Abstract
A simple, rapid, and regioselective approach for the synthesis of N-(methoxy-carbonylmethyl)- and N-(n-propoxycarbonylmethyl) nucleobases was developed. By using DMF as the solvent and in the presence of K2CO3 as the base, all the desired products were obtained in moderate yields within 8 min under microwave irradiation.
Introduction
In recent years, modified nucleoside analogues have become of great interest due to their intriguing biological and pharmacological properties [1,2,3,4,5]. For example, a number of agents that exhibit potent anti-viral and anti-tumor activities such as AZT [6], Acyclovir [7,8], Neplanocin A [9], Peptide Nucleic Acids (PNA) [10,11,12] and so on have been prepared by modification of the carbohydrate ring of the natural nucleosides. PNA is a potent DNA mimic, in which the sugar-phosphate backbone of natural nucleic acids is replaced by a polyamide backbone. Since it was first reported by Nielsen and coworkers in 1991, PNA has attracted wide attention in medicinal chemistry for the development of gene therapy drugs or molecular probes. As a result, several groups have developed a variety of methods for the preparation of N-(alkoxycarbonylmethyl) nucleobases and their derivatives [13,14,15,16,17], which are important building blocks for PNA. However, the reported methods have some drawbacks, such as poor yields and regioselectivity, long reaction times and harsh reaction conditions.
In order to expand our research on the modification of nucleosides [18,19,20] and obtain these building blocks in higher yields with shorter reaction times and under milder reaction conditions, we turned our attention to microwave irradiation (MWI). The use of microwave-assisted organic syntheses has attracted considerable interest over the last two decades, leading to remarkable decreases in reaction times, significant enhancements of yields, easier workups and better regioselectivity [21,22,23,24,25,26,27,28,29]. Herein, we report a rapid, facile and practical protocol for the formation of N-(methoxycarbonylmethyl)- and N-(n-propoxycarbonylmethyl) nucleobases.
Results and Discussion
Initially, we selected the reaction of uracil with methyl chloroacetate as a model system to investigate the influence of base and irradiation time on the yield, as summarized in Table 1.

Table 1.
Optimization of Base and Irradiation Time a. 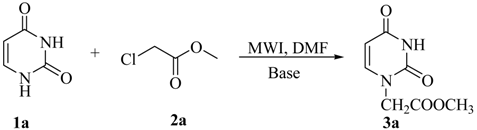

| Entry | Base | Irradiation time/min | Yield/% b |
|---|---|---|---|
| 1 | DMAP | 6 | 52 |
| 2 | CH3ONa | 6 | 41 |
| 3 | NaH | 6 | 50 |
| 4 | K2CO3 | 6 | 64 |
| 5 | K2CO3 | 8 | 76 |
| 6 | K2CO3 | 9 | 74 |
| 7 | K2CO3 | 10 | 68 |
a Reaction conditions: 1a (2 mmol), 2a (6 mmol), base (2 mmol), DMF (5 mL), MWI 250 W (160 oC);b Isolated yields based on 1a.
All reactions were carried out in DMF, as it is an excellent solvent both for dissolving nucleobases and absorbing microwave energy. To our delight, 3a was obtained in 52% yield by using DMAP as base (entry 1). Changing the base to CH3ONa or NaH only led to worse results (entries 2 and 3). An obvious yield improvement was observed when K2CO3 was employed (entry 4). Consequently, K2CO3 was selected as the best base, not only because it gave rise to the best results, but also because it is very cheap and easy to handle. The irradiation time had also significant effect on the yield, but it seemed that the reaction reached chemical equilibrium after being irradiated for some 8 min, as only slight yield variations were detected after longer irradiation times (entries 5 and 6). With prolonged reaction times (entry 7) a lower yield of 3a resulted and some N3-alkylated product was obtained. Further screening of irradiation power and reaction temperatures confirmed that 250 W and 160 °C were the best conditions. With this promising procedure in hand, we then extended the scope of substrates to include other uracil derivatives, as outlined in Table 2.

Table 2.
