Introduction
Although with the development of chemical and pharmacological analysis methods we can study natural compounds more thoroughly, a great number of compounds and plants still remain unexplored. One such plant is
Paulownia tomentosa. In this plant only a few compounds have been identified, mostly of polar character and divided into five groups: phenolic glycosides, furofuran lignanes, furanoquinones, iridoides and flavonoids [
1,
2,
3,
4,
5,
6]. A large group of essential oil substances has also been identified in the flowers [
7]. These compounds and especially the flavonoids were identified from different species, where they probably serve as UV irradiation protectors. The increase in free radical species (corresponding with a high UV irradiation) is nowadays considered to be the true cause and effect of many metabolic disorders connected to such diseases as neurodegeneration, cancer or diabetes mellitus [
8,
9,
10]. Nowadays a lot of different plant species are used as nutritional additives to add antioxidants to the organism to improve the immunity against these diseases [
11]. As a part of our efforts to find new antioxidative and antiradical active compounds we have investigated in some depth the family Scrophullariaceae. A screening assay of
P. tomentosa fruit extracts showed an antiradical effect; therefore we prepared a larger portion of EtOH extract from
P. tomentosa fruits, which was separated into three parts by immiscible liquid/liquid extraction. The CHCl
3, EtOAc and
n-BuOH fractions of the extract showed the presence of a large number of phenolic compounds after TLC and HPLC analyses, identified as prenylated or geranylated flavonoids on the basis of their UV spectra and retention times. We thus identified acteoside (
