Abstract
Selenium-containing heterocyclic compounds have been well recognized, not only because of their remarkable reactivities and chemical properties, but also because of their diverse pharmaceutical applications. In this context, isoselenocyanates have been emerged as a powerful tool for the synthesis of selenium-containing heterocycles, since they are easy to prepare and store and are safe to handle. In this review the recent advances in the development of synthesis methods for selenium-containing heterocycles from isoselenocyanates are presented and discussed.
Contents:
| 1. Introduction | 505 |
| 2. Synthesis of isoselenocyanates and acylisoselenocyanates | 505 |
| 3. Reactions with amines | 507 |
| 4. Reactions with alcohols and thiols | 513 |
| 5. Reactions with selenolates | 516 |
| 6. Reactions with carbanions | 518 |
| 7. Reactions with azodicaboxylates and diazomethanes | 521 |
| 8. Cycloaddition reactions | 523 |
| 9. Iodo- and acid-catalyzed cyclization reactions | 525 |
| 10. Synthesis of pentalenes | 525 |
| 11. Conclusions | 528 |
1. Introduction
Selenium was discovered in 1817 by J.J. Berzelius [1] and the first organoselenium compound, i.e., ethylselenol, was reported by F. Wöhler and C. Siemens in 1847 [2]. Most progress in the area of the synthetic organic chemistry of Se was accomplished more than 100 years later, in contrast to the chemistry of O- and S-containing organic molecules, which is much better developed. Although the chemistry of Se-containing compounds is often similar to that of the corresponding S analogues, some significant differences are also known, and because of the toxicity and instability of many Se compounds, the synthesis of Se heterocycles is much less developed. Despite the high toxicity of many selenium compounds, organic derivatives of selenium have been synthesized as anticancer [3,4,5], and for other medicinal applications [6], as well as biologically active substances exhibiting antiviral [7], antibacterial [8], antihypertensive [9], and fungicidal properties [10]. As a result, selenium-containing heterocycles are of increasing interest because of their chemical properties and biological activities. Also, new approaches for the synthesis of selenium heterocycles by using more stable, less toxic, and easily accessible Se reagents are of great interest. In this context, isoselenocyanates have emerged as a powerful tool for the synthesis of selenium-containing heterocycles, since they are easy to prepare and store, less-toxic and safe to handle. In this review article, the recent progress in the development of synthesis methods of selenium-containing heterocycles from isoselenocyanates is presented and discussed in the form of their reactions with various nucleophiles.
2. Synthesis of isoselenocyanates and acylisoselenocyanates
The literature to date contains few descriptions of the preparation of isoselenocyanates 1 (Scheme 1) [11,12,13,14,15,16,17,18,19]. The classical method of synthesis of organic isoselenocyanates involves the addition of elemental selenium to isonitriles 3 [11] or synthesis from the corresponding formamides 4 [12,13,14] Several other methods have also been investigated. An apparently more convenient procedure consists of treatment of a primary amine 5 with equimolar amounts of CSe2 and HgCl2 in the presence of triethylamine to give the corresponding isoselenocyanate 1 in reasonable yields [15]. The disadvantages of this method are that the presence of isoselenocyanate and the amine-mercuric chloride adduct leads to the formation of the corresponding selenourea and carbodiimide (R-N=C=N-R) as major side products, which make the purification of the desired material difficult [15]. Other methods of only limited applicability include, the alkylation of selenocyanate ion [16], the reaction of N-aryl-carbimidic dichlorides with sodium selenide [17], the treatment of isocyanates with phosphorous(V) selenide [17b], photochemical rearrangement of selenocyanates 6 [18] and via cycloaddition by the reaction of nitrile oxides 7 with primary selenoamides [19].
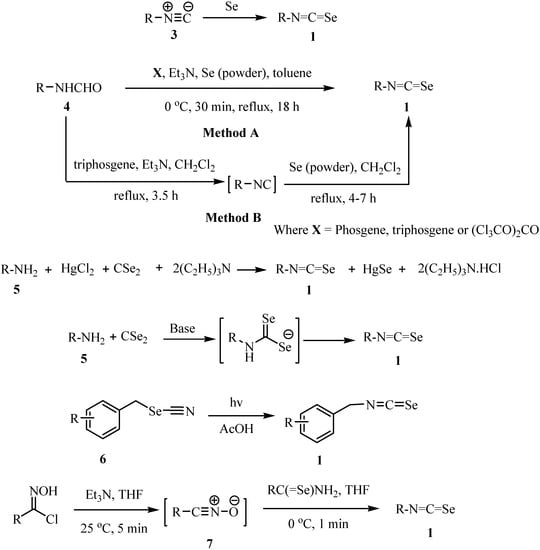
Scheme 1.
Scheme 1.
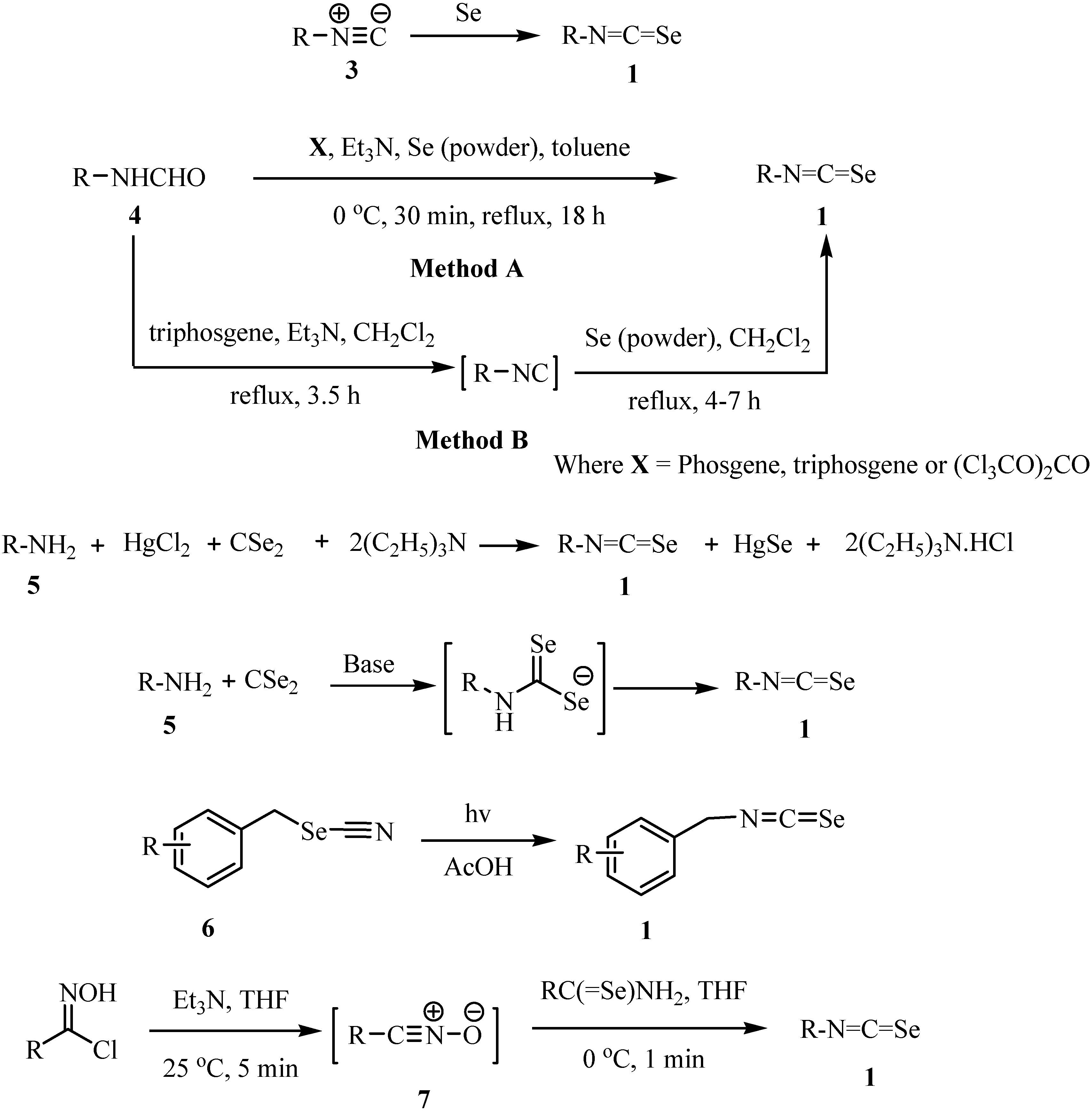
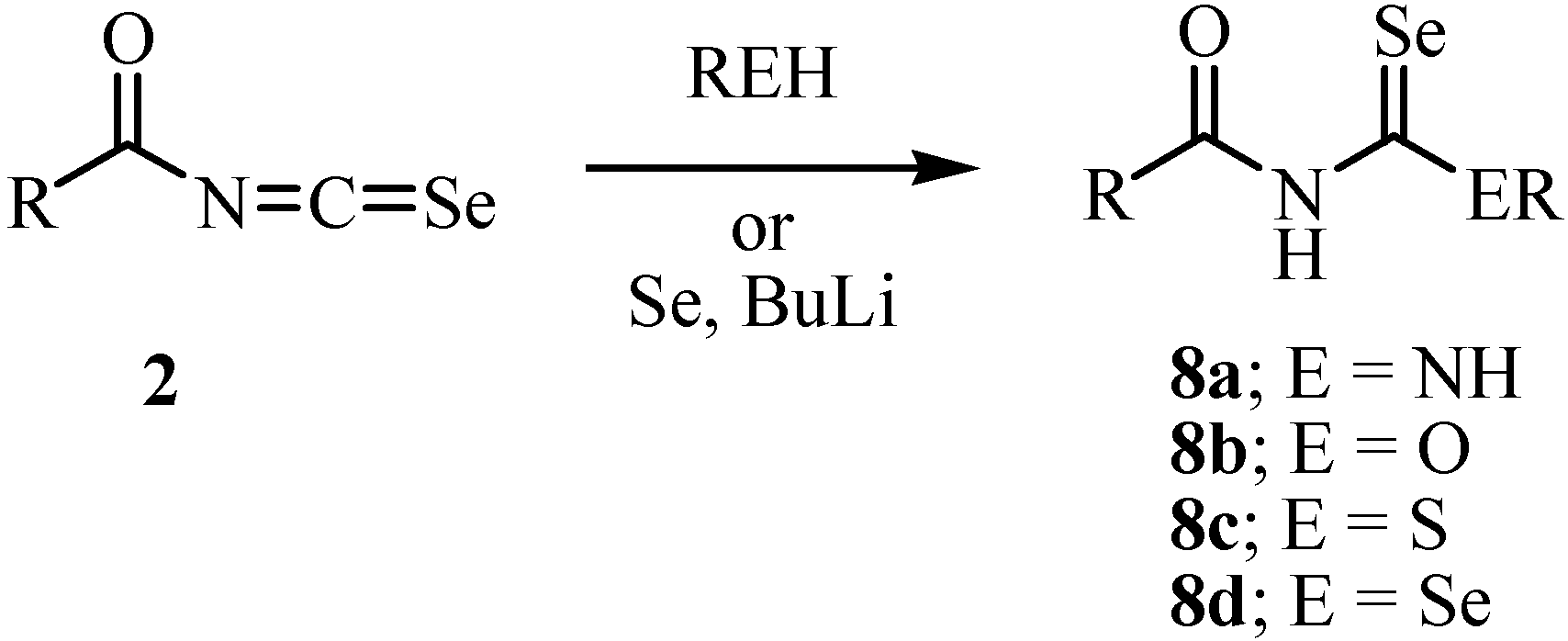
Acylisoselenocyanates 2 were prepared by a reaction of acyl chloride with potassium selenocyanate (Scheme 2), a method first investigated by Douglas [20]. The acylisoselenocyanates were never isolated. It was assumed that a polymeric form was present in equilibrium with the monomer that underwent the observed reaction. The generation of the acylisoselenocyanates was confirmed by a subsequent reaction with nucleophiles (Scheme 3) [21]. Douglas reported a reaction of the acylisoselenocyanates with amine in 1937 [20].