Alkylation of Various Uracil Derivatives in DMF under MWI a. 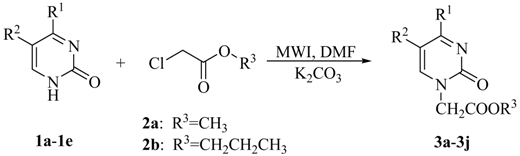

| Entry | Uracil derivative | R1 | R2 | R3 | Product | Yield/% b |
|---|---|---|---|---|---|---|
| 1 | 1a | OH | H | CH3 | 3a | 76 |
| 2 | 1b | OH | CH3 | CH3 | 3b | 78 |
| 3 | 1c | OH | Cl | CH3 | 3c | 73 |
| 4 | 1d | OH | I | CH3 | 3d | 72 |
| 5 | 1e | NH2 | H | H | 3e | - c |
| 6 | 1f | NHAc | H | CH3 | 3f | 64 |
| 7 | 1a | OH | H | CH2CH2CH3 | 3g | 70 |
| 8 | 1b | OH | CH3 | CH2CH2CH3 | 3h | 67 |
| 9 | 1c | OH | Cl | CH2CH2CH3 | 3i | 74 |
| 10 | 1d | OH | I | CH2CH2CH3 | 3j | 68 |
a Reaction conditions: 1 (2 mmol), 2 (6 mmol), base (2 mmol), DMF (5 mL), MWI 250 W (160 oC);b Isolated yields based on 1.c the desired product 3e was not obtained.
To our delight, all the uracil derivatives were exclusively alkylated at N-1, as confirmed by HMBC spectra, suggesting that our method was highly regioselective. It is worth mentioning that the same results were achieved when 2a was employed as the alkylating agent under the same conditions (entries 6-10). Substituting 5-H with CH3, Cl, or I only resulted in slight variations in yield, indicating that no obvious substitutent-effect existed [26]. Disappointingly, alkylation of 1e gave very poor results and only starting material was recovered. In order to increase the solubility and prevent side reactions, N4-acetyl cytosine (1f) was then utilized as the precursor of 1e and treated with 2a as described above to afford 3f in 64% yield (entry 5).
Interestingly, the procedure developed for uracil derivatives also worked well for purine derivatives. As can be seen from Table 3, the length of alkyl chain in the chloroacetate reagent did not affect the yield. The target N9-alkylated products were produced in high regioselectivity and good yields. To our surprise, no obvious changes in yields were observed when the 6-Cl and 2-H in 4a were substituted by 6-benzylamino (4c) and 2-Cl groups (4b), respectively. A possible explanation is that substitutents in these positions do not affect the electronic nature of N-9.
In order to investigate the capability and selectivity of our method compared with the conventional heating method, the formation of 3a was carried out in a pre-heated oil bath under the same conditions used with the microwave irradiation. It was shown the reaction afforded only 13% yield after 8 min and 50% yield after 6 h, and that the product was associated with the N3-alkylated byproduct, clearly indicating that our method was superior to the conventional method.

Table 3.
Alkylation of Various Purine Derivatives in DMF under MWI a. 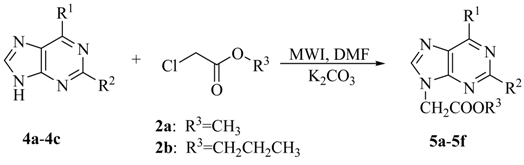

| Entry | Purine derivatives | R1 | R2 | R3 | Product | Yield/% b |
|---|---|---|---|---|---|---|
| 1 | 4a | Cl | H | CH3 | 5a | 72 |
| 2 | 4b | Cl | Cl | CH3 | 5b | 74 |
| 3 | 4c | benzylamino | H | CH3 | 5c | 78 |
| 4 | 4a | Cl | H | CH2CH2CH3 | 5d | 68 |
| 5 | 4b | Cl | Cl | CH2CH2CH3 | 5e | 70 |
| 6 | 4c | benzylamino | H | CH2CH2CH3 | 5f | 76 |
a Reaction conditions: 4 (2 mmol), 2 (6 mmol), base (2 mmol), DMF (5 mL), MWI 250 W (160 oC);b Isolated yields based on 4.
Conclusions
In summary, we have developed a procedure for the preparation of N-(alkoxycarbonylmethyl) nucleobases that is rapid, simple and highly efficient in terms of yield and regioselectivity. Our method has several additional advantages, such as milder reaction conditions, short reaction times and lack of side products. The use of this method to synthesize other PNA building blocks is currently under study in our laboratory and the results will be reported in due course.