1) and isoacteoside (
2) in the
n-BuOH and EtOAc extracts and mimulone (
3) and diplacone (
4) in MeOH extract and we quantified them. The antiradical activity of these extracts was confirmed by anti DPPH (diphenylpicrylhydrazyl) and peroxynitrite scavenging assays. The total amounts of polyphenolics was also determined.
Results and Discussion.
Fruits of
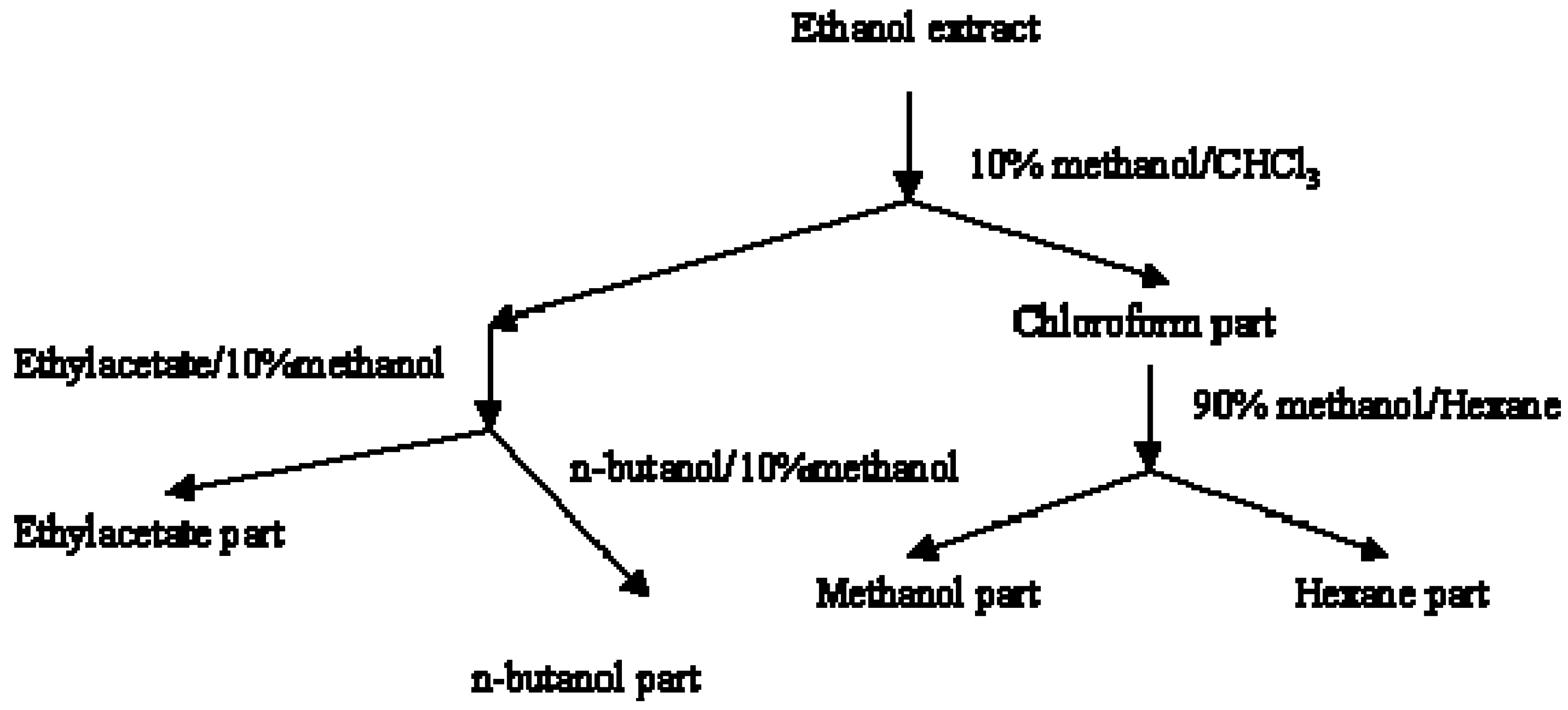
P. tomentosa were extracted with EtOH. The EtOH extract was then separated to fractions of similar polarity (
Figure 1).
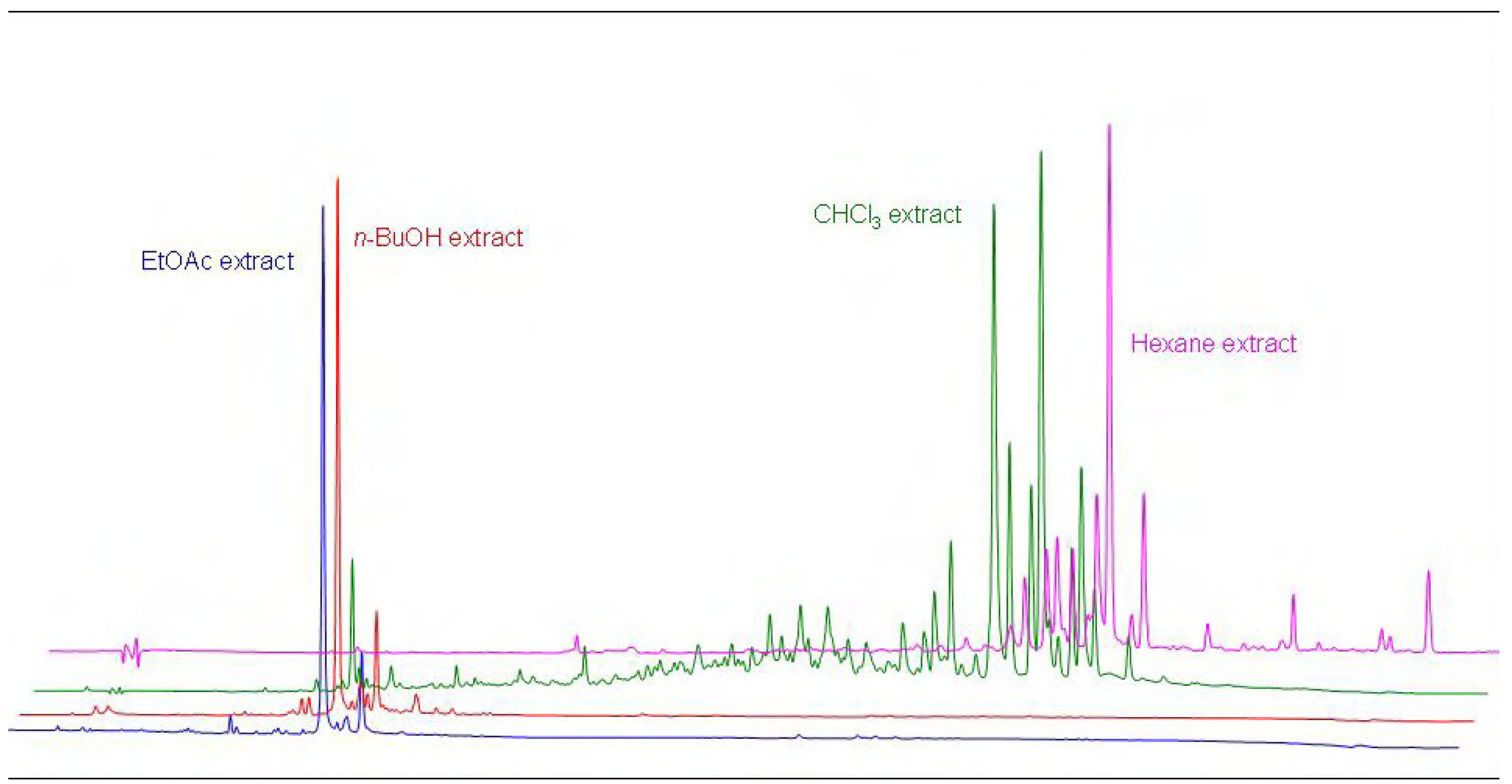
The separated fractions were analyzed by means of TLC and HPLC (
Figure 2). These chromatographic analyses showed that thanks to the good choice of extraction solvents, a good separation of compounds of similar polarity was achieved. We identified the main components of the MeOH,