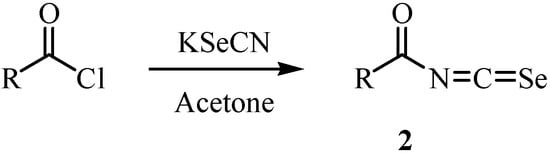
Scheme 2.
Scheme 2.
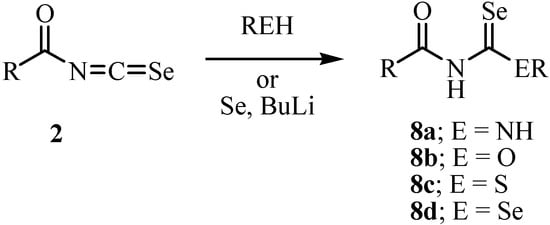
Scheme 3.
Scheme 3.
3. Reactions with amines
Treatment of ω-halo-alkylamines with aryl and alkylisoselenocyanates 1 in the presence of triethylamine (Scheme 4) gave the corresponding 1,3-selenazolidin-2-imines 9 [22], 1,3-selenazin-2-imines 10 [23], and 1,3-selenazepane-2-imines 11 [24], respectively.
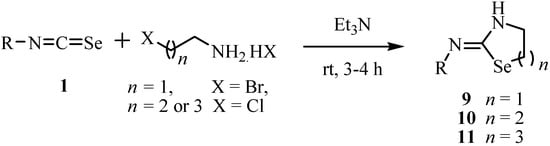
Scheme 4.
Scheme 4.
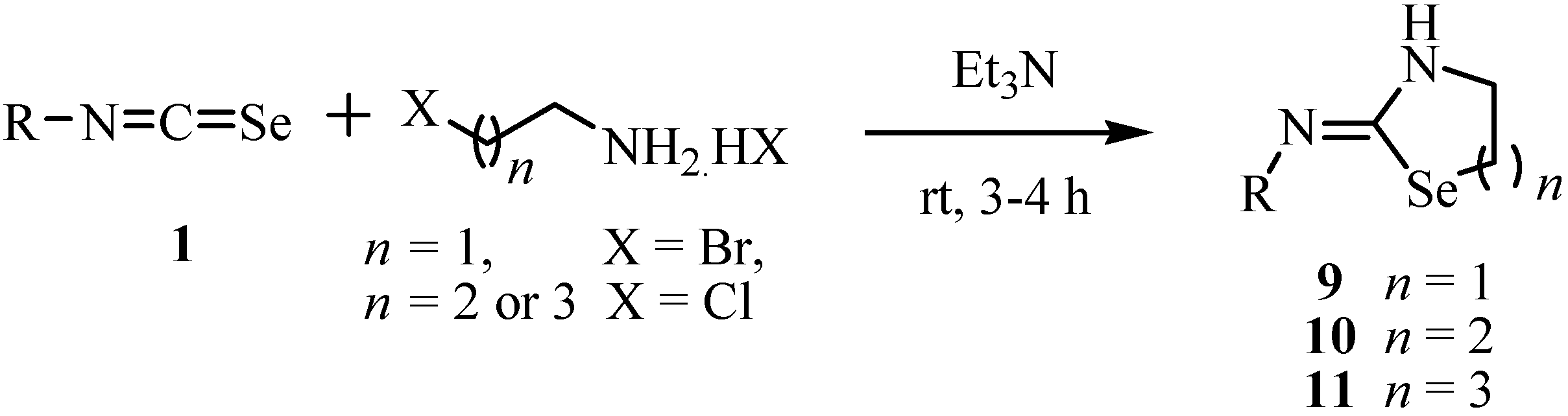
On the other hand, the reaction of haloamines with two equiv. of isoselenocyanates in an organic solvent in the presence of a stronger base such as sodium hydroxide gave 2-(phenylimino)-1,3-selenazolidine-3-carboselenoic anilide 12 and 2-(phenylimino)-1,3-selenazane-3-carboselenoic anilide 13 in excellent yields (Scheme 5) [23].
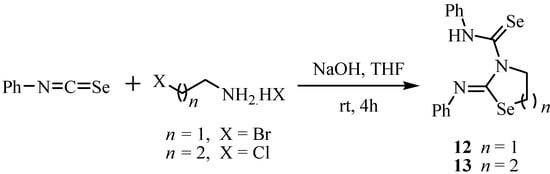
Scheme 5.
Scheme 5.
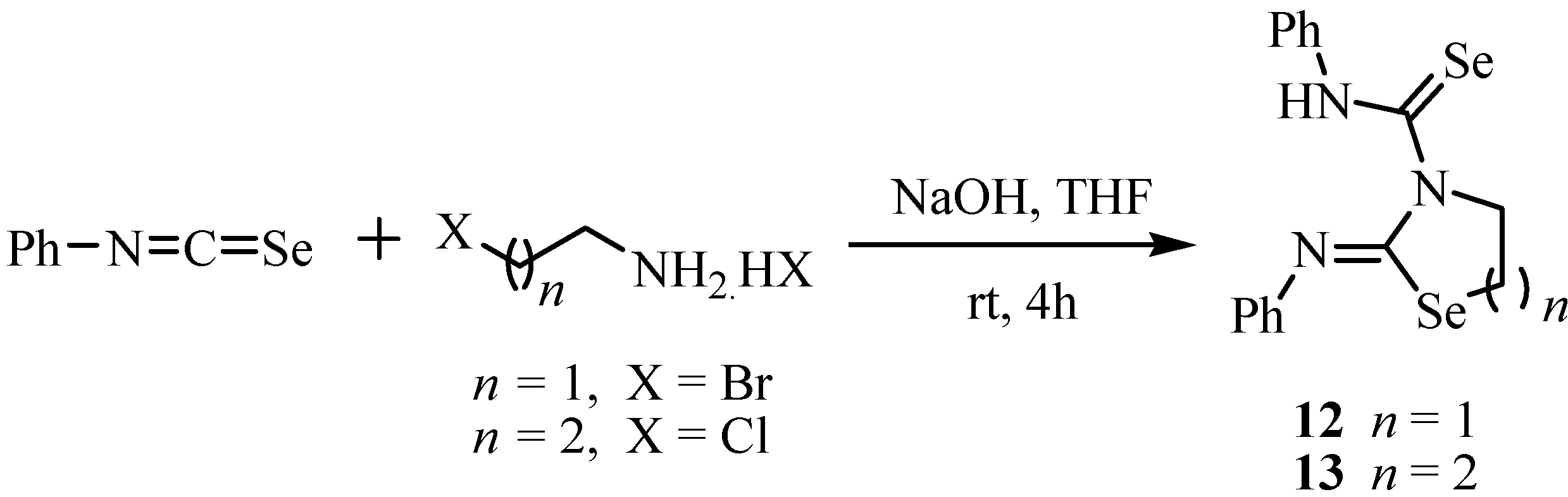
A one-pot synthesis of 2-imino-5-methylene-1,3-selenazolidines in high yields has been achieved by the reaction of alkylisoselenocyanates with propargylamines [25,26]. In these reactions primary amines gave higher yields, as compared to secondary amines (Scheme 6).
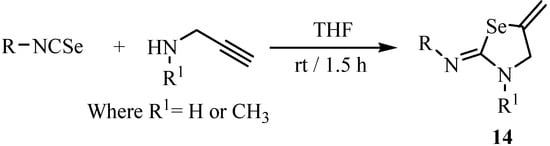
Scheme 6.
Scheme 6.
In the 1H-NMR spectra of 14 in CDCl3, selenium coupling with the H5b proton 3J(77Se-1H) = 23.9 Hz was observed, but the same coupling has not observed with the H5a proton. The H5b proton is more downfield as compared to the H5a proton (Figure 1).