Experimental Section
General
All reagents and solvents were purchased from commercial sources and used without further purification. The nucleobases were a gift of Xinxiang Tuoxin Biochemical Technology & Science Co. Ltd, P. R. China. Melting points were determined on an XRC-1 micro melting point apparatus and are uncorrected. 1H- and 13C-NMR spectra were recorded in DMSO-d6 solutions on a Bruker DPX-400 spectrometer (at 400 MHz and 100 MHz, respectively) using TMS as internal standard. High resolution mass spectra were obtained on the electrospray ionization (ESI) mass spectrometer. Elemental analyses were performed on an EA-1110 (CE Instruments) instrument. All reactions were performed in a commercially available single-mode microwave apparatus equipped with a high sensitivity IR sensor for temperature control and measurement (MAS-I, Sineo Microwave Chemical Technology Co. Ltd., Shanghai, China).
General Procedure for the preparation of 3a (microwave method)
A mixture of uracil (2 mmol, 0.224 g), K2CO3 (2 mmol, 0.276 g) and methyl chloroacetate (6 mmol, 0.55 mL) in DMF (5 mL) was placed in a 50 mL round-bottom glass flask. After being irradiated at 250 W (160 oC) for 8 min, the reaction mixture was concentrated to dryness under reduced pressure and the residue was purified by column chromatography (1:1 ethyl acetate-cyclohexane) to afford 1-(methoxycarbonylmethyl) uracil (3a) as a white power in 76% yield; M.p.180-182 oC (ref. 30); 1H-NMR δ: 3.69 (s, 3H, CH3), 4.52 (s, 2H, CH2), 5.62 (d, 1H, J=8 Hz, H-5), 7.62 (d, 1H, J=8 Hz, H-6), 11.40 (s, 1H, H-3); 13C-NMR δ: 48.7 (CH2), 52.4 (CH3), 101.3 (C-5), 146.0 (C-6), 151.1 (C-2), 163.9 (C-4), 168.8 (C=O). The following compounds were similarly prepared:
1-(Methoxycarbonylmethyl) thymine (3b): Colorless needles; M.p. 189–190 oC (lit. [31] M.p. 189-191 oC); 1H-NMR δ: 1.76 (d, J=0.4 Hz, CH3-5), 3.69 (s, 3H, OCH3), 4.48 (s, 2H, CH2), 7.50 (d, 1H, J=0.4 Hz, H-6), 11.39 (s, 1H, H-3); 13C-NMR δ: 12.0 (CH3-5), 48.5 (CH2), 52.4 (CH3), 108.8 (C-5), 141.7 (C-6), 151.1 (C-2), 164.5 (C-4), 168.9 (C=O).
5-Chloro-1-(methoxycarbonylmethyl) uracil (3c): Colorless needles; M.p. 203-204 oC; 1H-NMR δ: 3.68 (s, 3H, CH3), 4.53 (s, 2H, CH2), 8.16 (s, 1H, H-6), 11.10 (s, 1H, H-3); 13C-NMR δ 48.8 (CH2), 52.6 (CH3), 106.5 (C-5), 143.2 (C-6), 150.2 (C-2), 159.6 (C-4), 168.5 (C=O); HR-MS calcd. for C7H7ClN2O4 218.0094, found 218.0087; Anal. calcd. for C7H7ClN2O4 for C, 38.46, H, 3.23, N, 12.82, found C, 38.45, H, 3.18, N, 12.72.
5-Iodo-1-(methoxycarbonylmethyl) uracil (3d): Colorless plates; M.p. 197-199 oC [32]; 1H-NMR δ: 3.69 (s, 3H, CH3), 4.52 (s, 2H, CH2), 8.20 (s, 1H, H-6), 11.81 (s, 1H, H-3); 13C-NMR δ: 48.6 (CH2), 52.5 (CH3), 68.4 (C-5), 150.2 (C-6), 150.8 (C-2), 161.2 (C-4), 168.6 (C=O); HR-MS calcd. for C7H7IN2O4 309.9450, found 309.9446; Anal. calcd. for C7H7IN2O4 for C, 27.12, H, 2.28, N, 9.04, found C 27.16, H 2.23, N 9.08.
