Different Supramolecular Coordination Polymers of [N,N'-di(pyrazin-2-yl)-pyridine-2,6-diamine]Ni(II) with Anions and Solvent Molecules as a Result of Hydrogen Bonding
Abstract
:Introduction
Results and Discussion
Crystal structure description
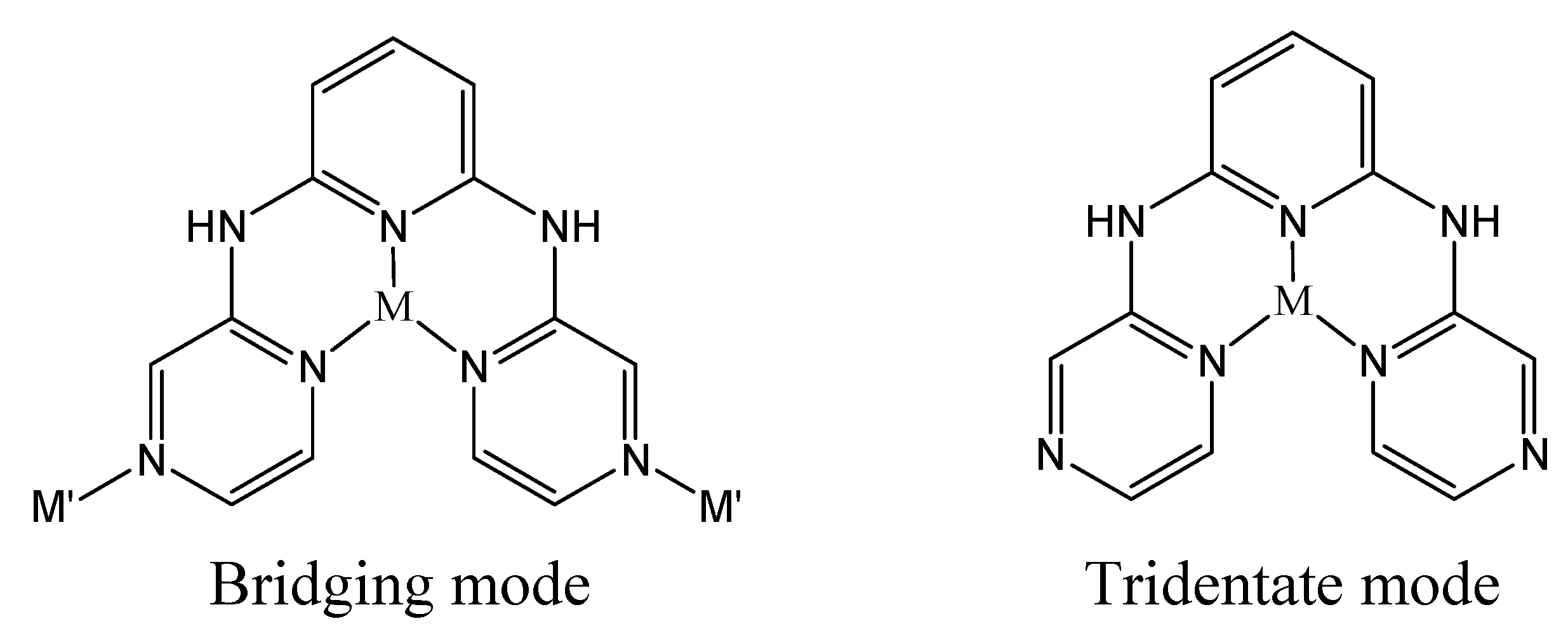
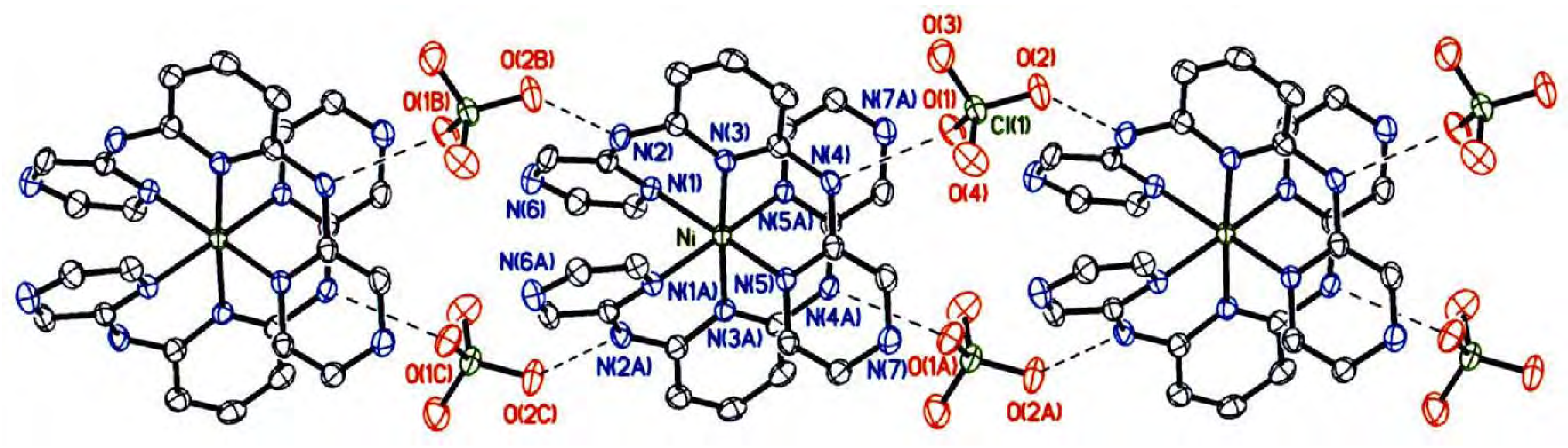
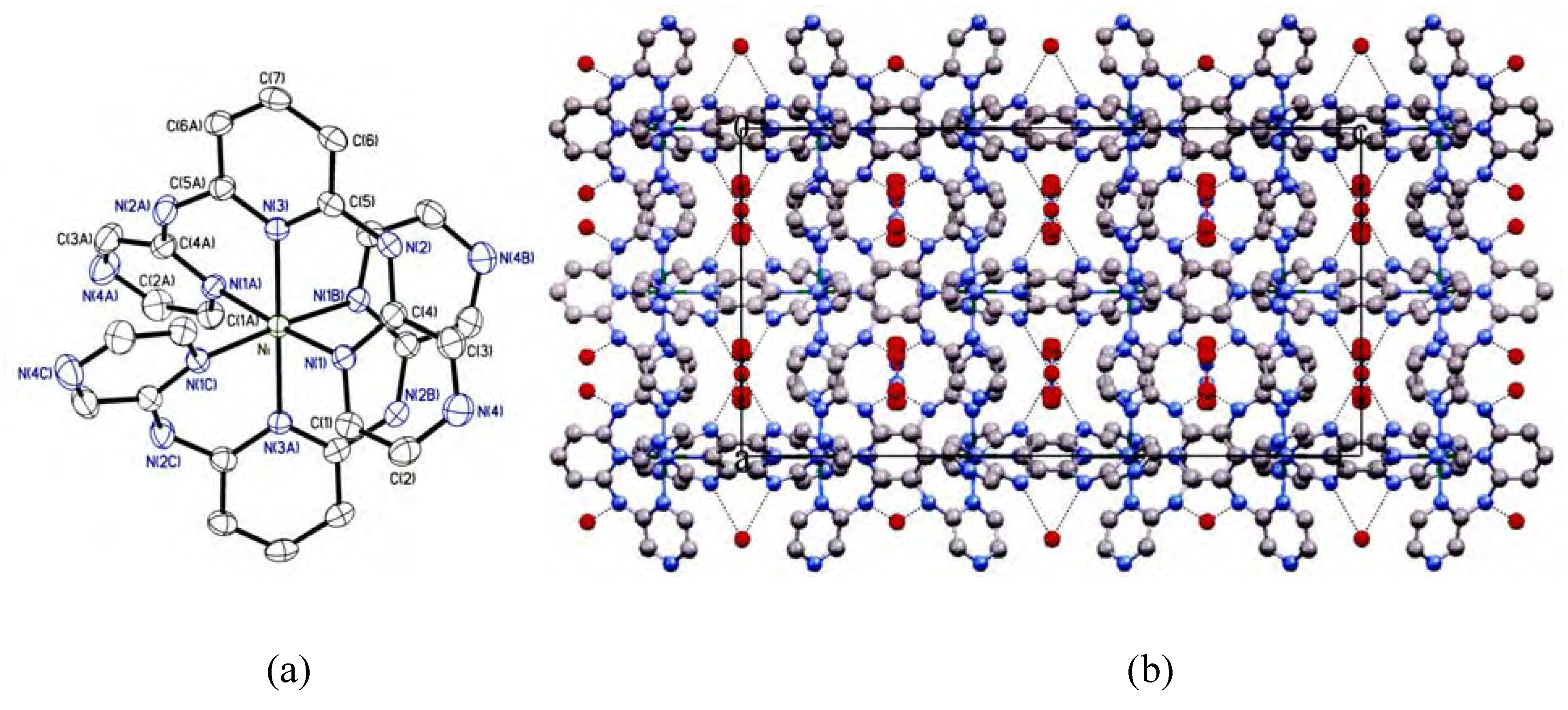
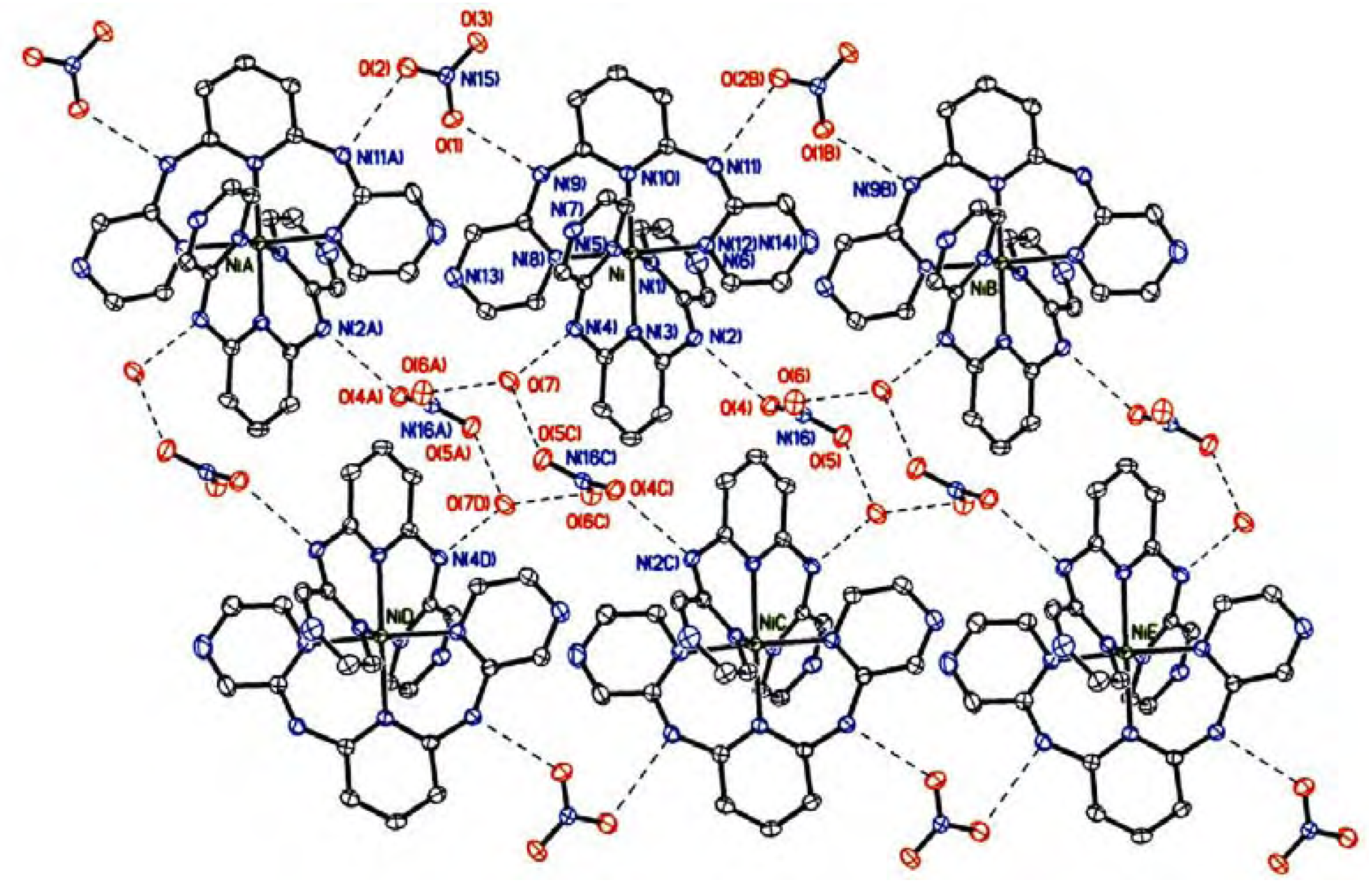
| 1 | |||
| Ni-N(1) | 2.1003(17) | Ni-N(3) | 2.0797(16) |
| Ni-N(5) | 2.0654(17) | ||
| N(1)-Ni-N(3) | 87.48(7) | N(1)-Ni-N(5) | 172.91(6) |
| N(1)-Ni-N(1A) | 85.75(9) | N(1)-Ni-N(3A) | 95.42(7) |
| N(1)-Ni-N(5A) | 89.65(7) | N(3)-Ni-N(5) | 87.59(7) |
| N(3)-Ni-N(3A) | 176.05(10) | N(3)-Ni-N(5A) | 89.76(6) |
| N(5)-Ni-N(5A) | 95.42(9) | ||
| 2 | |||
| Ni-N(1) | 2.0708(17) | Ni-N(3) | 2.076(2) |
| N(1)-Ni-N(1A) | 90.91(8) | N(1)-Ni-N(1B) | 175.31(7) |
| N(1)-Ni-N(1C) | 89.29(8) | N(1)-Ni-N(3) | 87.65(4) |
| N(1)-Ni-N(3A) | 92.35(4) | N(3)-Ni-N(3A) | 180.0 |
| 2·H2O | |||
| Ni-N(1) | 2.0626(17) | Ni-N(3) | 2.0884(16) |
| Ni-N(5) | 2.0637(17) | Ni-N(8) | 2.0911(17) |
| Ni-N(10) | 2.1030(16) | Ni-N(12) | 2.0781(17) |
| N(1)-Ni-N(3) | 89.11(7) | N(1)-Ni-N(5) | 176.11(6) |
| N(1)-Ni-N(8) | 91.45(6) | N(1)-Ni-N(10) | 91.90(6) |
| N(1)-Ni-N(12) | 90.04(6) | N(3)-Ni-N(5) | 87.18(6) |
| N(3)-Ni-N(8) | 89.66(6) | N(3)-Ni-N(10) | 178.34(6) |
| N(3)-Ni-N(12) | 92.63(6) | N(5)-Ni-N(8) | 89.71(6) |
| N(5)-Ni-N(10) | 91.84(6) | N(5)-Ni-N(12) | 88.95(6) |
| N(8)-Ni-N(10) | 89.00(6) | N(8)-Ni-N(12) | 177.28(6) |