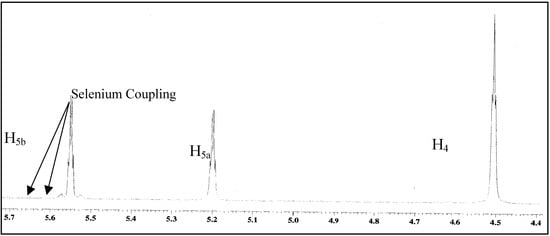
Figure 1.
Figure 1.
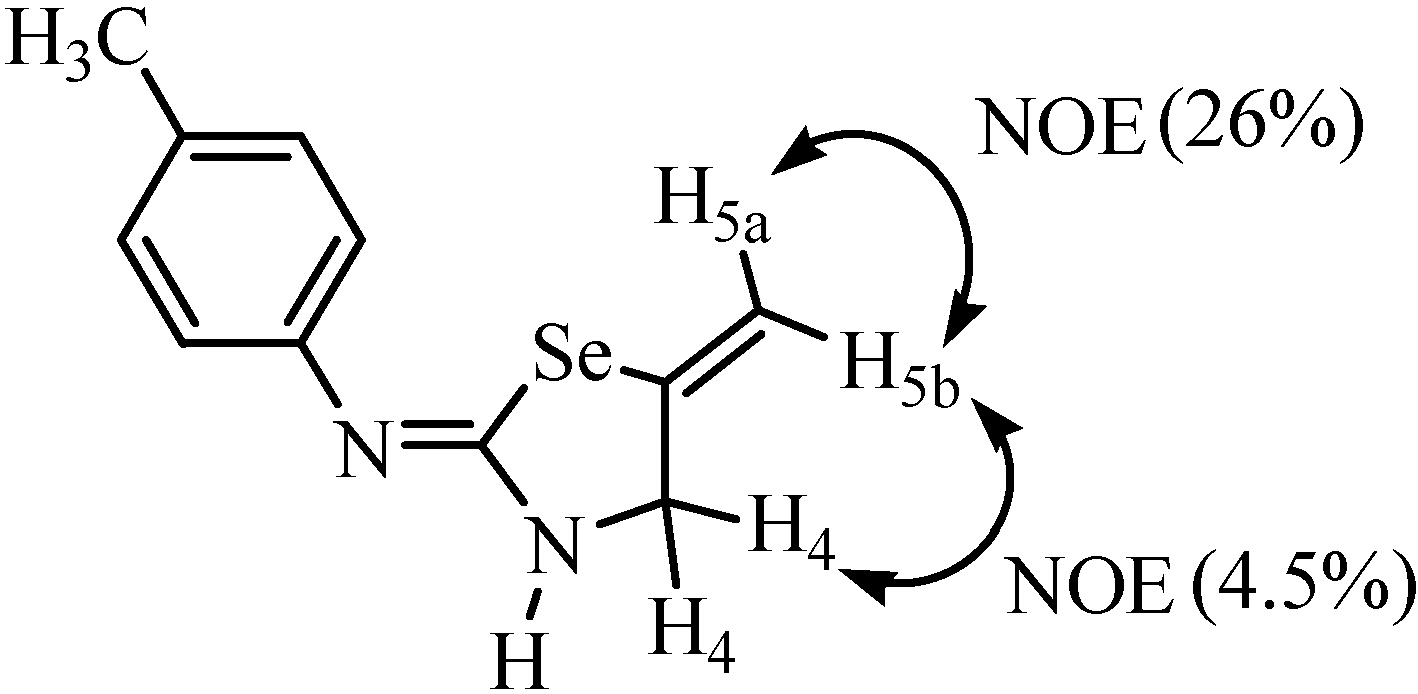
The NOE experiment of compound 14 showed a NOE of H5a proton with H5b (26%) and NOE of the H5b proton with H4 protons (4.5%) (Figure 2). From the above result it was confirmed that selenium shows coupling with the trans proton [26]. This observation is an important aid for determining structures and conformations of organoselenium compounds for which such NMR information is not available.
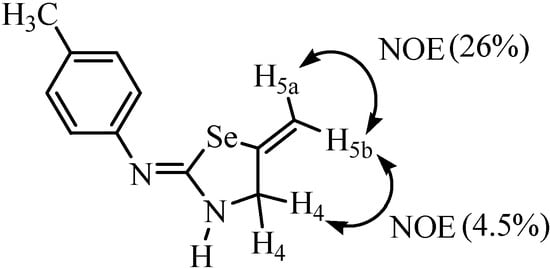
Figure 2.
Figure 2.
The selenazoles 15 were synthesized by the reaction of allenyl isoselenocyanate with a nitrogen-containing nucleophile (Scheme 7) [27]. Due to their pronounced tendency to polymerize, the isoselenocyanates can only be handled in solution. The synthesis of selenazoles 15 shows that allenyl isoselenocyanate reacts distinctly more slowly with nucleophiles than the unusually reactive allenyl isothiocyanate [28].
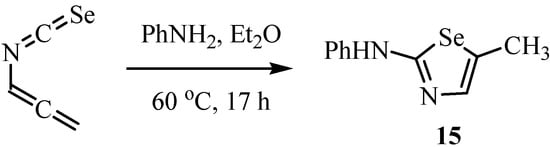
Scheme 7.
Scheme 7.
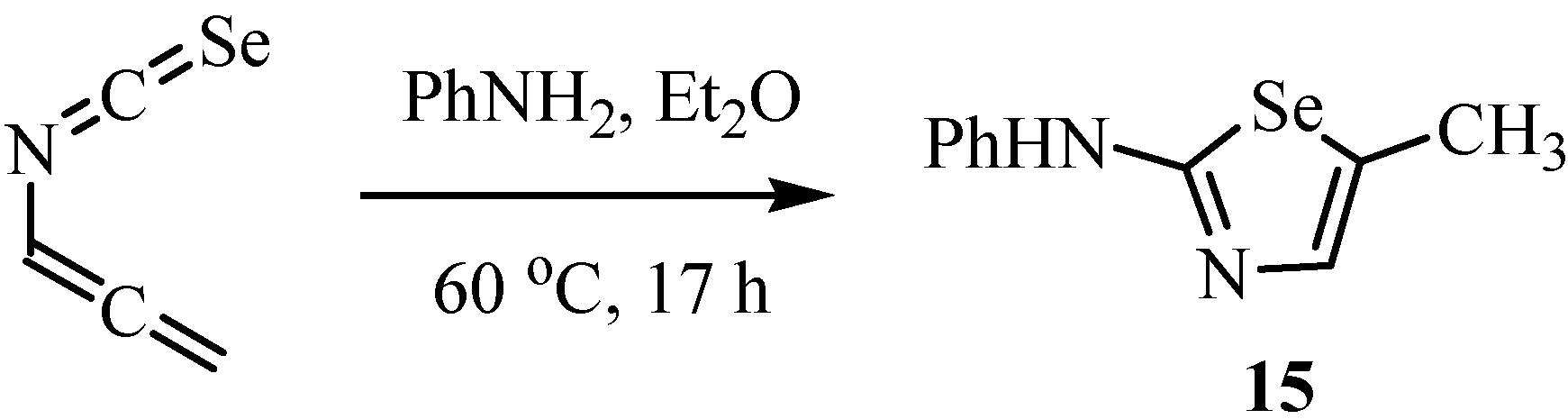
The reaction of chirally pure isoselenocyanate with 28% ammonia aqueous solution in dioxane gave (4R,5R)-5-ethyl-2-imino-4-methylselenazolidine (16, Scheme 8) [29]. The order of inhibitory activity against iNOS of the series of 16 was (4R,5R) > (4S,5S) > (4R,5S) > (4S,5R). Inversion of the R-configuration at the 4-position of 16 to the S-configuration reduced the inhibitory activity against iNOS and nNOS and the selectivity for iNOS. Among the oxazolidines [30], thiazolidines [31] and selenazolidines synthesized so far, compound 16 showed the best selectivity for iNOS (IC50nNOS/IC50iNOS = 85).
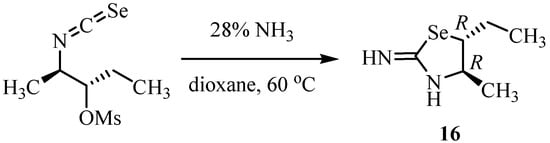
Scheme 8.
Scheme 8.
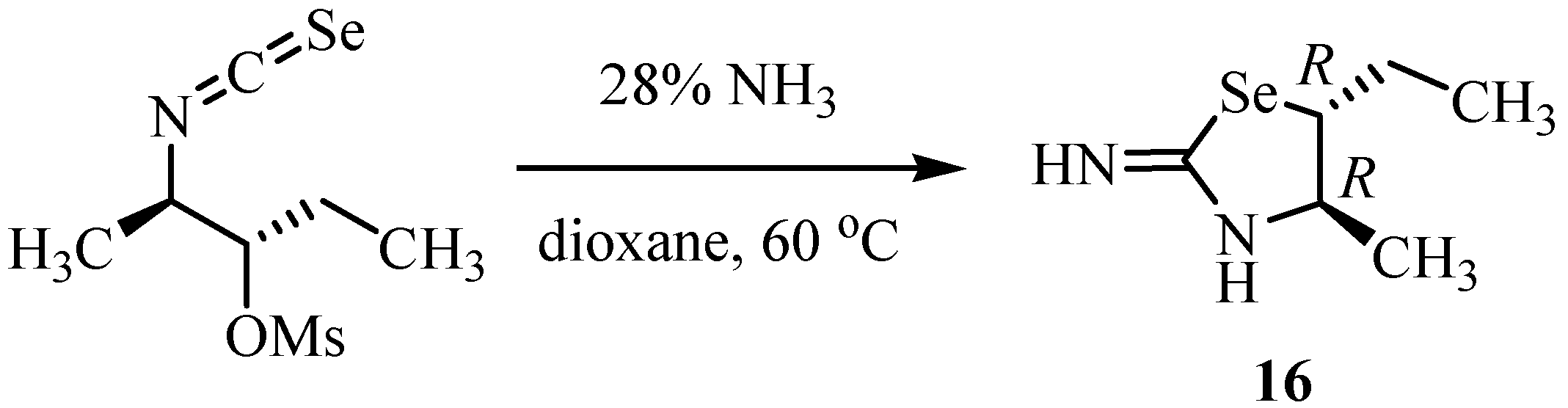
The reaction of N-arylbenzimidoyl isoselenocyanates with primary and secondary amines in acetone at room temperature, followed by treatment with a base, led to 6H-(5,1,3)-benzoselenadiazocine derivatives of type 17 (Scheme 9) [32].