N4-Acetyl-1-(methoxycarbonylmethyl) cytosine (3f): Colorless needles; M.p.186-188 oC; 1H-NMR δ: 2.10 (s, 3H, COCH3), 3.68 (s, 3H, OCH3), 4.63 (s, 2H, CH2), 7.20 (d, 1H, J=7.6 Hz, H-5), 8.05 (d, 1H, J=7.6 Hz, H-5), 10.89 (s, 1H, NH); 13C-NMR δ: 24.5 (COCH3), 50.8 (OCH3), 52.4 (NCH2), 95.4 (C-5), 150.7 (C-6), 155.3 (C-2), 163.2 (C-4), 168.6 (COCH3), 171.1 (C=O); HR-MS calcd. for C9H11N3O4 225.0750, found 225.0746; Anal. calcd. for C9H11N3O4 for C, 48.00; H, 4.92; N, 18.66; O, 28.42, found C, 47.91, H, 4.87, N 18.59.
1-(n-Propoxycarbonylmethyl) uracil (3g): Colorless plates; M.p. 124-125 oC; 1H-NMR δ: 0.88 (t, 3H, J=7.2 Hz, CH3), 1.60 (m, 2H, CH2CH3), 4.07 (t, 2H, J=7.2 Hz, OCH2), 4.52 (s, 2H, NCH2), 5.61 (d, 1H, J=8 Hz, H-5), 7.62 (d, 1H, J=8 Hz, H-6), 11.38 (s, 1H, H-3); 13C-NMR δ: 10.3 (CH3), 21.6 (CH2CH3), 48.8 (NCH2), 66.7 (OCH2), 101.2 (C-5), 146.0 (C-6), 151.1 (C-2), 163.9 (C-4), 168.3 (C=O); HR-MS calcd. for C9H12N2O4 212.0797, found 212.0290; Anal. calcd. for C9H12N2O4 for C, 50.94; H, 5.70; N, 13.20, found C, 50.90; H, 5.61; N, 13.30.
1-(n-Propoxycarbonylmethyl) thymine (3h) Colorless plates; M.p. 140-141 oC; 1H-NMR δ: 0.88 (t, 3H, J=7.2Hz, CH2CH3), 1.60 (m, 2H, CH2CH3), 1.76 (s, 3H, CH3-5), 4.06 (t, 2H, J=7.2 Hz, OCH2), 4.47 (s, 2H, NCH2), 7.50 (d, 1H, J=1.2 Hz, H-6), 11.36 (s, 1H, H-3); 13C-NMR δ: 10.3 (CH2CH3), 12.0 (CH3-5), 21.6 (CH2CH3), 48.6 (NCH2), 66.6 (OCH2), 108.7 (C-5), 141.7 (C-6), 151.1 (C-2), 164.5 (C-4), 168.4 (C=O); HR-MS calcd. for C10H14N2O4 226.0954, found 226.0950; Anal. calcd. for C10H14N2O4 for C, 53.09; H, 6.24; N, 12.38, found C, 53.00; H, 6.15; N, 12.30.
5-Chloro-1-(n-propoxycarbonylmethyl) uracil (3i) White power; M.p. 148-149 oC; 1H-NMR δ: 0.88 (t, 3H, J=7.2 Hz, CH2CH3), 1.60 (m, 2H, CH2CH3), 4.06 (t, 2H, J=7.2 Hz, OCH2), 4.52 (s, 2H, NCH2), 8.16 (d, 1H, J=4 Hz, H-6), 11.97 (s, 1H, H-3); 13C-NMR δ: 10.3 (CH2CH3), 21.6 (CH2CH3), 48.9 (NCH2), 66.8 (OCH2), 106.5 (C-5), 143.3 (C-6), 150.3 (C-2), 159.6 (C-4), 168.0 (C=O); HR-MS calcd. for C9H11ClN2O4 246.0407, found 246.0401; Anal. calcd. for C9H11ClN2O4 for C, 43.83; H, 4.50; N, 11.36, found C, 43.78; H, 4.41; N, 11.30.