| N(10)-Ni-N(12) | 88.68(6) |
| D−H···A | d(D−H) | d(H···A) | d(D···A) | < (DHA) |
|---|---|---|---|---|
| 1 | ||||
| N(2)−H(2)···O(2B) | 0.88 | 2.081 | 2.933(2) | 162.6 |
| N(4)−H(4)···O(1) | 0.88 | 2.053 | 2.895(2) | 159.9 |
| 2 | ||||
| N(2)−H(2)···O(1) | 0.88 | 2.127 | 2.998(2) | 170.6 |
| 2•H2O | ||||
| N(2)−H(2)···O(4) | 0.88 | 2.150 | 2.896(2) | 142.2 |
| N(4)−H(4)···O(7) | 0.88 | 2.070 | 2.820(2) | 142.6 |
| N(9)−H(9)···O(1) | 0.88 | 2.157 | 2.805(2) | 123.0 |
| N(11)−H(11)···O(2B) | 0.88 | 2.234 | 2.892(2) | 131.4 |
| O(7)−H(7)···O(6A) | 0.80 | 1.986 | 2.781(2) | 170.0 |
| O(7)−H(7’)···O(5C) | 0.80 | 2.036 | 2.830(2) | 168.0 |
Magnetic and spectroscopic properties


Conclusions
Experimental
General
Preparation of compounds
Crystal Structure Determinations
| Compound | [Ni(H2dpzpda)2](ClO4)2 (1) | [Ni(H2dpzpda)2](NO3)2 (2) | [Ni(H2dpzpda)2](NO3)2•H2O (2•H2O) |
| Formula | C26H22N14O8C2Ni | C26H22N16O6Ni | C26H24N16O7Ni |
| Formula weight | 788.19 | 713.31 | 731.32 |
| Crystal system | Monoclinic | Tetragonal | Triclinic |
| Space group | C2/c | I41/acd | P ī |
| Formula per unit cell, Z | 4 | 8 | 2 |
| Unit-cell dimensions | a = 18.0619(5) Å | a = 14.6836(3) Å | a = 10.3287(6) Å |
| b = 11.0820(3) Å | b = 14.6836(3) Å | b = 10.3728(6) Å | |
| c = 16.9851(4) Å | c = 28.3900(5) Å | c = 16.6563(9) Å | |
| α = 90° | α = 90° | α = 73.506(1)° | |
| β = 118.082(2)° | β = 90° | β = 82.367(1)° | |
| γ = 90° | γ = 90° | γ = 61.950(1)° | |
| Unit-cell volume, V (Å 3) | 2999.5(1) | 6121.1(2) | 1510.1(2) |