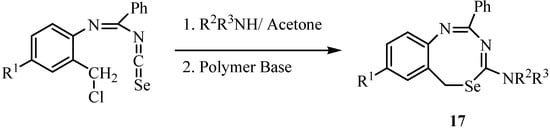
Scheme 9.
Scheme 9.
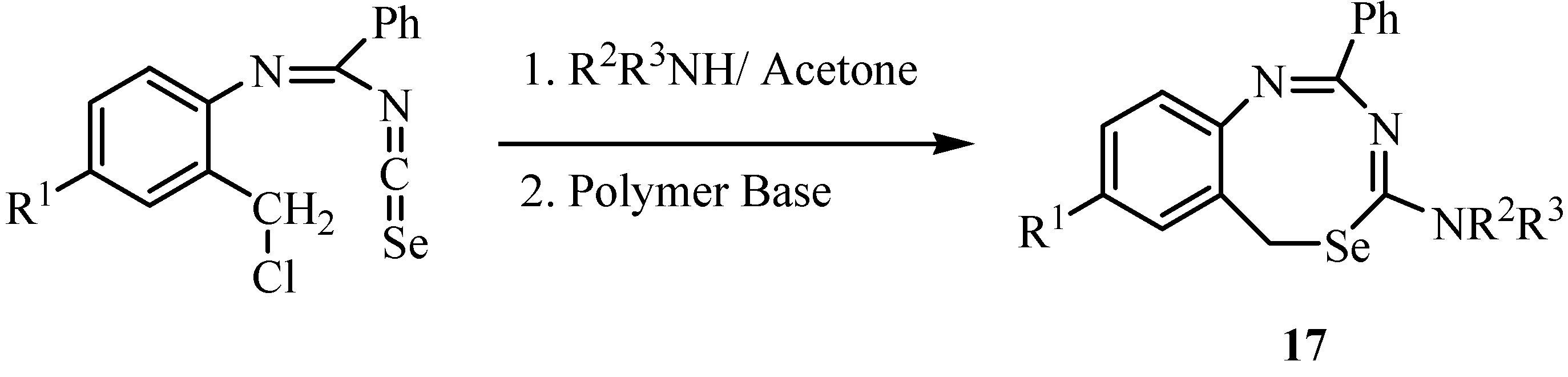
Reaction of isoselenocyanates with methylaminoacetate hydrochloride in the presence of excess of triethylamine afforded selenohydantoins, i.e., 2-selenoxoimidazolidin-4-ones 18 (Scheme 10) [33].
Scheme 10.
Scheme 10.
The reaction of 3-amino-2-chloropyridine with aryl isoselenocyanates in refluxing 2-propanol gave the hydrochlorides of 2-arylaminoselenazolo[5,4-b]pyridines in good yields (Scheme 11) [34]. The free bases 19 were obtained after treatment with aqueous NaOH and recrystallization.
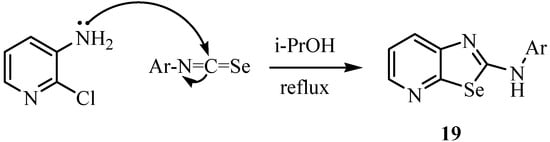
Scheme 11.
Scheme 11.
2-Chloronicotinoyl isoselenocyanate (20) and 2,6-dimethyl-4-chloronicotinoyl isoselenocyanate (21) react with arylamines to give 2-arylimino-4-oxopyrido[3,2-e]-1,3-selenazine (22) and 2-aryl-imino-5,7-dimethyl-4-oxopyrido[3,4-e]-1,3-selenazine (23) (Scheme 12) [35].
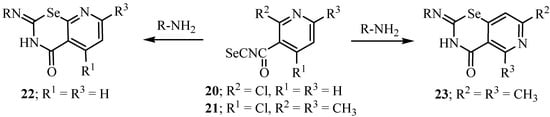
Scheme 12.
Scheme 12.
The reaction of aryl isoselenocyanates with methyl 3-amino-4-chloro-1-ethylpyrrolo[3,2-c]quinoline-2-carboxylate in boiling pyridine leads to tetracyclic selenoheterocycles of type 24 in high yields via an intermediate selenoureido derivative and cyclization via nucleophilic substitution of Cl by Se (Scheme 13) [36].
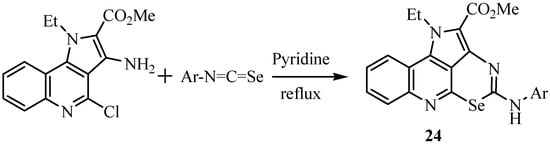
Scheme 13.
Scheme 13.

1-Selenapurine derivatives 25 and 1-substituted 1,6-dihydro-6-imino-9H-purine-2(3H)-chalcogenone (26) were synthesized by the reaction in pyridine of 5(4)-aminoimidazole-4(5)-carbonitrile with various isoselenocyanates (Scheme 14). The outcome of cyclization reactions involving 5(4)-iminoimidazole-4(5)-carbonitrile and isoselenocyanates depends to a remarkable extent on the R portion of the isoselenocyanates. The predominant formation of purine-type products presumably comes from preferential nucleophilic attack on the imino group by the nitrogen atom of the selenoureido intermediate [37].
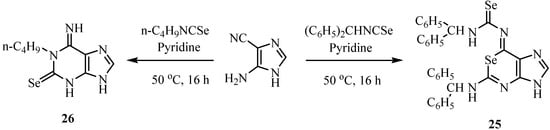
Scheme 14.
Scheme 14.
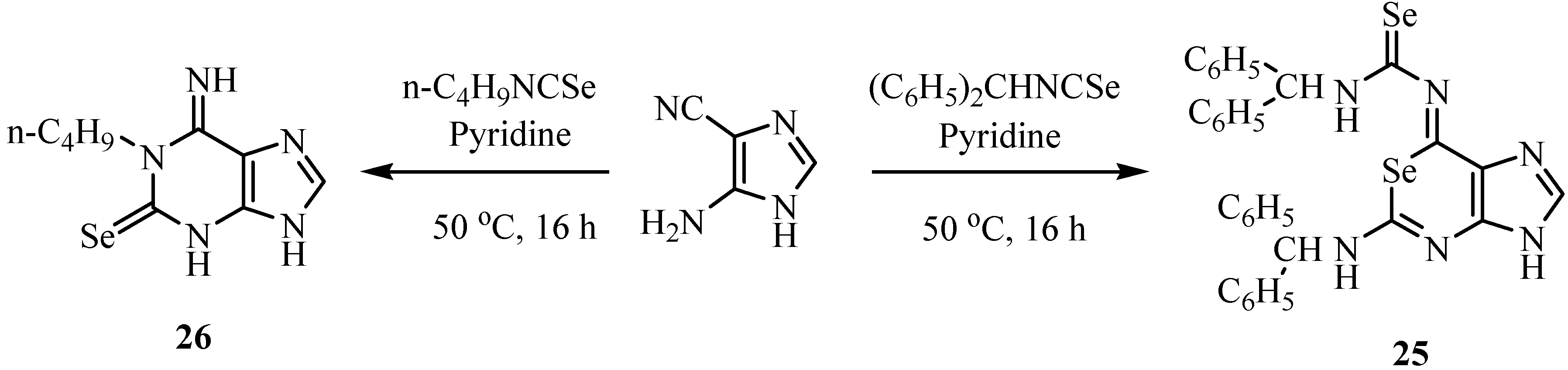
The reaction of anthranilonitriles with phenyl isoselenocyanate in dry pyridine under reflux gave 4-(phenylamino)quinazoline-2(1H)-selones 27 (Scheme 15) [38]. These compounds are easily oxidized and converted to diselenides of type 28. A possible reaction mechanism involving a Dimroth rearrangement of the primarily formed intermediate is presented in Scheme 16.
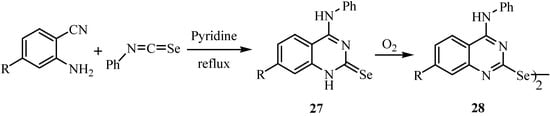
Scheme 15.
Scheme 15.
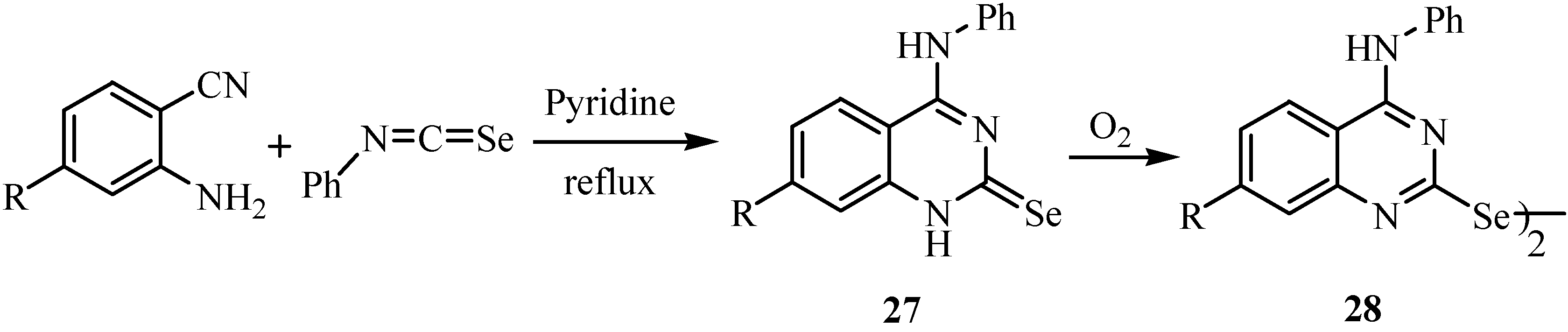
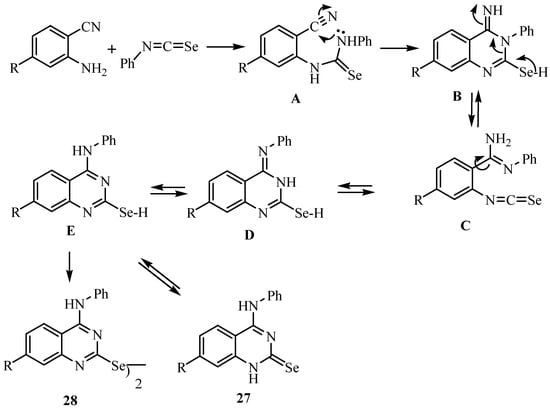
Scheme 16.