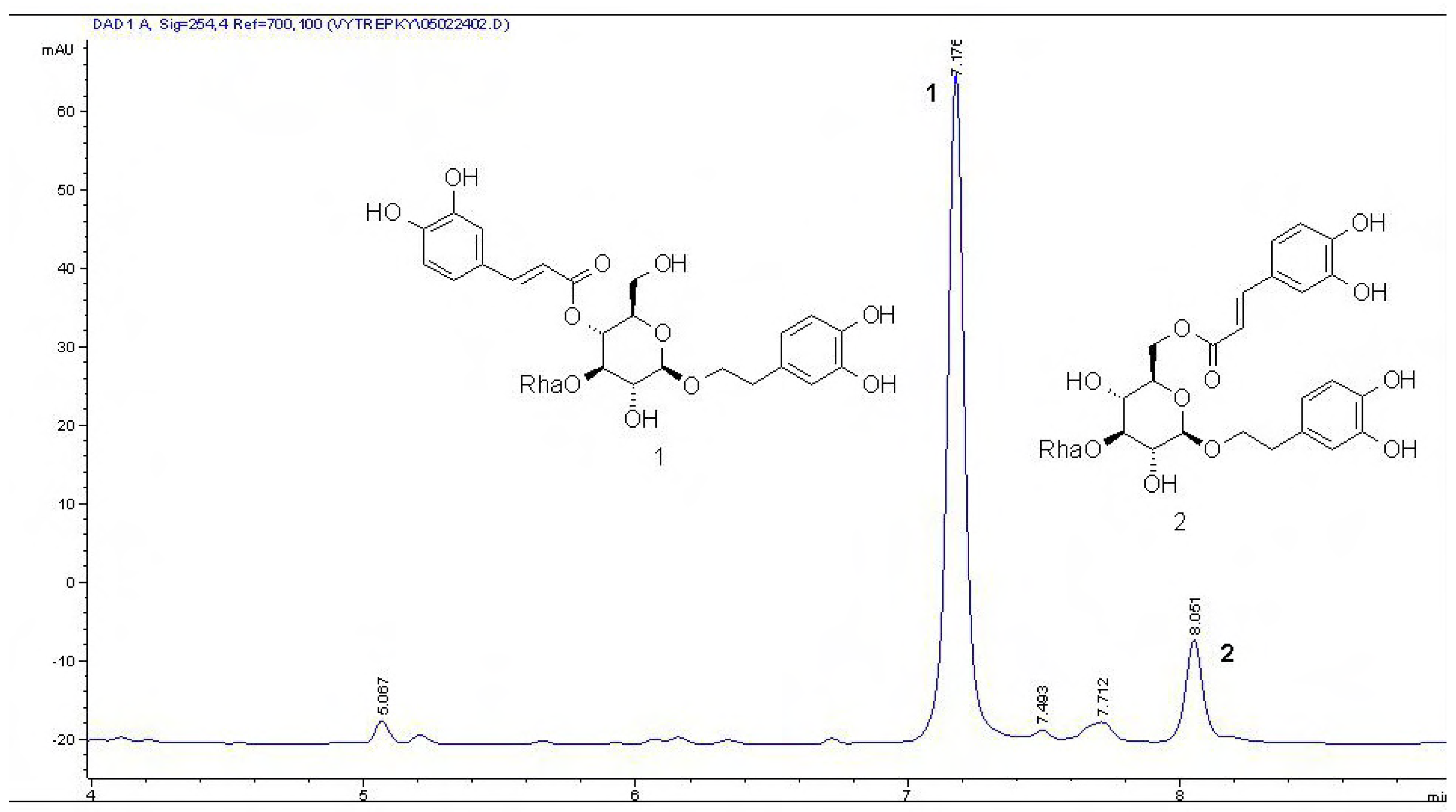
n-BuOH and EtOAc extracts (by comparison with standards) and we quantified these compounds with help of HPLC. The components of the EtOAc and
n-BuOH extracts are represented mainly by acteoside (
1) and isoacteoside (
2) (
Figure 3; EtOAc extract
1 43.1 % and
2 24.3 % by dry weight;
n-BuOH
1 55.1 % and
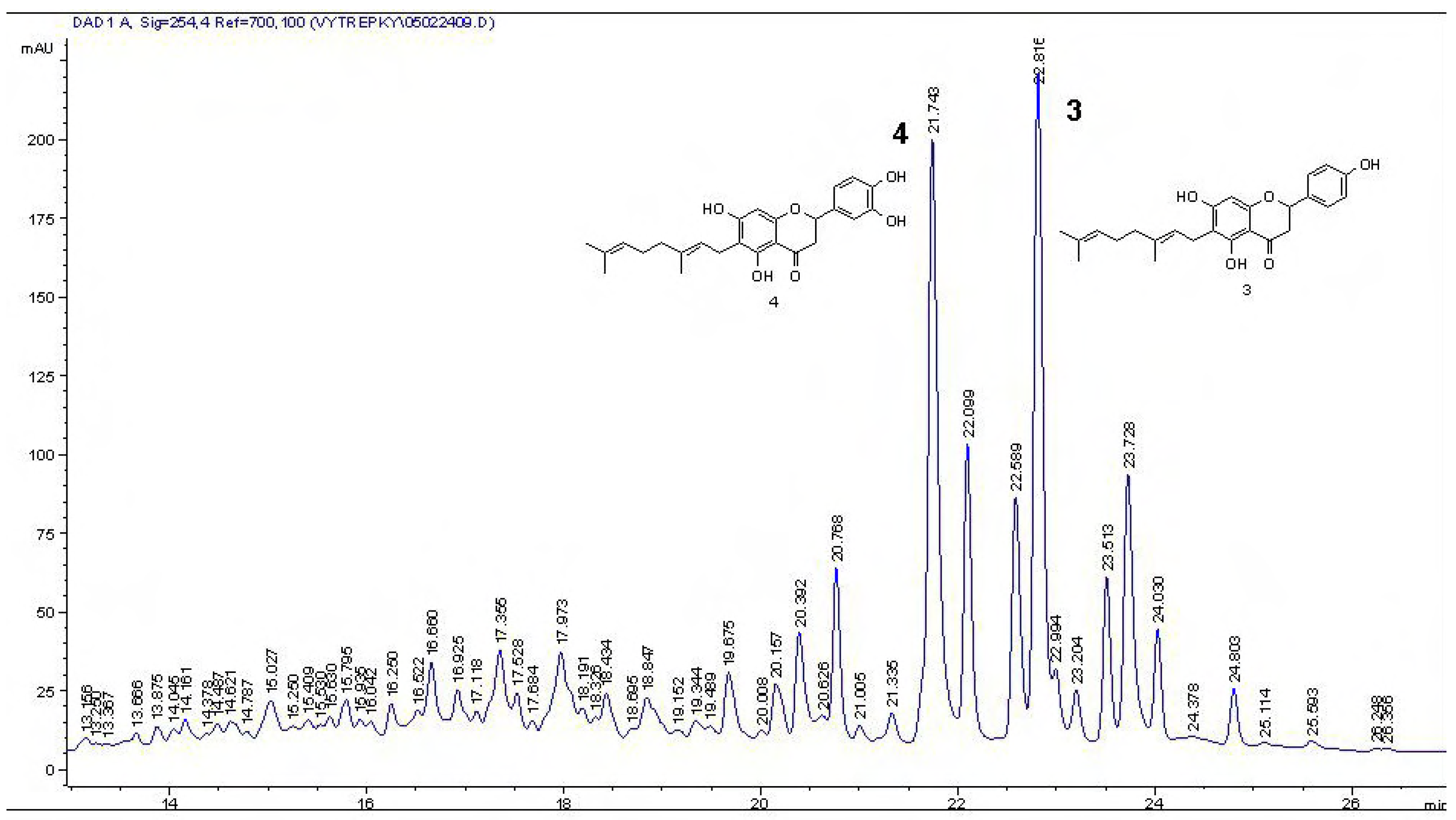
2 34.5 % of dry weight). The MeOH extract contained mimulone (
3) and diplacone (
4) (
Figure 4, 4.3 % of
3 and 15.3 % of
4 in the dry extract, respectively). These compounds have been previously isolated from
P. tomentosa [
2,
6]. Quantification was made by means of calibration curves derived from peak areas and the amounts of compound in the samples. The content of compounds in
P. tomentosa extracts is summarized in
Table 1.
