Synthesis and X-ray Structure of the Inclusion Complex of Dodecamethylcucurbit[6]uril with 1,4-Dihydroxybenzene
Abstract
:Introduction

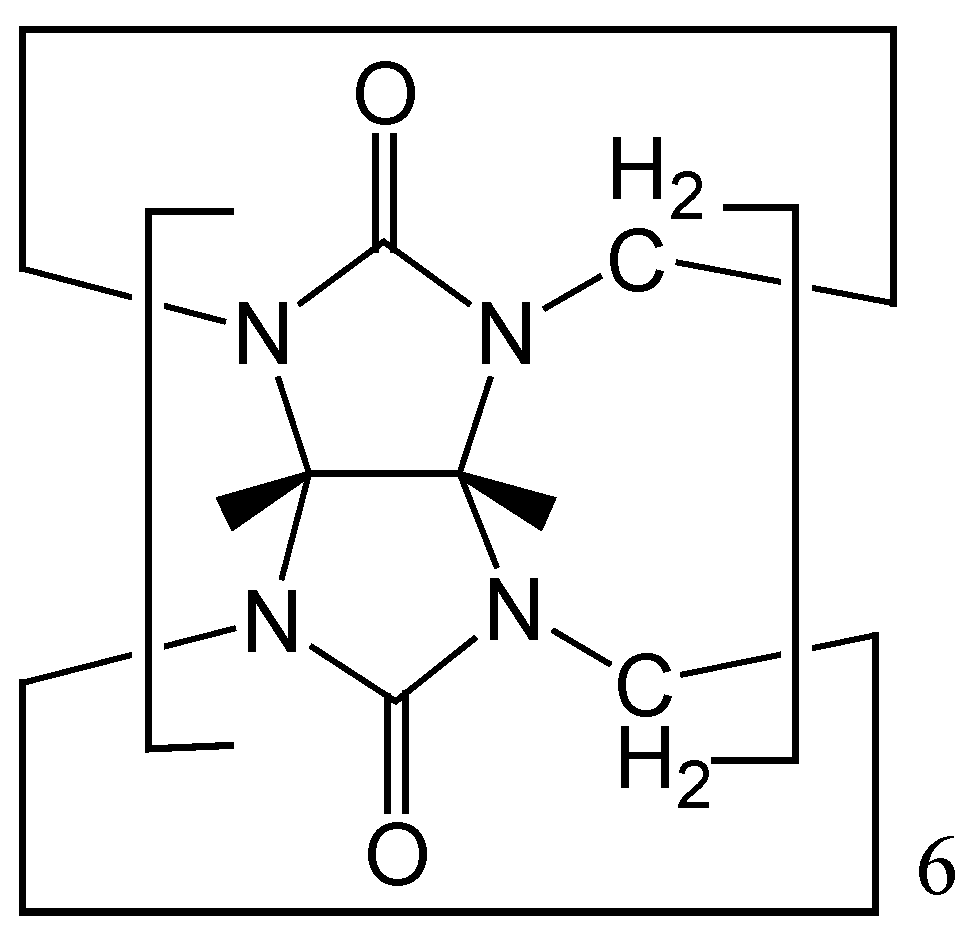
Results and Discussion

Experimental
General
Syntheses and separation
X-ray analysis of inclusion complex
| Chemical formula | C78H90N24O36 |
| Formula weight | 139.74 |
| Crystal Color, Habit | colorless, diamond like |
| Crystal Dimensions | 0.1° 0.1 ° 0.15 |
| Crystal System | triclinic |
| Lattice Type | primitive |
| Space Group | P-1 |
| Lattice Parameters | a = 12.2847(4) Å |
| b = 12.6895(4) Å | |
| c = 15.1310(4) Å | |
| α =74.6960(10)° | |
| β=71.4090(10)° | |
| γ =86.5090(10)° | |
| Volume | 2155.66(11) Å3 |
| Z value | 1 |
| Dcalc | 1.494 g/cm3 |
| F(000) | 1014 |
| μ (MoKα) | 0.120 mm-1 |
| Reflections/restraints/parameters | 7477 / 0 / 640 |
| Residuals: R1, wR2 [I > 2 σ (I)] | 0.0767, 0.2216 |
| Goodness of Fit, S | 1.079 |
| Max. shift/error | 0.000 |
| Max. peak in final Δρ synthesis | 0.825 |
| Min. peak in final Δρ synthesis | -0.812 |
Acknowledgments
References and Notes
- Kim, J.; Jung, I. S.; Kim, S.-Y.; Lee, E.; Kang, J.-K.; Sakamoto, S.; Yamaguchi, K.; Kim, K. New cucurbituril homologues: syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7, and 8). J. Am. Chem. Soc. 2000, 122, 540–541. [Google Scholar]
- Day, A. I.; Arnold, A. P.; Blanch, R. J.; Snushall, B. Controlling factors in the synthesis of cucurbituril and its homologues. J. Org. Chem. 2001, 66, 8094–8100. [Google Scholar] [CrossRef]
- Day, A. I.; Blanch, R. J.; Arnold, A. P.; Lorenzo, S.; Lewis, G. R.; Dance, I. A cucurbituril-based gyroscane: a new supramolecular form. Angew. Chem., Int. Ed. 2002, 41, 275–277. [Google Scholar] [CrossRef]
- Jon, S. Y.; Selvapalam, N.; Oh, D. H.; Kang, J. -K.; Kim, S. -Y.; Jeon, Y. J.; Lee, J. W.; Kim, K. Facile synthesis of cucurbit[n]uril derivatives via direct functionalization: expanding utilization of cucurbit[n]uril. J. Am. Chem. Soc. 2003, 125, 10186–10187. [Google Scholar]
- Jeon, Y. J.; Kim, H.; Jon, S.; Selvapalam, N.; Oh, D. H.; Seo, I.; Park, C. S.; Jung, S. R.; Koh, D.-S.; Kim, K. Artificial ion channel formed by cucurbit[n]uril derivatives with a carbonyl group fringed portal reminiscent of the selectivity filter of K+ channels. J. Am. Chem. Soc. 2004, 126, 15944–15945. [Google Scholar] [CrossRef]
- Lee, H.-K.; Park, K. M.; Jeon, Y. J.; Kim, D.; Oh, D. H.; Kim, H. S.; Park, C. K.; Kim, K. Vesicle formed by amphiphilc cucurbit[6]uril: Versatile, noncovalent modification of the vesicle surface, and multivalent binding of sugar-decorated vesicles to lectin. J. Am. Chem. Soc. 2005, 127, 5006–5007. [Google Scholar]