5-Iodo-1-(n-propoxycarbonylmethyl) uracil (3j) Colorless needles; M.p. 156-157 oC; 1H-NMR δ: 0.88 (t, 3H, J=7.2 Hz, CH3), 1.60 (m, 2H, CH2CH3), 4.07 (t, 2H, J=7.2 Hz, OCH2), 4.52 (s, 2H, NCH2), 8.22 (d, 1H, J=4 Hz, H-6), 11.80 (s, 1H, H-3); 13C-NMR δ: 10.3 (CH2CH3), 21.7 (CH2CH3), 48.7 (NCH2), 66.8 (OCH2), 68.3 (C-5), 150.2 (C-6), 150.8 (C-2), 161.2 (C-4), 168.1 (C=O); HR-MS calcd. for C9H11IN2O4 337.9763, found 337.9759; Anal. calcd. for C9H11IN2O4 for C, 31.97; H, 3.28; N 8.29, found C, 31.89; H, 3.20; N, 8.25.
6-Chloro-9-(methoxycarbonylmethyl) purine (5a) Colorless plates; M.p. 109-110 oC [33]; 1H-NMR δ: 3.73 (s, 3H, CH3), 5.29 (s, 2H, CH2), 8.68 (s, 1H, H-8), 8.80 (s, 1H, H-2); 13C-NMR δ: 44.74 (CH2), 52.8 (CH3), 130.7 (C-5), 148.0 (C-8), 149.4 (C-4), 152.0 (C-6), 152.2 (C-2), 168.0 (C=O).
2,6-Dichloro-9-(methoxycarbonylmethyl) purine (5b) Colorless plates; M.p. 142-144 oC [34]; 1H-NMR δ: 3.74 (s, 3H, CH3), 5.27 (s, 2H, CH2), 8.71 (s, 1H, H-8); 13C-NMR δ: 44.48 (CH2), 52.9 (CH3), 130.3 (C-5), 149.0 (C-8), 150.1 (C-4), 151.5 (C-6), 153.7 (C-2), 167.8 (C=O).
6-Benzylamino-9-(methoxycarbonylmethyl) purine (5c) Paleyellow power; M.p. 138-139 oC; 1H-NMR δ: 3.70 (s, 3H, CH3), 4.72 (s, 2H, CH2Ph), 5.10 (s, 2H, NCH2), 7.19~7.36 (m, 5H, Ph), 8.14 (s, 1H, H-8), 8.19 (s, 1H, H-2), 8.39 (s, 1H, NH); 13C-NMR δ: 43.1 (CH2Ph), 44.0 (NCH2), 52.6 (CH3), 119.5 (C-5), 126.8, 127.7, 128.4 (Ph), 140.3 (C-8), 141.4 (C-4), 152.8 (C-2), 154.6 (C-6), 168.6 (C=O); HR-MS calcd. for C15H15N5O2 297.1226, found 297.1220; Anal. calcd. for C15H15N5O2 for C, 60.60; H, 5.09; N, 23.56, found C, 60.53; H, 5.02; N, 23.60.
6-Chloro-9-(n-propoxycarbonylmethyl) purine (5d) Yellow power; M.p. 56-58 oC; 1H-NMR δ: 0.84 (t, 3H, J=7.2 Hz, CH3), 1.59 (m, 2H, CH2CH3), 4.09 (t, 2H, J=7.2 Hz, OCH2), 5.29 (s, 2H, NCH2), 8.69 (s, 1H, H-8), 8.19 (s, 1H, H-2); 13C-NMR δ: 10.2 (CH2CH3), 21.5 (CH2CH3), 44.8 (NCH2), 67.1 (OCH2), 130.7 (C-5), 148.0 (C-8), 149.4 (C-4), 152.0 (C-6), 152.2 (C-2), 167.5 (C=O); HR-MS calcd. for C10H11ClN4O2 254.0571, found 254.0562; Anal. calcd. for C10H11ClN4O2 for C, 47.16; H, 4.35; N, 22.00, found C, 47.09; H, 4.27; N, 21.89.