Figure 1.
Liquid/liquid fractionation of EtOH extract of fruits of P.tomentosa.
Figure 1.
Liquid/liquid fractionation of EtOH extract of fruits of P.tomentosa.
Table 1.
The content of compounds isolated from P. tomentosa fruit in prepared extracts. Data expressed as mols of compound per 100 g dry weight of extract.
Table 1.
The content of compounds isolated from P. tomentosa fruit in prepared extracts. Data expressed as mols of compound per 100 g dry weight of extract.
| Extract | 1 | 2 | 3 | 4 |
|---|
| MeOH extract | - | - | 0.01 | 0.04 |
| EtOAc extract | 0.07 | 0.04 | - | - |
| n-BuOH extract | 0.09 | 0.06 | - | - |
Figure 2.
Comparison of the HPLC chromatograms of the MeOH, EtOAc, n-BuOH, and hexane extracts of P. tomentosa fruits, 1 g L-1 (10 µL injection).
Figure 2.
Comparison of the HPLC chromatograms of the MeOH, EtOAc, n-BuOH, and hexane extracts of P. tomentosa fruits, 1 g L-1 (10 µL injection).
Figure 3.
HPLC chromatograms of the EtOAc extract of P. tomentosa fruits, 1 g L-1 (1 µL injection) showing acteoside (1) and isoacteoside (2).
Figure 3.
HPLC chromatograms of the EtOAc extract of P. tomentosa fruits, 1 g L-1 (1 µL injection) showing acteoside (1) and isoacteoside (2).
Figure 4.
HPLC chromatograms of the MeOH extract of P. tomentosa fruits, 1 mg/mL (10 µL injection): mimulone (3), diplacone (4).
Figure 4.
HPLC chromatograms of the MeOH extract of P. tomentosa fruits, 1 mg/mL (10 µL injection): mimulone (3), diplacone (4).
Separated fractions were tested for anti free radical activity. Both anti DPPH and antiperoxynitrite assays identified the promising activity of the CHCl
3, EtOAc and
n-BuOH extracts. After separation of the CHCl
3-fraction only the MeOH one displayed antiradical activity. Antiperoxynitrite activity expressed as the percentage of inhibition of tyrosine nitration is shown in
Table 2.
Table 2.
Antioxidant activity and total phenolic content of tested extracts.
Table 2.
Antioxidant activity and total phenolic content of tested extracts.
| Extract | Total Polyphenolicsa,c | Antiperoxynitrite activitya,b | Anti DPPH activity EC50d (mg/mL) | Anti DPPH activityb |
|---|
| CHCl3 | 43.8±1.2 | 178±10 | 0.017 | 13.7 |
| EtOAc | 49.4±0.9 | 211±15 | 0.007 | 325.3 |
| n-BuOH | 47.3±0.8 | 190±1 | 0.008 | 284.7 |
| Hexane | 7.0±1.3 | 10±2 | 0.740 | 3.1 |
| MeOH | 31.3±1.1 | 203±4 | 0.032 | 72.1 |
Anti DPPH activity assays showed similar results, as also expressed in
Table 2. With the assistance of measurements of samples of different concentrations we acquired graphs for obtaining the EC
50 values of extracts. At EC
50 we established a course of scavenging in 30 minutes. We expressed also anti-DPPH activity as TEAC.