| Dcalcd. (g/cm3) | 1.745 | 1.548 | 1.608 |
| Absorption coefficient (mm-1) | 0.903 | 0.705 | 0.719 |
| F(000) | 1608 | 2928 | 752 |
| Crystal size (mm) | 0.40 x 0.20 x 0.15 | 0.18 x 0.15 x 0.15 | 0.28 x 0.25 x 0.25 |
| θ ranges for data collection (o) | 2.24 ~ 27.50 | 2.43 ~ 27.50 | 1.27 ~ 27.50 |
| Reflections collected | 15189 | 13603 | 19579 |
| Independent reflections | 3444 (Rint = 0.0476) | 1765 (Rint = 0.0475) | 6901 (Rint = 0.0273) |
| Completeness to θ=27.50 (%) | 99.8 | 100 | 99.7 |
| Absorption correction | Multi-scan | Multi-scan | Multi-scan |
| Max. and min. transmission | 0.876 and 0.714 | 0.907 and 0.862 | 0.841 and 0.824 |
| Refinement method | Full-matrix L. S. on F2 | Full-matrix L. S. on F2 | Full-matrix L. S. on F2 |
| Data / restraints / parameters | 3444 / 0 / 231 | 1765 / 2 / 123 | 6901 / 0 / 453 |
| Goodness-of-fir on F2 | 1.043 | 1.099 | 1.082 |
| R1, wR2 [I > 2σ(I)] | 0.0341, 0.0866 | 0.0336, 0.0904 | 0.0405, 0.0987 |
| R1, wR2 (all data) | 0.0487, 0.0936 | 0.0633, 0.0999 | 0.0456, 0.1017 |
| Largest diff. peak and hole | 0.401 and -0.493 e/ Å 3 | 0.275 and -0.331 e/ Å 3 | 0.526 and -0.595 e/ Å 3 |
Acknowledgments
References
- Ferlay, S.; Hosseini, W. Crystalline molecular alloys. Chem. Commun. 2004, 788–789. [Google Scholar] [CrossRef] [PubMed]
- Dechambenoit, P.; Ferlay, S.; Hosseini, M. W. From tectons to composite crystals. Cryst. Growth Des. 2005, 5, 2310–2312. [Google Scholar] [CrossRef]