2,6-Dichloro-9-(n-propoxycarbonylmethyl) purine (5e) Colorless rod-like crystals; M.p. 117-119 oC; 1H-NMR δ: 0.86 (t, 3H, J=7.2 Hz, CH2CH3), 1.60 (m, 2H, CH2CH3), 4.11 (t, 2H, J=7.2Hz, OCH2), 5.27 (s, 2H, NCH2), 8.71 (s, 1H, H-8); 13C-NMR δ: 10.2 (CH3), 21.6 (CH2CH3), 45.0 (NCH2), 67.2 (OCH2), 130.3 (C-5), 149.0 (C-8), 150.1 (C-4), 151.5 (C-6), 153.8 (C-2), 167.3 (C=O); HR-MS calcd. for C10H10Cl2N4O2 288.0181, found 288.0178; Anal. calcd. for C10H10Cl2N4O2 for C, 41.54; H, 3.49; N, 19.38, found C, 41.45; H, 3.41; N, 19.46.
6-Benzylamino-9-(n-propoxycarbonylmethyl) purine (5f) Colorless plates; M.p. 179-180 oC; 1H-NMR δ: 0.85 (t, 3H, J=7.2 Hz, CH3), 1.59 (m, 2H, CH2CH3), 4.07 (t, 2H, J=7.2 Hz, OCH2), 4.72 (s, 2H, CH2Ph), 5.09 (s, 2H, NCH2), 7.19~7.36 (m, 5H, Ph), 8.14 (s, 1H, H-8), 8.19 (s, 1H, H-2), 8.34 (s, 1H, NH); 13C-NMR δ: 10.2 (CH3), 21.6 (CH2CH3), 43.1 (CH2Ph), 44.1 (NCH2), 66.8 (OCH2), 118.8 (C-5), 126.8, 127.3, 128.3 (Ph), 140.3 (C-8), 141.4 (C-4), 152.8 (C-6), 154.8 (C-2), 168.1 (C=O); HR-MS calcd. for C17H19N5O2 325.1539, found 325.1536; Anal. calcd. for C17H19N5O2 for C, 62.75; H, 5.89; N, 21.52, found C, 62.68; H, 5.80; N, 21.60.
Synthesis of 3a (conventional method)
A mixture of uracil (2 mmol, 0.224 g), K2CO3 (2 mmol, 0.276 g) and methyl chloroacetate (6 mmol, 0.55 mL) in DMF (5 mL), contained in a 50 mL round-bottom glass flask, was stirred under reflux in an oil-bath (160 oC) for 8 min or 6 h. The workups were performed as described above for the microwave method.
Acknowledgments
We are grateful for financial support from the Natural Science Foundation of China (No. 20372018) for financial support.
References
- Haines, D. R.; Tseng, C. K. H.; Marquez, V. E. Synthesis and biological activity of unsaturated carboacyclic purine nucleoside analogs. J. Med. Chem. 1987, 30, 943–947. [Google Scholar] [CrossRef]
- Bisacchi, G. S.; Singh, J.; Godfrey, J. D., Jr.; Kissick, T. P.; Mitt, T.; Malley, M. F.; Di Marco, J. D.; Gougoutas, J. Z.; Mueller, R. H.; Zahler, R. Regioselective Coupling of Tetraalkylammonium Salts of 6-Iodo-2-aminopurine to a Cyclobutyl Triflate: Efficient Preparation of Homochiral BMS-180,194, a Potent Antiviral Carbocyclic Nucleoside. J. Org. Chem. 1995, 60, 2902–2905. [Google Scholar] [CrossRef]
- Paoli, M.L.; Piccini, S.; Rodriquez, M.; Sega, A. Sensible Improvements Induced by Ionic Liquids in the Reaction of Modified Carbasugars with Bases for the Building of Constrained Carbanucleosides. J. Org. Chem. 2004, 69, 2881–2883. [Google Scholar] [CrossRef]
- Freer, R.; Geen, G. R.; Ramsay, T. W.; Share, A. C.; Slater, G. R.; Smith, N. M. A New Route to Famciclovir via Palladium Catalysed Allylation. Tetrahedron 2000, 56, 4589–4595. [Google Scholar] [CrossRef]
- Lanver, A.; Schmalz, H.-G. Microwave-Assisted Amination of a Chloropurine Derivative in the Synthesis of Acyclic Nucleoside Analogues. Molecules 2005, 10, 508–515. [Google Scholar] [CrossRef]
- Mitsuya, H.; Weinhold, K.J.; Furman, P.A. 3'-Azido-3'-deoxythymidine (BW A509U): An antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Pro. Natl. Acad. Sci. USA. 1985, 82, 7096–7100. [Google Scholar] [CrossRef]