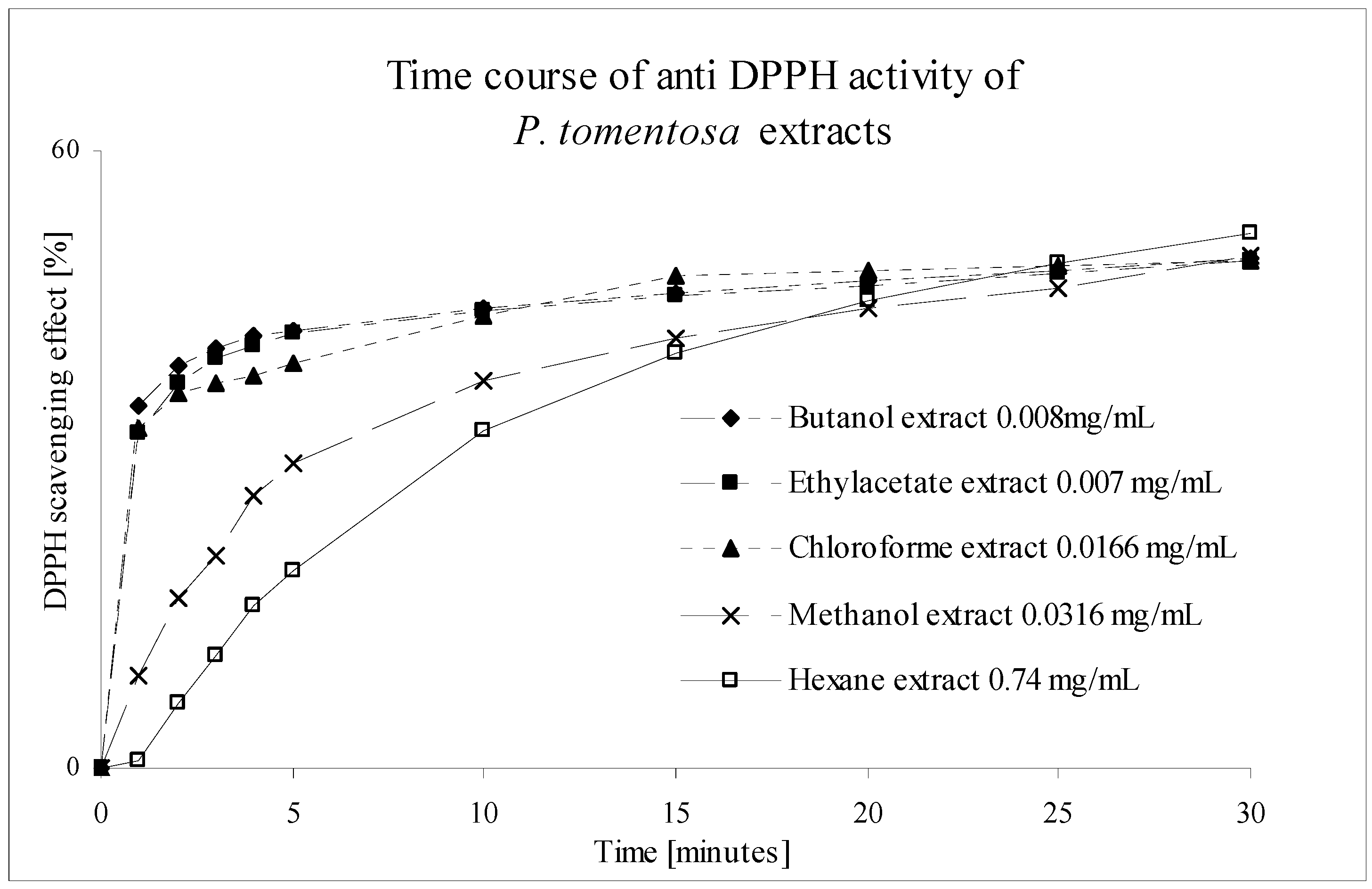
Figure 5.
Time course of the anti DPPH activity of P. tomenotosa extracts. EC50 concentrations used, values determined from three parallel measurements.
Figure 5.
Time course of the anti DPPH activity of P. tomenotosa extracts. EC50 concentrations used, values determined from three parallel measurements.