- Nguyen, T. L.; Fowler, F. W.; Lauher, J. W. Commensurate and Incommensurate Hydrogen Bonds. An Exercise in Crystal Engineering. J. Am. Chem. Soc. 2001, 123, 11057–11064. [Google Scholar] [CrossRef] [PubMed]
- Prior, T. J.; Bradshaw, D.; Teat, S. J.; Rosseinsky, M. J. Designed layer assembly: a three-dimensional framework with 74% extra-framework volume by connection of infinite two-dimensional sheets. Chem. Commun. 2003, 500–501. [Google Scholar] [CrossRef]
- Lee, G. H.; Wang, H. T. Synthesis and Molecular Structures of Two [1,4-bis(3-pyridyl)-2,3-diazo-1,3-butadiene]-dichloro-Zn(II) Coordination Polymers. Molecules 2006, 11, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Lee, G. H.; Wang, H. T. Synthesis and Crystal Structure of a Three Dimensional Nickel(II) Coordination Polymer with 1,4-Bis(3-pyridyl)-2,3-diazo-1,3-butadiene as Ligand. Anal. Sci. 2007, 23, x1–x2. [Google Scholar] [CrossRef]
- Lu, Y. L.; Wu, J. Y.; Chan, M. C.; Huang, S. M.; Lin, C. S.; Chiu, T. W.; Liu, Y. H.; Wen, Y. S.; Ueng, C. H.; Chin, T. M.; Hung, C. H.; Lu, K. L. Influence of Water Content on the Self-Assembly of Metal-Organic Frameworks Based on Pyridine-3,5-dicarboxylate. Inorg. Chem. 2006, 45, 2430–2437. [Google Scholar] [CrossRef] [PubMed]
- Ismayilov, R. H.; Wang, W. Z.; Lee, G. H.; Peng, S. M. One-, two- and three-dimensional Cu(II) complexes built via new oligopyrazinediamine ligands: from antiferromagnetic to ferromagnetic coupling. Dalton Trans. 2006, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C. Y.; Chou, C. H.; Pan, K. C.; Wang, C. C.; Lee, G. H.; Su, Y. O.; Peng, S. M. Linear Pentacobalt Complexes: Synthesis, Structures, and Physical Properties of Both Neutral and One-electron Oxidation Compounds. J. Chem. Soc. Dalton Trans. 2002, 2670–2677. [Google Scholar] [CrossRef]