- Isobe, H.; Sato, S.; Nakamura, E. Synthesis of disubstituted cucurbit[6]uril and its rotaxane derivative. Org. Lett. 2002, 4, 1287–1289. [Google Scholar] [CrossRef]
- Day, A. I.; Arnold, A. P.; Blanch, R. J. A method for synthesizing partially substituted cucurbit[n]uril. Molecules 2003, 8, 74–84. [Google Scholar] [CrossRef]
- Zhao, Y.-J.; Xue, S. -F.; Zhu, Q. -J.; Tao, Z.; Zhang, J. -X.; Wei, Z. -B.; Long, L. -S.; Hu, M.-L.; Xiao, H. -P.; Day, A. I. Synthesis of a symmetrical tetrasubstituted cucurbit[6]uril and its host-guest compound with 2,2'-bipyridine. Chin. Sci. Bull. 2004, 49, 1111–1116. [Google Scholar] [CrossRef]
- Lagona, J.; Fettinger, J. C.; Isaacs, L. Cucurbit[n]uril analogues. Org. Lett. 2003, 5, 3745–3747. [Google Scholar] [CrossRef]
- Wagner, B. D.; Boland, P. G.; Lagona, J.; Isaacs, L. A cucurbit[6]uril analogue: host properties monitored by fluorescence spectroscopy. J. Phys. Chem. B 2005, 109, 7686–7691. [Google Scholar] [CrossRef]
- Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. The cucurbit[n]uril family. Angew. Chem. Int. Edit. 2005, 44, 4844–4870. [Google Scholar] [CrossRef]
- Huang, F. H.; Gibson, H. W. Polypseudorotaxanes and polyrotaxanes. Prog. Polym. Sci. 2005, 30, 982–1018. [Google Scholar] [CrossRef]
- Sliwa, W.; Zujewska, T. Interlocked molecules containing quaternary azaaromatic moieties. Heterocycles 2005, 65, 1713–1739. [Google Scholar] [CrossRef]
- Gerasko, O. A.; Sokolov, M. N.; Fedin, V. P. Mono- and polynuclear aqua complexes and cucurbit[6]uril: versatile building blocks for supramolecular chemistry. Pure Appl. Chem. 2004, 76, 1633–1646. [Google Scholar]
- Sokolov, M. N.; Dybtsev, D. N.; Fedin, V. P. Supramolecular compounds of cucurbituril with molybdenum and tungsten chalcogenide cluster aqua complexes. Russ. Chem. Bull. 2003, 52, 1041–1060. [Google Scholar] [CrossRef]
- Kim, K. Mechanically interlocked molecules incorporating cucurbituril and their supramolecular assemblies. Chem. Soc. Rev. 2002, 31, 96–107. [Google Scholar] [CrossRef]
- Buschmann, H. J.; Mutihac, L.; Jansen, K. Complexation of some amine compounds by macrocyclic receptors. J. Incl. Phenom. Macro. 2001, 39, 1–11. [Google Scholar] [CrossRef]
- Mock, W. L. Cucurbituril. Top. Curr. Chem. 1995, 175, 1–24. [Google Scholar] [CrossRef]