- Vandenriessche, F.; Snoeck, R.; Janssen, G.; Hoogmartens, J.; Aerschot, A. V.; De Clercq, E.; Herdewijn, P. Synthesis and antiviral activity of acyclic nucleosides with a 3(S),5-dihydroxypentyl or 4(R)-methoxy-3 (S),5-dihydroxypentyl side chain. J. Med. Chem. 1992, 35, 1458–1465. [Google Scholar] [CrossRef]
- Schaeffer, H.J.; Beauchamp, L.; De Miranda, P. 9-(2-Hydroxyethoxymethyl)guanine activity against viruses of the herpes group. Nature 1978, 272, 583–585. [Google Scholar] [CrossRef]
- Ono, M.; Nishimura, K.; Tsubouchi, H.; Nagaoka, Y.; Tomioka, K. Total Synthesis of (-)-Neplanocin A by Using Lithium Thiolate-Initiated Michael-Aldol Tandem Cyclization Reaction. J. Org. Chem. 2001, 66, 8199–8203. [Google Scholar]
- Nielsen, P.E.; Egholm, M.; Berg, R.H.; Buchardt, O. Sequence-selective recognition of DNA by strand displacement with thymine-substituted polyamide. Science 1991, 254, 1497–1500. [Google Scholar]
- Egholm, M.; Buchardt, O.; Christensen, L.; Behrens, C.; Freier, S.M.; Driver, D.A.; Berg, R.H.; Kim, S.K.; Norden, B.; Nielsen, P.E. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature 1993, 365, 566–568. [Google Scholar]
- Nielsen, P. E. Peptide Nucleic Acid. A Molecule with Two Identities. Acc. Chem. Res. 1999, 32, 624–630. [Google Scholar] [CrossRef]
- Austin, R. E.; Waldraff, C.; Al-Obeidi, F. Microwave assisted solid-phase synthesis of trisubstituted 2-(2,6-purin-9-yl) acetamides. Tetrahedron Lett. 2005, 46, 2873–2875. [Google Scholar]
- Dueholm, K.L.; Egholm, M.; Behrens, C.; Christensen, L.; Hansen, H.F.; Vulpius, T.; Petersen, K.H.; Berg, R.H.; Nielsen, P.E.; Buchardt, O. Synthesis of Peptide Nucleic Acid Monomers Containing the Four Natural Nucleobases: Thymine, Cytosine, Adenine, and Guanine and Their Oligomerization. J. Org. Chem. 1994, 59, 5767–5773. [Google Scholar] [CrossRef]
- Thomson, S.A.; Josey, J.A.; Cadilla, R.; Gaul, M.D.; Hassman, C.F.; Luzzio, M.J.; Pipe, A.J.; Reed, K.L.; Ricca, D.J.; Wiethe, R.W.; Noble, S.A. Fmoc mediated synthesis of peptide nucleic acids. Tetrahedron 1995, 51, 6179–6194. [Google Scholar]
- Egholm, M.; Buchardt, O.; Nielsen, P.E.; Berg, R.H. Peptide nucleic acids (PNA). Oligonucleotide analogs with an achiral peptide backbone. J. Am. Chem. Soc. 1992, 114, 1895–1897. [Google Scholar] [CrossRef]
- Will, D.W.; Breipohl, G.; Langner, D.; Knolle, J.; Uhlmann, E. The synthesis of polyamide nucleic acids using a novel monomethoxytrityl protecting-group strategy. Tetrahedron 1995, 51, 12069–12082. [Google Scholar] [CrossRef]
- Qu, G.-R.; Li, Y.; Han, S.-H. Microwave assisted synthesis of N-(ethoxycarbonylmethyl) nucleobases: Building blocks for PNAs. J. Chem. Res. 2005, 167–168. [Google Scholar]
- Qu, G.-R.; Han, S.-H; Zhang, Z.; Geng, M.; Xue, F. Microwave assisted synthesis of 6-substituted aminopurine analogs in water. J. Braz. Chem. Soc. 2006, 17, 915–922. [Google Scholar] [CrossRef]
