | 0.5000 | 0.0159 (3) |
| I2 | −0.24076 (7) | 0.5000 | −0.17764 (10) | 0.0145 (3) |
| Cu1 | −0.5000 | 0.5000 | 0.0000 | 0.0127 (4) |
| Cu2 | −0.00269 (16 | 0.5000 | 0.2230 (2) | 0.0227 (4) |
| N1 | −0.3879 (8) | 0.6107 (5) | 0.2179 (11) | 0.0174 (13) |
| C1 | −0.4199 (10) | 0.7046 (6) | 0.0819 (15) | 0.0208 (16) |
Conclusions
Experimental
Apparatus
Reagents
Characterization of the Complex [22]
Electrosynthesis
Microanalysis
Acknowledgments
References and Notes
- Hannon, M. J.; Painting, C. L.; Plummer, E. A.; Childs, L. J.; Alcock, N. W. Competing Supramolecular Interactions Give a New Twist to Terpyridyl Chemistry: Anion- and Solvent-Induced Formation of Spiral Arrays in Silver (I) Complexes of a Simple Terpyridine. Chem. Eur. J. 2002, 8, 2226–2238. [Google Scholar] [CrossRef]
- Nather, C.; Jess, I. Synthesis, Crystal Structure and Thermal Reactivity of New Copper (I) Halide Pyrimidine-Containing Coordination Polymers. Eur. J. Inorg. Chem. 2004, 14, 2868–2876. [Google Scholar]
- Graham, P. M.; Pike, R. D.; Sabat, M.; Bailey, R. D.; Pennington, W. T. Coordination Polymers of Copper (I) Halides. Inorg. Chem. 2000, 39, 5121–5132. [Google Scholar] [CrossRef] [PubMed]
- Blake, A. J.; Brooks, N. R.; Champness, N. R.; Hanton, L. R.; Hubberstey, P.; Schroder, M. Copper (I) Halide Supramolecular Networks Linked by N-Heterocyclic Donor Bridging Ligands. Pure Appl. Chem. 1998, 70, 2351–2357. [Google Scholar] [CrossRef]
- Zheng, Y.; Du, M.; Li, J. R.; Zhang, R. H.; Bu, X. H. Tuning the Framework Formation of Silver(I) Coordination Architectures with Heterocyclic Thioethers. Dalton Trans. 2003, 8, 1509–1514. [Google Scholar]
- Long, L. S.; Cai, J. W.; Ren, Y. P.; Tong, Y. X.; Chen, X. M.; Ji, L. N.; Huang, R. B.; Zheng, L. S. Hydrogen Bond Induced Change of Geometry and Crystallized Form of Copper(II)Complexes: Syntheses and Crystal Structure of Complexes with Schiff-Base Ligands Containing Two Imidazolyl Groups. J. Chem. Soc. Dalton Trans. 2001, 6, 845–849. [Google Scholar] [CrossRef]
- Su, C. Y.; Cai, Y. P.; Chen, C. L.; Smith, M. D.; Kaim, W.; Zoye, H. C. Z. Ligand-Directed Molecular Architectures: Self-Assembly of Two-Dimensional Rectangular Metallacycles and Three-Dimensional Trigonal or Tetragonal Prisms. J. Am. Chem. Soc. 2003, 125, 8595–8613. [Google Scholar]
- Battaglia, L. P.; Corradi, A. B.; Menabue, L. Structure Magnetism Correlation in Dimeric Copper (II) Carboxylates-Crystal and Molecular-Structure of Tetra-Mu-(Propanoato-O,O’)-bis[aquacopper (II)]. J. Chem. Soc. Dalton Trans. 1986, 1653–1657. [Google Scholar] [CrossRef]
- Harcourt, R. D.; Skrezenek, F. L.; Maclagan, R. Nonempirical Valence Bond Studies of the Origin of the Antiferromagnetism of Copper (II) Carboxylate Dimers. J. Am. Chem. Soc. 1986, 108, 5403–5408. [Google Scholar] [CrossRef]
- Gonzalez-Alvarez, M.; Alzuet, G.; Borras, J.; Macias, B.; Castineiras, A. Oxidative Cleavage of DNA by a New Ferromagnetic Linear Trinuclear Copper (II) Complex in the Presence of H2O2/Sodium Ascorbate. Inorg. Chem. 2003, 42, 2992–2998. [Google Scholar]
- Chung, Y. H.; Wei, H. H.; Liu, Y. H.; Lee, G. H.; Wang, Y. Reinvestigation of the Crystal Structure and Cryomagnetic Behaviour of Copper (II) Propionates. Polyhedron 1998, 17, 449–455. [Google Scholar] [CrossRef]
- Mirkhani, V.; Harkema, S.; Kia, R. Bis[N,N '-bis(diphenylmethylene)ethylenediamine-kappa N-2,N ']copper(I) Dichlorocuprate(I). Acta Cryst. C 2004, 60, m343–344. [Google Scholar] [CrossRef]
- Goreshnik, E.; Schollmeyer, D.; Mys’kiv, M. Bis(2-methylbenzimidazole-kappa N-1)copper(I)Dichlorocuprate (I). Acta Cryst. E 2004, 60, m279–281. [Google Scholar] [CrossRef]
- Su, C. Y.; Kang, B. S.; Sun, J. Synthesis and Crystal Structure of the Tetranuclear Copper(I) Complex [Cu4I4(MPTQ)(2)] with a N,S,N'-Tridentate Ligand (MPTQ=8-((2-pyridylmethyl)thio)-quinoline). Chem. Lett. 1997, 8, 821–822. [Google Scholar]