- We have found that the symmetry of fully methyl-substituted Q[5] (DEMeQ[5]) is higher than that of the partially methyl-substituted Q[5]; the corresponding dipole (0.0393 Debye) is quite smaller than that of dimethyl-substituted Q[5], (0.7444 Debye), or hexamethyl-substituted Q[5] (1.2115 Debye). The normal Q[5] has the smallest dipole, 0.0216 Debye, due to its high symmetry. On the other hand, among the four Q[5]s, HMeQ[5] has the best water solubility (up to 17 g/100 g water); while the Q[5] has the least water solubility (only 0.03 g/100 g water). The order of dipoles of the four Q[5]s is qualitatively consistent with that of the solubility of these Q[5]s, that is HMeQ[5] > DMeQ[5] > DEMeQ[5] > Q[5].
- Flinn, A.; Hough, G. C.; Stoddart, J. F.; Williams, D. J. Decamethylcucurbit[5]uril. Angew. Chem. Int. Ed. 1992, 31, 1475–7. [Google Scholar] [CrossRef]
- Sanjita, S.; Mantosh, K.; Sinha, E. K. Facile purification of rare cucurbiturils by affinity chromatography. Org. Lett. 2004, 6, 1225–1228. [Google Scholar] [CrossRef]
- Ma, P.-H.; Dong, J.; Xiang, S.-C.; Xue, S.i-F.; Zhu, Q.-J.; Tao, Z.; Zhang, J.-X.; Zhou, X. Interaction of host-guest complexes of cucurbit[n]urilswith double probe guests. Sci. China Ser. B. 2004, 47, 301–310. [Google Scholar] [CrossRef]
- Fu, H.-Y.; Xue, S.-F.; Zhu, Q.-J.; Tao, Z.; Zhang, J.-X.; Day, A. I. Investigation of host-guest compounds of cucurbit[n = 5-8]uril with some ortho pyridiniumammonium Ions. J. Incl. Phenom. Macro. 2005, 52, 101–107. [Google Scholar] [CrossRef]
- Sample availability: Contact the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Lu, L.-B.; Zhang, Y.-Q.; Zhu, Q.-J.; Xue, S.-F.; Tao, Z. Synthesis and X-ray Structure of the Inclusion Complex of Dodecamethylcucurbit[6]uril with 1,4-Dihydroxybenzene. Molecules 2007, 12, 716-722. https://doi.org/10.3390/12040716
Lu L-B, Zhang Y-Q, Zhu Q-J, Xue S-F, Tao Z. Synthesis and X-ray Structure of the Inclusion Complex of Dodecamethylcucurbit[6]uril with 1,4-Dihydroxybenzene. Molecules. 2007; 12(4):716-722. https://doi.org/10.3390/12040716
Chicago/Turabian StyleLu, Li-Bin, Yun-Qian Zhang, Qian-Jiang Zhu, Sai-Feng Xue, and Zhu Tao. 2007. "Synthesis and X-ray Structure of the Inclusion Complex of Dodecamethylcucurbit[6]uril with 1,4-Dihydroxybenzene" Molecules 12, no. 4: 716-722. https://doi.org/10.3390/12040716
APA StyleLu, L.-B., Zhang, Y.-Q., Zhu, Q.-J., Xue, S.-F., & Tao, Z. (2007). Synthesis and X-ray Structure of the Inclusion Complex of Dodecamethylcucurbit[6]uril with 1,4-Dihydroxybenzene. Molecules, 12(4), 716-722. https://doi.org/10.3390/12040716