Table 2 shows the values of EC
50 and comparison of scavenging effect to effect of Trolox C. EtOAc,
n-BuOH and MeOH extracts are good scavengers in comparison to Trolox C. Graph (
Figure 5) presents the time course of reaction showing that tested extracts represent fast scavengers in the exception of hexane extract. Hexane extract is designated as slow scavenger.
Total amount of polyphenols in extracts was established by standard colorimetric methods. The results are presented as GAE in
Table 1. The amount of polyphenolics was in good correspondence with the antiradical activity assay results, and hexane extraction removed nonactive and non-phenolic substances. The content of phenolic substances in chloroform (MeOH), EtOAc and
n-BuOH extracts was comparable with those of the traditional Chinese medicine plant extracts
Rhus chinensis and
Acacia catechu [
12]. Generally, the phenylpropanoid glycosides display a number of biological activities, for instance antiestrogenic [
13], vasorelaxant [
14] and anti-inflammatory [
15]. The phenylpropanoid glycosides acteoside (
1) and isoacteoside (
2) are also known as excellent radical scavengers isolated from many plants [
16]. Their antiradical potential was previously established with help of various methods including DPPH scavenging activity assays [
17,
18]. In relation to the content of
1 and
2 in the
n-BuOH and EtOAc extract this leads to the valid premise that
1 and
2 are the main compounds responsible for antiradical activity of these extracts. Mimulone (
3) and diplacone (
4) had also been previously isolated from
P. tometosa fruits and their anti DPPH activity was established [
19,
20]. Compound
4 had been proved as antiradical active compound whereas activity of
3 was much lower due to the different substitution pattern of flavonoid B ring. The chromatographic analysis of MeOH portion of extract showed presence of variety of compounds, some of them could be more active in comparison with
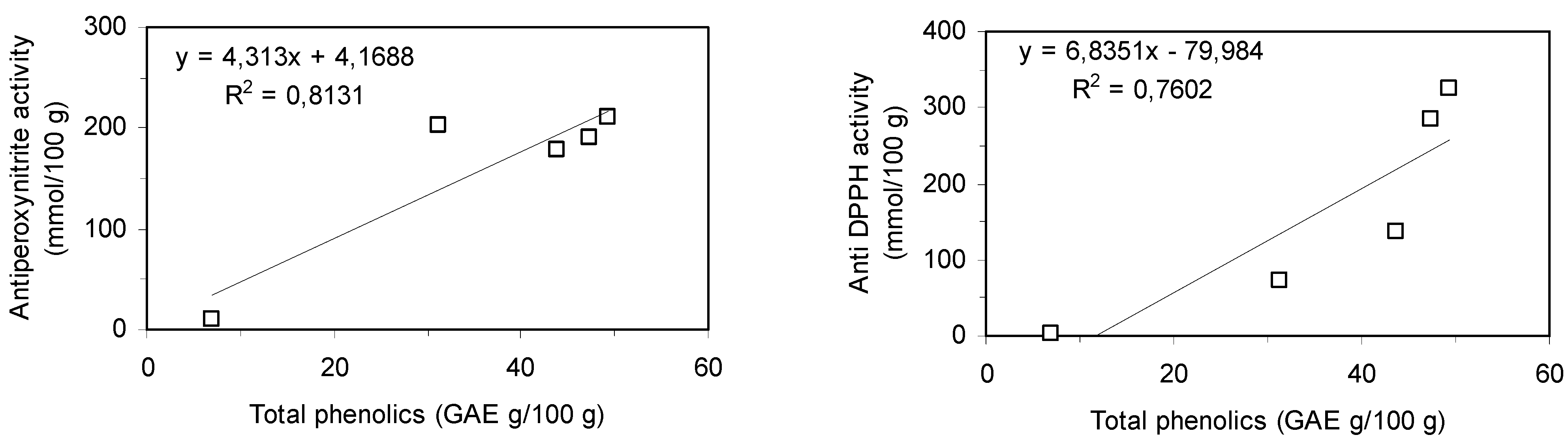
4 and could be responsible for at least part of the antiradical activity of MeOH extract. The correlation between antiradical activity and content of phenolic compounds of extracts was stated. As shown in the
Figure 6, there is a significant linear correlation (r > 0.87, p ≤ 0.05). Presented results show the suitability of MeOH,
n-BuOH and EtOAc extracts for use as radical scavenger sources after further work and confirm
P. tomentosa fruits as a rich source of acteoside (
1) and diplacone (
4).