- Carlin, R. L.; O’Connor, C. J.; Bhatia, S. N. Large Zero Field Splitting and Subcritical Antiferro-magnetic Interactions in [Ni(C5H5N0)6](Cl04)2. J. Am. Chem. Soc. 1976, 98, 3523–3526. [Google Scholar] [CrossRef]
- Boča, R. Zero-field splitting in metal complexes. Coord. Chem. Rev. 2004, 248, 757–815. [Google Scholar] [CrossRef]
- Xu, J. Y.; Bian, H. D.; Gu, W.; Yan, S. P.; Cheng, P.; Zheng, D. Z.; Jiang, Z. H.; Shen, P. W. X-ray structural and spectroscopic studies of a mononuclear nickel(II) complex with tris(2-pyridyl-methyl)amine and thiocyanate. J. Mol. Struct. 2003, 646, 237–242. [Google Scholar] [CrossRef]
- Mašlejová, A.; Boca, R.; Dlhán, L.; Papánková, B.; Svoboda, I.; Fuess, H. Structure and zero-field splitting in bis(1,2-dimethylimidazole)bis(acetato)nickel(II) molecular complex. Chem. Phys. Lett. 2001, 347, 397–402. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Meth. Enzymol. 1997, 276, 307–326.
- SAINT. Program for cell refinement and data reduction; Bruker AXS Inc.: Madison, Wisconsin, USA, 2005.
- Blessing, R. H. An empirical correction for absorption anisotropy. Acta. Cryst. Sect. A. 1995, 51, 33–38. [Google Scholar] [CrossRef]
- Sheldrick, G. M. Program for absorption correction; University of Göttingen: Göttingen, Germany, 2003. [Google Scholar]