Scheme 16.
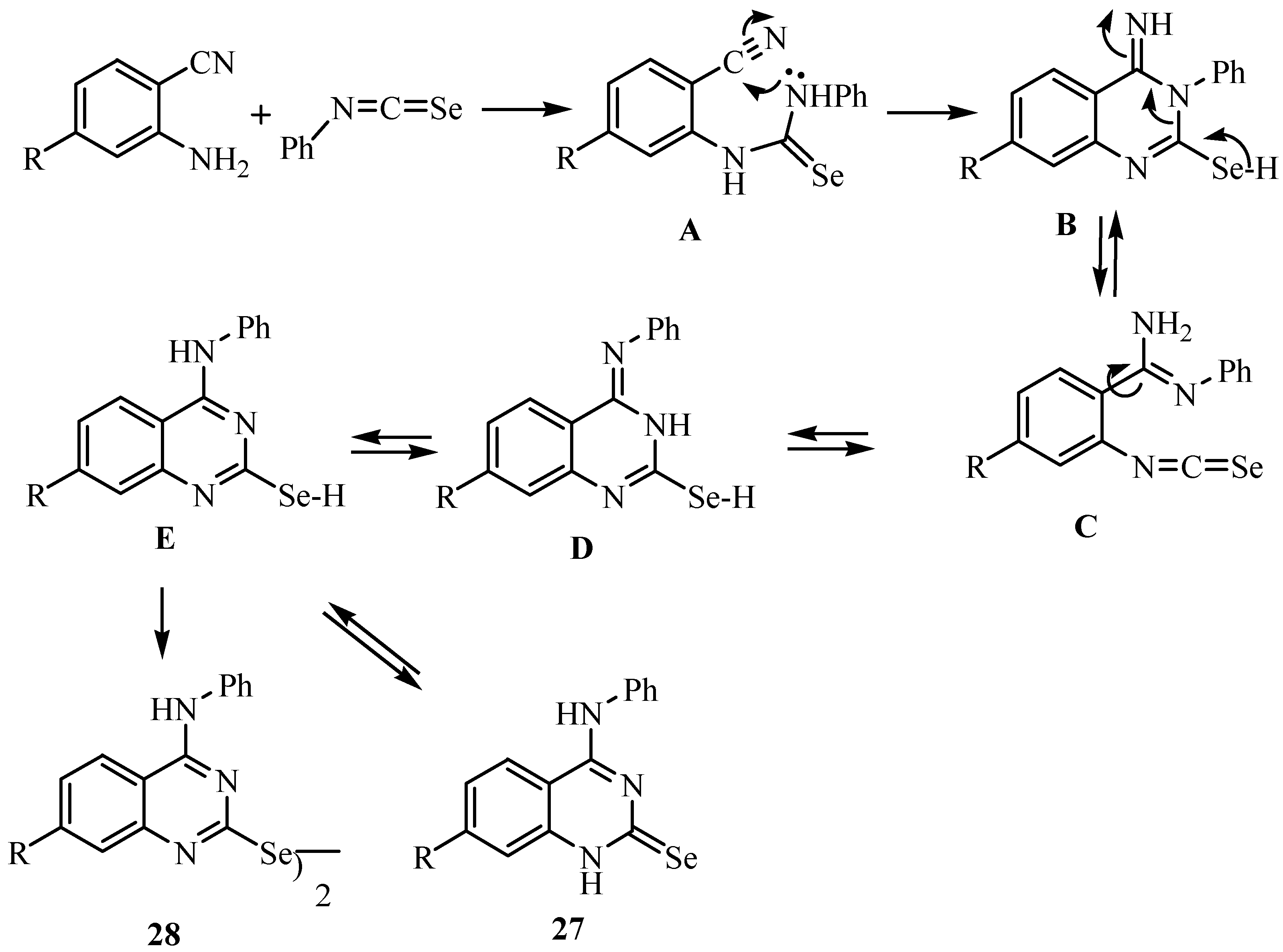
Addition of the NH2 group of anthranilonitriles to phenyl isoselenocyanate leads to the selenourea derivative A, which undergoes a ring closure to give B (or its tautomer). An isomerization via ring opening to C and a new ring closure leads to D, which tautomerizes to give 27 [36], a reaction similar to the Dimroth rearrangement [39]. An analogous isomerization has been reported by Taylor and Ravindranathan [40], who obtained the imino derivative of type B as a stable compound.
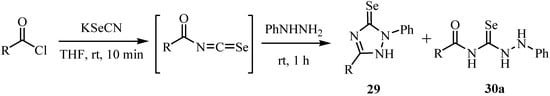
Scheme 17.
Scheme 17.
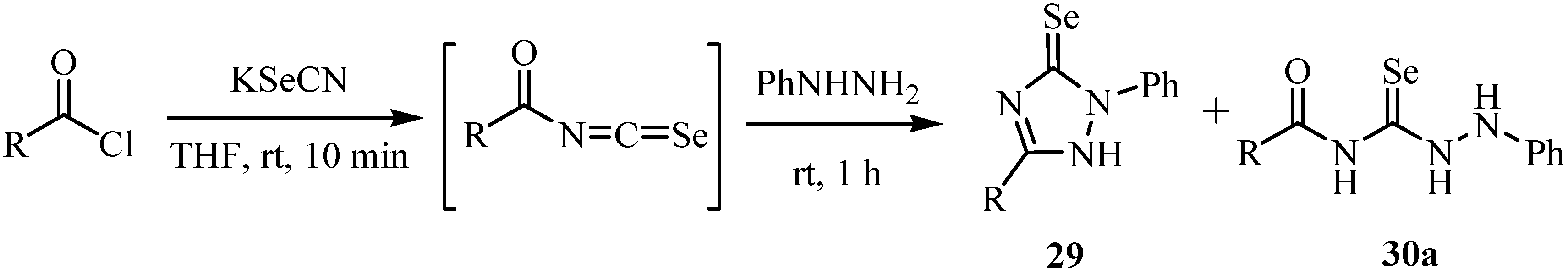
Reactions of the acylisoselenocyanates with phenylhydrazine at room temperature gave 4-acyl-phenylselenosemicarbazides 30a as the major products and 2-phenyl-1,2-dihydro-3H-1,2,4-triazole-3-selones 29 as minor ones, whereas reaction at -80ºC gave selenosemicarbazides in moderate yields (Scheme 17) [41].
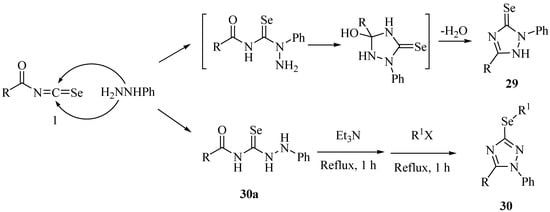
The mechanism of the reaction is as shown in Scheme 18. The formation of 29 is initiated on the nucleophilic addition of the phenylate amine of phenylhydrazine to the isoselenocyanate carbon, affording the 2-phenyl-1,2-dihydro-3H-1,2,4-triazole-3-selones 29, whereas the formation of 30a is initiated by the nucleophilic addition of terminal amine of the phenylhydrazine to the isoselenocyanate carbon, affording 1-phenylselenosemicarbazides 30a. The cyclization of 30a in the presence of triethylamine took place and the product was then trapped by alkyl halides at reflux to afford 3-alkylseleno-1-phenyl-5-p-tolyl-1H-1,2,4-triazoles 30.
Scheme 18.
Scheme 18.