- Qu, G.-R.; Han, S.-H; Zhang, Z.; Geng, M.; Xue, F. Microwave-assisted regioselective synthesis of acyclic nucleosides through an alkylating reaction with 2-oxa-1,4-butanediol diacetate. Can. J. Chem. 2006, 84, 819–824. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem., Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Loupy, A. Microwaves in organic synthesis; Wiley-VCH: Weinheim, 2002; pp. 253–290. [Google Scholar]
- Ricardo, A.W.; Neves, F.; Rajendra, M.S. A Handy and Solventless Direct Route to Primary 3-[3-Aryl)-1,2,4-oxadiazol-5-yl]propionamides Using Microwave Irradiation. Molecules 2006, 11, 318–324. [Google Scholar] [CrossRef]
- Varma, R.S. Solvent-free organic syntheses: Using supported reagents and microwave irradiation. Green Chem. 1999, 1, 43–55. [Google Scholar]
- Liu, G.; Yang, S.; Song, B.; Xue, W.; Hu, D.; Jin, L.; Lu, P. Microwave Assisted Synthesis of N-Arylheterocyclic Substituted-4-aminoquinazoline Derivatives. Molecules 2006, 11, 272–278. [Google Scholar] [CrossRef]
- Khalafi-Nezhad, A.; Zare, A.; Parhami, A.; Soltani Rad, M.N.; Nejabat, G.R. Microwave-assisted N-nitroarylation of Some Pyrimidine and Purine Nucleosides. Can. J. Chem. 2006, 84, 979–985. [Google Scholar] [CrossRef]
- Le, H.P.; Muller, C.E. Rapid Microwave-assisted Fluorination Yielding novel 5'-deoxy-5'-Fluorouridine Derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 6139–6142. [Google Scholar] [CrossRef]
- Paolini, L.; Petricci, E.; Corelli, F.; Botta, M. Microwave-Assisted C-5 Iodination of Substituted Pyrimidinones and Pyrimidine Nucleosides. Synthesis 2003, 1039–1042. [Google Scholar]
- Khalafi-Nezhad, A.; Zarea, A.; Soltani Rad, M.N.; Mokhtari, B.; Parhami, A. Microwave-Assisted Michael Addition of Some Pyrimidine and Purine Nucleobases with α, β-Unsaturated Esters: A Rapid Entry into Carboacyclic Nucleoside Synthesis. Synthesis 2005, 419–424. [Google Scholar]
- Wyrzykiewicz, E.; Kazimierczuk, Z. An electron-impact mass spectral (EIMS) study of N-1 and C-6 carboxyalkyl- and alkoxycarbonylalkyl-substituted derivatives of uracil. J. Heterocycl. Chem. 1998, 35, 349–358. [Google Scholar] [CrossRef]
- Aldrian-Herrada, G.; Rabie, A.; Wintersteiger, R.; Brugidou, J. Solid-phase Synthesis of Peptide Nucleic Acid (PNA) Monomers and Their Oligomerization Using Disulphide Anchoring Linkers. J. Peptide Sci. 1998, 4, 266–281. [Google Scholar] [CrossRef]
- Bergmann, F.; Herrmann, R.; Seidel, C.; Koch, T. Novel monomer elements for marking peptidic nucleic acids. WO Patent 9842735, (1998). [Google Scholar]
- Kim, B. Y.; Ahn, J. B.; Lee, H. W.; Kang, S. K.; Lee, J. H.; Shin, J. S.; Ahn, S. K.; Hong, C. I.; Yoon, S. S. Synthesis and biological activity of novel substituted pyridines and purines containing 2,4-thiazolidinedione. Eur. J. Med. Chem. 2004, 39, 433–447. [Google Scholar] [CrossRef]
- Brik, A.; Wu, C-Y; Best, M. D.; Wong, C-H. Tetrabutylammonium fluoride-assisted rapid N9-alkylation on purine ring: Application to combinatorial reactions in microtiter plates for the discovery of potent sulfotransferase inhibitors in situ. Bioorg. & Med. Chem. 2005, 13, 4622–4626. [Google Scholar]
- Sample Availability: Samples of the compounds mentioned are available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.