- Xu, Y. Q.; Luo, J. H.; Yuan, D. Q.; Xu, Y.; Cao, R.; Hong, M. C. [Cu(dca)(2)(en)](n): A Two-Dimensional Copper (II) Coordination Polymer with Both Mu (1,5)-dca and Pseudo-mu(1,3)-dca Bridges. J. Mol. Struct. 2003, 658, 223–228. [Google Scholar]
- Cavicchioli, M.; Massabni, A. C.; Ferreira, A. M. D.; Castellano, E. E.; Crespi, M. S. Synthesis, Structure and Redox Properties of an Unexpected Trinuclear Copper(II) Complex with Aspartame: [Cu(apm)(2)Cu(mu-N,O : O '-apm)(2)(H2O)Cu(apm)(2)(H2O)]center Dot 5H(2)O. Inorg. Chem. Acta 2005, 358, 4431–4436. [Google Scholar] [CrossRef]
- Beloso, I.; Borras, J.; Castro, J.; Garcia-Vazquez, J. A.; Perez-Lourido, P.; Romero, J.; Sousa, A. Flexidentate Behaviour of 2-Pyridylsulfonamides - Direct Electrochemical Synthesis and Spectroscopic and X-Ray Characterization of Neutral Copper (II) Complexes of N-(2-Pyridyl) Sulfonamides. Eur. J. Inorg. Chem. 2004, 3, 635–645. [Google Scholar] [CrossRef]
- Hanton, L.R.; Hellyer, R. M.; Spicer, M. D. Structural Variations in Copper(I) Iodide Coordination Polymers of Sulfide and Disulfide Containing Flexible 3-Substituted Pyridine Ligands. Inorg. Chem. Acta 2006, 359, 3659–3665. [Google Scholar]
- Goher, M. A. S.; Hafez, A. K.; Mak, T. C. W. A Copper(I) Complex Containing a New Structure of the [Cu2I3](-) Anion. Reaction of CuI with Quinaldic Acid and the Crystal Structure of Tris-(2-carboxyquinoline) triiododicopper(I) Monohydrate. Polyhedron 2001, 20, 2583–2587. [Google Scholar]
- Feng, H.; Zhou, X. P.; Wu, T.; Li, D.; Yin, Y. G.; Ng, S. W. Hydrothermal Synthesis of Copper Complexes of 4 '-Pyridyl Terpyridine: From Discrete Monomer to Zigzag Chain olymer. Inorg. Chem. Acta 2006, 359, 4027–4035. [Google Scholar] [CrossRef]
- Romano, V.; Pizzino, T.; Gianguzza, A.; Maggio, F. Studies on Complex-Formation of Bis-(ethylenediamine)-copper (II) with Chloride, Bromide, Iodide, and Azide Ions. Inorg. Nucl. Chem. Lett. 1975, 11, 177–183. [Google Scholar] [CrossRef]
- CCDC No: 637522 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif."
- Stoe & Cie. X–RED, version 1.28b, Program for Data Reduction and Absorption Correction; Stoe & Cie GmbH: Darmatadt, Germany, 2005. [Google Scholar]
- Stoe & Cie. X–SHAPE, vesion 2.05: Program for Crystal Optimization for Numerical Absorption Correction; Stoe & Cie GmbH: Darmatadt, Germany, 2004. [Google Scholar]
- Sheldrick, G. M. SHELX97. Program for Crystal Structure Solution and Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sample Availability: Contact the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Fotouhi, L.; Dehghanpour, S.; Heravi, M.M.; Ardakani, M.D. Electrochemical Synthesis and Structural Characterization of a Novel Mixed-valence Copper (I)-copper (II) Complex: {[Bis(ethylenediamine) Copper (II)] Bis[diiodocuprate (I)]}. Molecules 2007, 12, 1410-1419. https://doi.org/10.3390/12071410
Fotouhi L, Dehghanpour S, Heravi MM, Ardakani MD. Electrochemical Synthesis and Structural Characterization of a Novel Mixed-valence Copper (I)-copper (II) Complex: {[Bis(ethylenediamine) Copper (II)] Bis[diiodocuprate (I)]}. Molecules. 2007; 12(7):1410-1419. https://doi.org/10.3390/12071410
Chicago/Turabian StyleFotouhi, Lida, Saeed Dehghanpour, Majid M Heravi, and Mahboobeh Dashti Ardakani. 2007. "Electrochemical Synthesis and Structural Characterization of a Novel Mixed-valence Copper (I)-copper (II) Complex: {[Bis(ethylenediamine) Copper (II)] Bis[diiodocuprate (I)]}" Molecules 12, no. 7: 1410-1419. https://doi.org/10.3390/12071410
APA StyleFotouhi, L., Dehghanpour, S., Heravi, M. M., & Ardakani, M. D. (2007). Electrochemical Synthesis and Structural Characterization of a Novel Mixed-valence Copper (I)-copper (II) Complex: {[Bis(ethylenediamine) Copper (II)] Bis[diiodocuprate (I)]}. Molecules, 12(7), 1410-1419. https://doi.org/10.3390/12071410