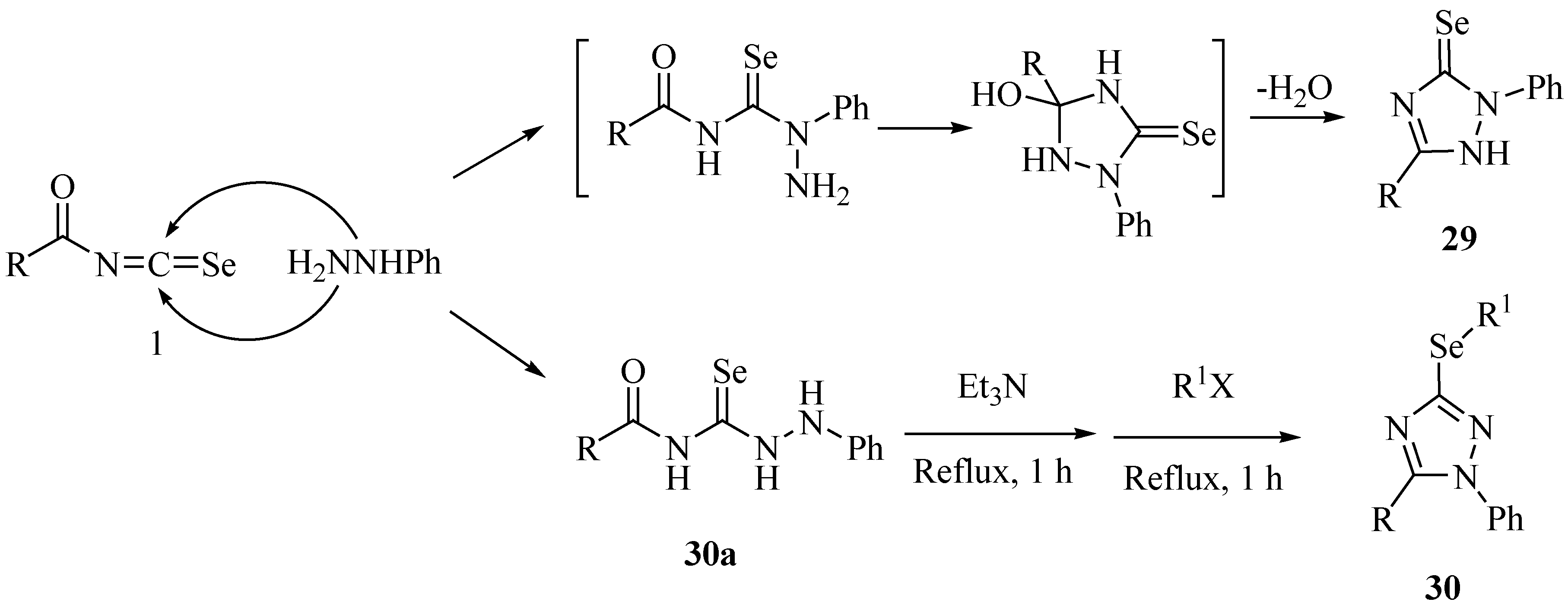
A three component reaction of arylisoselenocyanates, phenacyl halides and hydrazine hydrate resulted in the formation of 1,3,4-selenadiazines 31 in good-to-excellent yields (Scheme 19) [42].
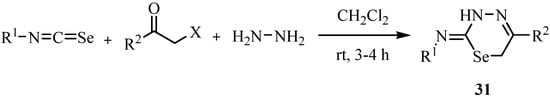
Scheme 19.
Scheme 19.
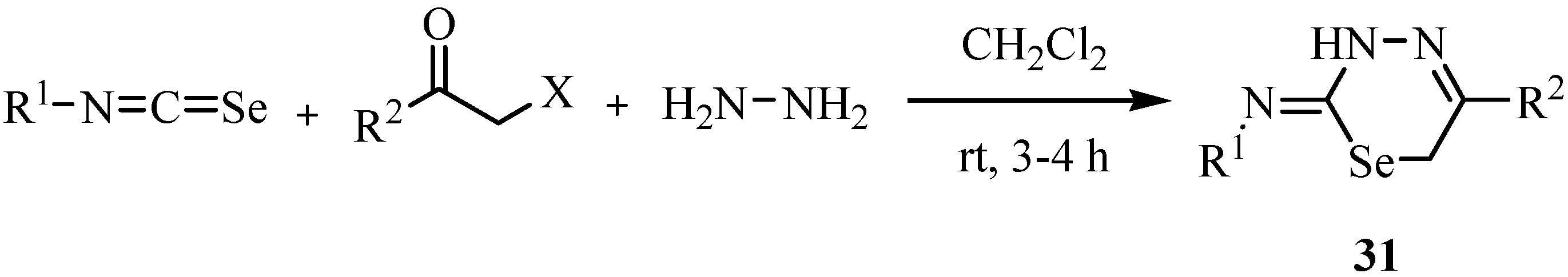
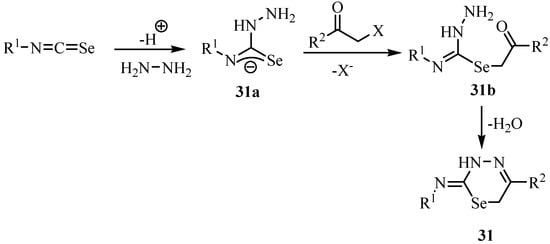
The mechanism of the reaction is shown in Scheme 20. The addition of hydrazine to the isoselenocyanate leads to the adduct 31a, which immediately reacts with the third component to give 31b. Finally, an intramolecular condensation with elimination of H2O, i.e., the formation of a hydrazone, leads to the selenium-containing heterocycles 31.
Scheme 20.
Scheme 20.
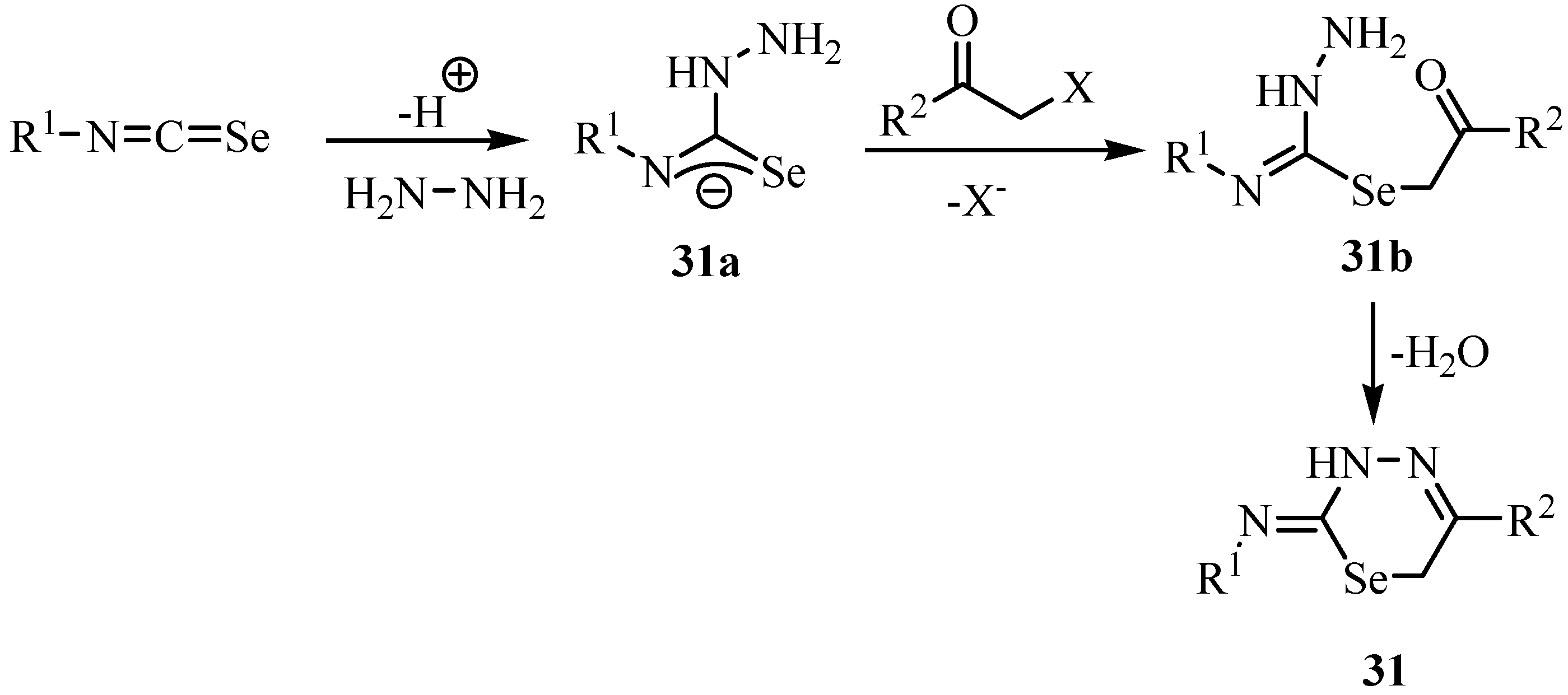
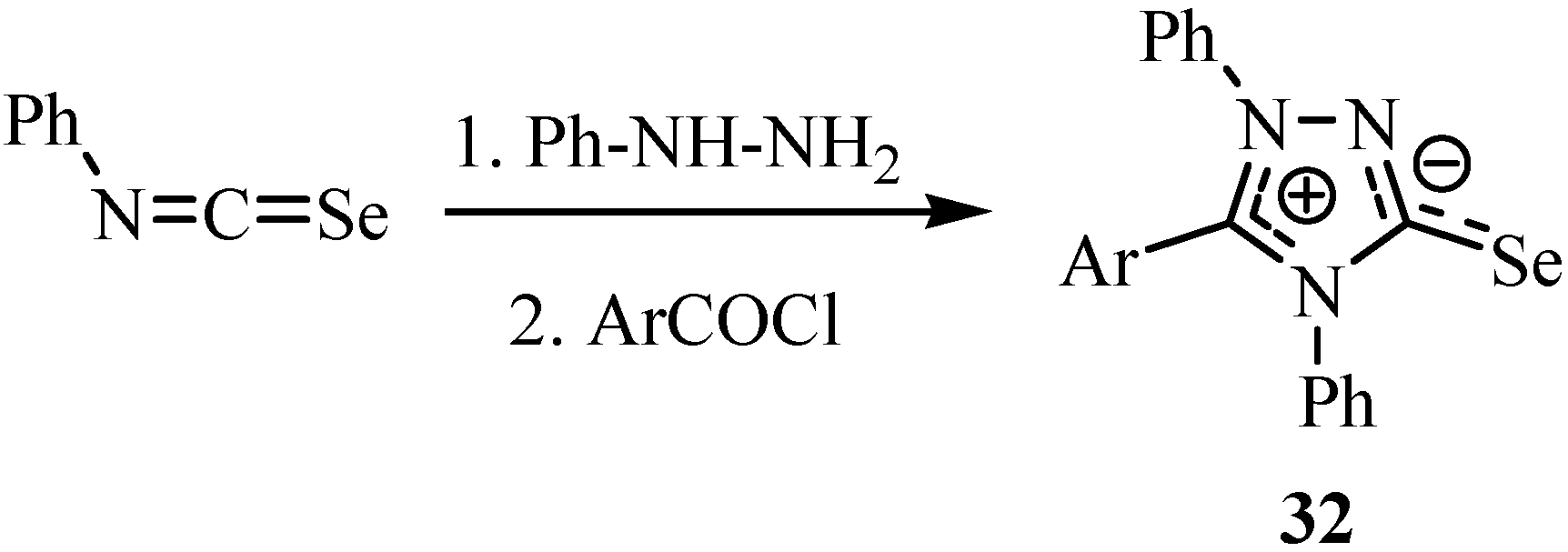
1,3,4-Triazolium-2-selenolates 32 were prepared by the reaction of isoselenocyanates and phenyl hydrazine and then treatment with an aroyl chloride (Scheme 21) [43]. The structures were interpreted according to the comparative method used by Stefaniak et al. [44,45], Bartels-Keith et al. [46] and by Miller and Montanari [47].