- Sheldrick, G. M. Phase annealing in SHELX-90: direct methods for larger structures. Acta Cryst. 1990, A46, 467. [Google Scholar] [CrossRef]
- Sheldrick, G. M. Program for the refinement of crystal structure; University of Göttingen,: Göttingen, Germany, 1997. [Google Scholar]
- SHELXTL. Software used to prepare material for publication; Bruker AXS Inc.: Madison, Wisconsin, USA, 2000.
- Sample Availability: Available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Lee, G.-H.; Wang, H.-T. Different Supramolecular Coordination Polymers of [N,N'-di(pyrazin-2-yl)-pyridine-2,6-diamine]Ni(II) with Anions and Solvent Molecules as a Result of Hydrogen Bonding. Molecules 2007, 12, 821-831. https://doi.org/10.3390/12040821
Lee G-H, Wang H-T. Different Supramolecular Coordination Polymers of [N,N'-di(pyrazin-2-yl)-pyridine-2,6-diamine]Ni(II) with Anions and Solvent Molecules as a Result of Hydrogen Bonding. Molecules. 2007; 12(4):821-831. https://doi.org/10.3390/12040821
Chicago/Turabian StyleLee, Gene-Hsiang, and Hsin-Ta Wang. 2007. "Different Supramolecular Coordination Polymers of [N,N'-di(pyrazin-2-yl)-pyridine-2,6-diamine]Ni(II) with Anions and Solvent Molecules as a Result of Hydrogen Bonding" Molecules 12, no. 4: 821-831. https://doi.org/10.3390/12040821
APA StyleLee, G.-H., & Wang, H.-T. (2007). Different Supramolecular Coordination Polymers of [N,N'-di(pyrazin-2-yl)-pyridine-2,6-diamine]Ni(II) with Anions and Solvent Molecules as a Result of Hydrogen Bonding. Molecules, 12(4), 821-831. https://doi.org/10.3390/12040821