Figure 6.
Correlation of antiperoxynitrite and anti DPPH activity of extracts and of total phenolics content.
Figure 6.
Correlation of antiperoxynitrite and anti DPPH activity of extracts and of total phenolics content.
Experimental
Plant material
Materials for extraction were fruits of Paulownia tomentosa Scrophulariaceae collected in autumn 2004 in the campus of VFU Brno. A voucher specimen (PT-02O) was deposited at the herbarium of the Department of Natural Drugs, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences Brno, Czech Republic.
22.5 kg of fruits were collected, cleaned and then extracted by maceration in EtOH (3× 20 L). The extract was filtered and the solution was evaporated in vacuo. The compounds 1-4 have been previously isolated from P. tomentosa.
TLC
Thin layer chromatography was performed on silica gel F254 Merck (Germany). Mobile phase was a 95:5 (v/v) mixture of benzene-acetone. For visualization the chromatogram was sprayed with Neu reagent (diphenylethyl ester boric acid in MeOH, 1% solution) and inspected visually or using UV (254 and 356 nm).
Analytical HPLC
HPLC was done using an Agilent 1100 UV-VIS DAD (Agilent, Germany), a Supelcosil ABZ+Plus column (15 cm × 4.6 mm, 3 μm; Supelco, USA), and a gradient elution of MeCN:HCOOH (0.2%) from 10:90 (v/v) at 0 min up to 100:0 (v/v) after 28 min, then up to 100 % of MeCN after 32 min. Flow rate was 1 mL·min-1, 40 °C. DAD (diode array detection) was performed at λ 254, 280 and 350 nm.
DPPH quenching assay
To quantify the DPPH quenching activity, the modified method of Braca
et al. [
21] was used. The MeOH DPPH (Sigma) solution was prepared at the concentration 22 g·L
-1. Tested extracts were dissolved in MeOH at different concentrations. A volume of test solution (0.2 mL) was mixed with DPPH solution (1.8 mL) and the absorbance of the mixture at 517 nm was measured each minute during the first 5 minutes of experiment and than each 5 minutes for the next 25 minutes. Using the recorded data, scavenging effect r was calculated [r = (1 - sample absorbance/control absorbance) × 100)] and the EC
50 of extracts and the time course of activity increase was drew and the differences between the tested extracts were compared. The percentage of DPPH scavenging was compared to that of the calibrated Trolox standard. Results were expressed in terms of Trolox equivalent antioxidant capacity (mmol Trolox equivalents per 100 g dry weight of plant extract).
Antiperoxynitrite assay
The measurement of inhibition of tyrosine nitration was performed in the following way [
22]: a 10 mM solution of peroxynitrite (8 µL) in 0.1 M NaOH was drawn and mixed up in HPLC injector with 1.0 mM solution of tyrosine in 0.05 M phosphate buffer pH 6.0 (42 µL) containing the sample (0.15 g L
-1) and DMSO in a 1:1 (v/v) ratio. The reaction mixture was then injected directly onto a HPLC column (Supelcosil ABZ+Plus, 25 cm × 4.6 mm, 5 μm; Supelco, USA); mobile phase was 90% 40 mM HCOOH: 10% MeCN (v/v) isocratic elution, flow rate 1 mL·min
-1, detection at 276 nm. Inhibition of tyrosine nitration was calculated relative to peak area of 3-nitrotyrosine founded in the control measurement. The percentage of inhibition of tyrosine nitration was compared to that of the calibrated Trolox standard. Results were expressed in terms of Trolox equivalent antioxidant capacity (mmol Trolox equivalents per 100 g dry weight of plant extract).
Total amount of polyphenolics
The total amount of polyphenolics was determined by a colorimetric method. Appropriately diluted extract solution (700 μL) was oxidized by 0.2 N Folin-Ciocalteu reagent (400 μL) and than a solution of Na2CO3 (75 g L-1) was added to a total volume of 10.0 mL. After 2 hours, the suspension was centrifuged (5000 rpm, 10 minutes) and the resulting absorption was measured (λ=760 nm). Quantification was made based on a gallic acid calibration curve. The results were expressed as gallic acid equivalents (GAE) per 100 g of dry weight of plant extract.