Scheme 21.
Scheme 21.
4. Reactions with alcohols and thiols
The reaction of isocyanides with selenium in the presence of DBU gave isoselenocyanates, which then were allowed to react with alk-2-yn-1-ols 33 to give, without purification, the selenium-containing heterocycles 2-imino-4-alkylidene-1,3-oxaselenolanes 34 and 2-selenoxo-1,3-oxolidine 35 (Scheme 22) [48].
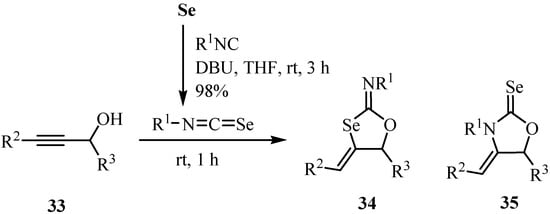
Scheme 22.
Scheme 22.
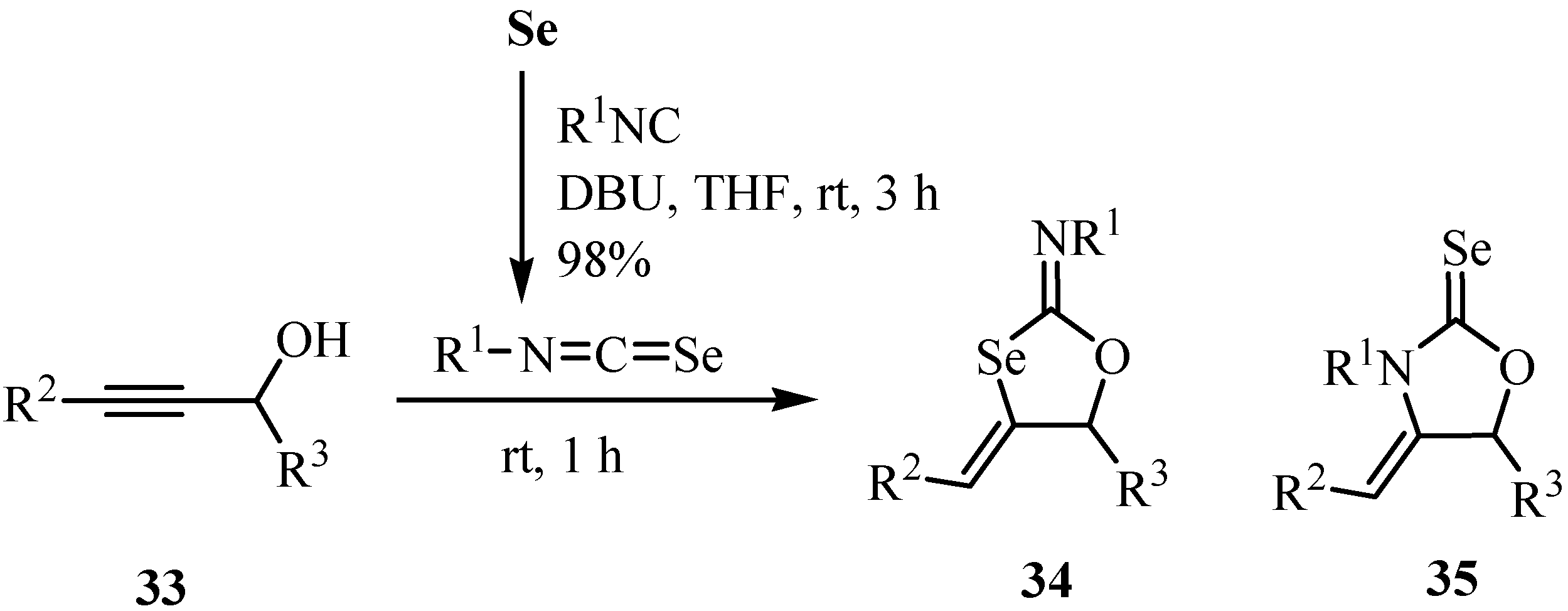
A plausible reaction mechanism for this transformation is shown in Scheme 23. First, alk-2-yn-1-ol 33 undergoes selenoimidoylation by the reaction with selenium and isocyanide to yield oxyimidoylselenoate 36, which underwent intramolecular cycloaddition affording new selenium-containing heterocycles 34. The stereoselectivity of the C=C double bonds of the products can be explained by a trans addition mechanism (36 => 37 => 34), where proton coordination to the carbon-carbon triple bond facilitates nucleophilic addition of selenium to this triple bond from the opposite side. 2-Selenoxo-1,3-oxolidine 35 is formed by the nucleophilic addition of nitrogen to the carbon-carbon triple bond of 36. The product selectivity observed is due to the higher nucleophilicity of the selenium atom. In fact, the product ratio was almost the same when the reaction time was shortened to 1 h. Isolated 34 and 35 were not interconverted under similar reaction conditions.
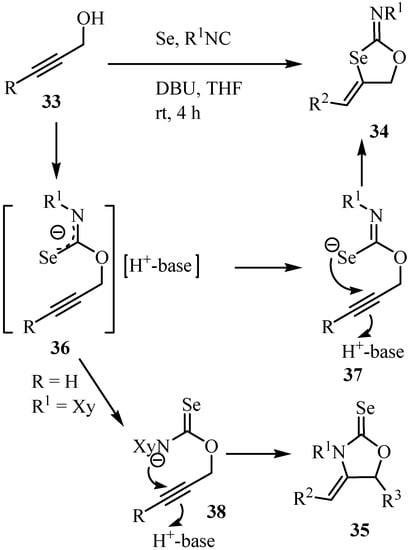
Scheme 23.
Scheme 23.
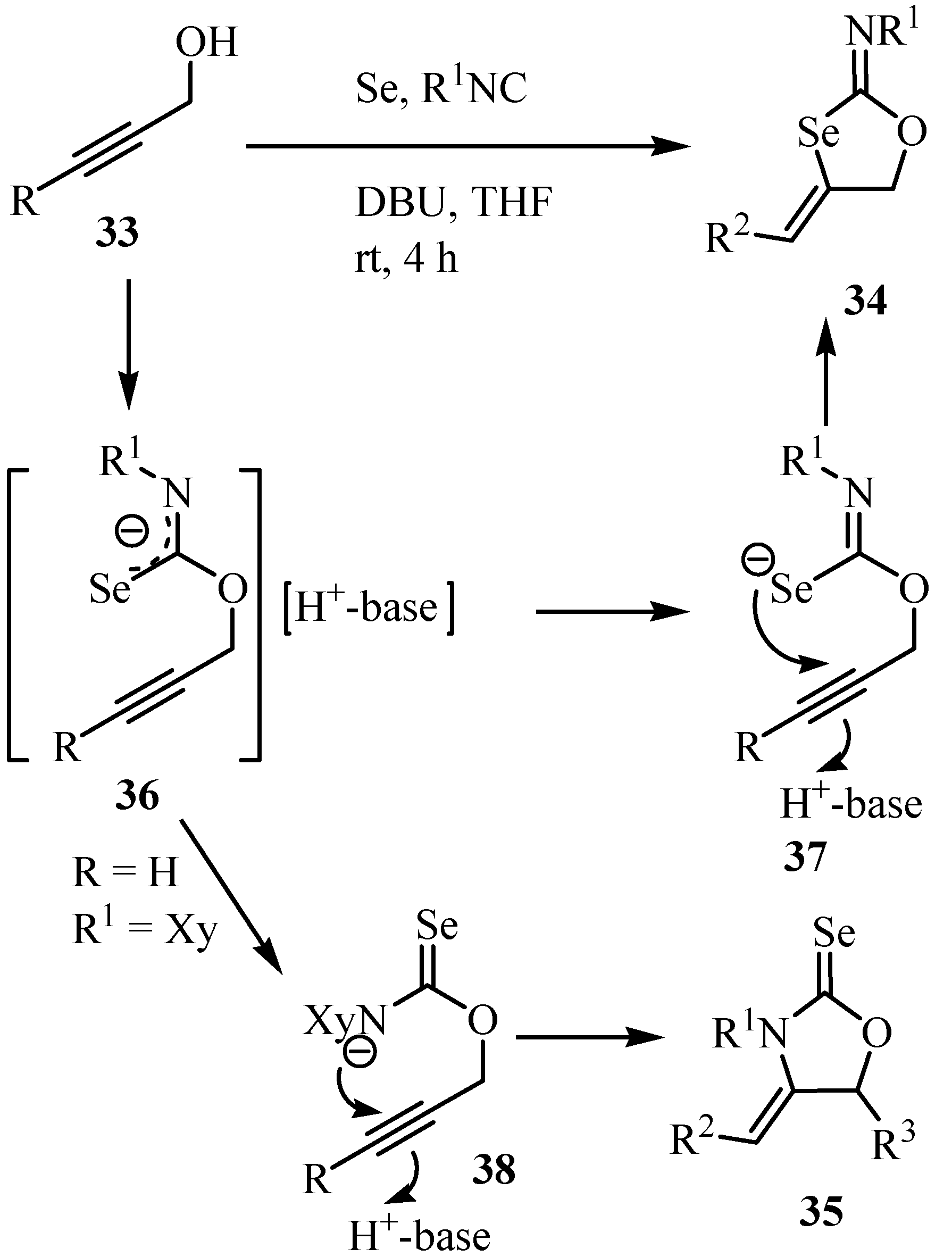
The reaction of but-3-yn-1-ol 39 under similar conditions did not result in the formation of the expected six-membered heterocycle, 4-methylidene-1,3-selenane 40, at all. Since it is known that CuI promotes the intramolecular cyclization of N-propargyl selenocarbamates [49] and O-propargyl thiocarbonates [50] the addition of CuI and subsequent heating of the reaction mixture at reflux afforded 40 in 65% yield (Scheme 24) [48].
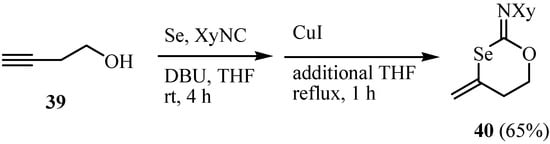
Scheme 24.
Scheme 24.
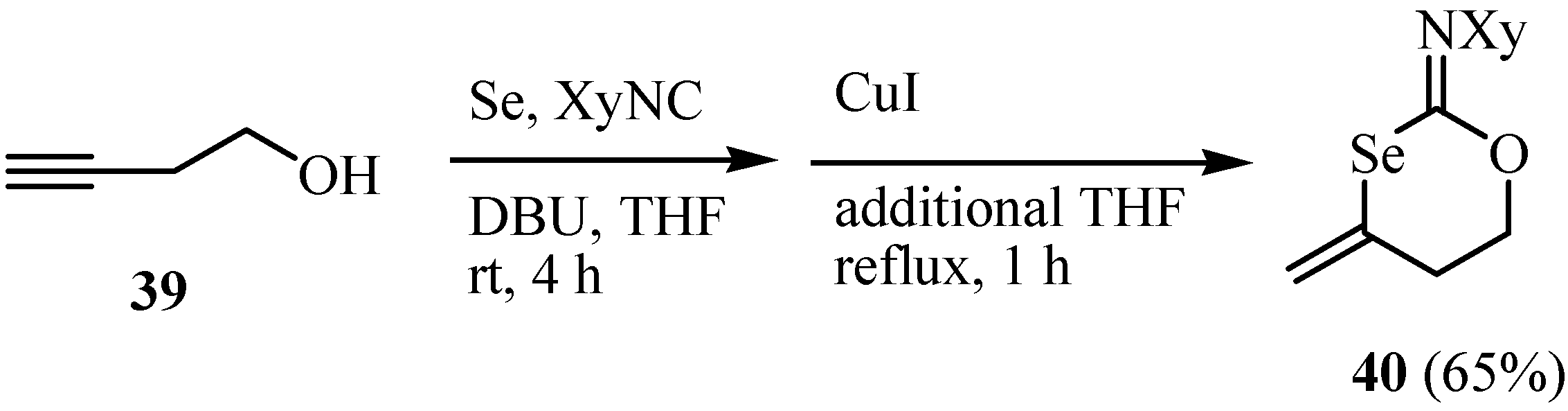
The selenazoles 41, 42 were synthesized by the reaction of allenyl isoselenocyanate with oxygen-containing necleophiles (Scheme 25) [27].
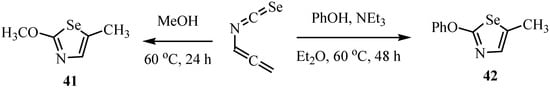
Scheme 25.
Scheme 25.
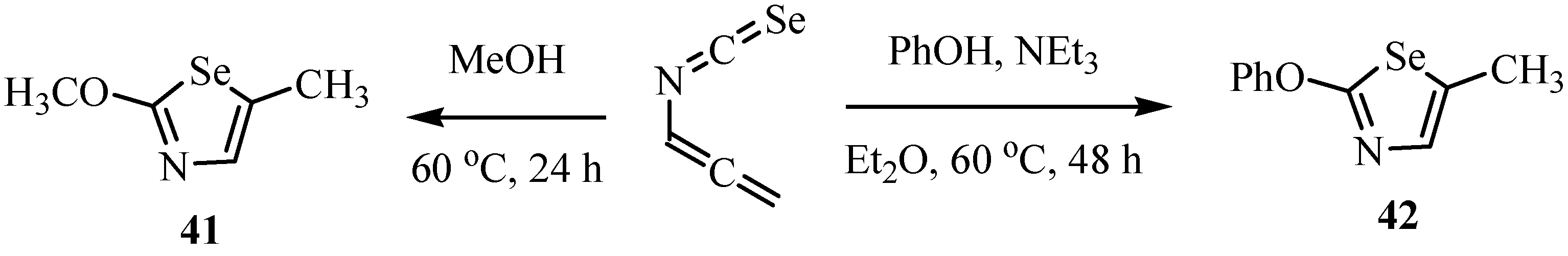
Cleavage of the silyl ether with tetrabutylammonium fluoride (TBAF) in tetrahydrofuran was followed by ring closure to give the (4S, 5R)-(-)-4-methyl-5-phenyloxazolidine-2-selone (43, Scheme 26) [51].
Scheme 26.
Scheme 26.
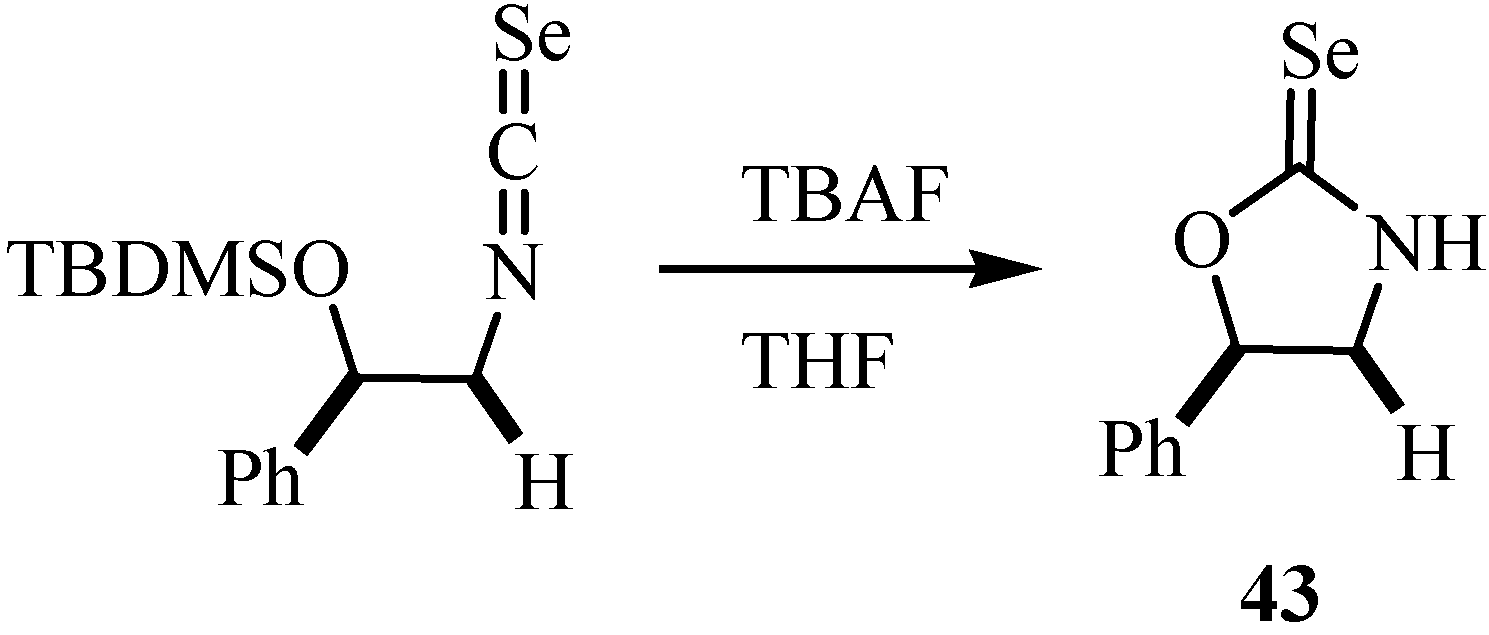
2-Selenoxo-1,3-thiazolidin-4-ones (selenorhodanines) 44 and 2-selenoxo-1,3-thiazinanes 45 were synthesized in a one pot reaction in 60-96% yields from arylisoselenocyanates and α- and β-mercapto carboxylic acids (Scheme 27). No additional base is needed to catalyse the reaction [52].
Scheme 27.
Scheme 27.

2-Chloronicotinoyl isoselenocyanate 20 and 2,6-dimethyl-4-chloronicotinoyl isoselenocyanate (21) react with sodium hydrogen sulfide to afford the respective 2-thioxo-4-oxopyrido-1,3-selenazines 46 and 47 (Scheme 28) [35].
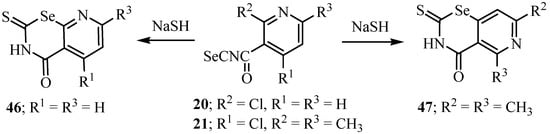
Scheme 28.
Scheme 28.
5. Reactions with selenolates
A reaction of potassium 2-arylethynethiolates 48 with acyl isoselenocyanates, which were prepared in situ from the reaction of acetyl or aroyl chloride with potassium selenocyanate in THF, yielded N-(4-aryl-1,3-thiaselenol-2-ylidene)-amides 50 (Y = S) and the reaction of compound 49 (Y = Se) with acylisoselenocyanates gave N-(4-aryl-1,3-diselenol-2-ylidene)-amides 51 (Y = Se) in high yields (Scheme 29) [53].
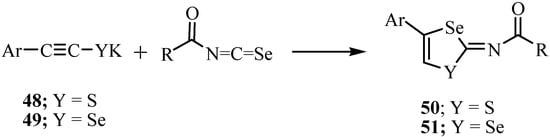
Scheme 29.
Scheme 29.
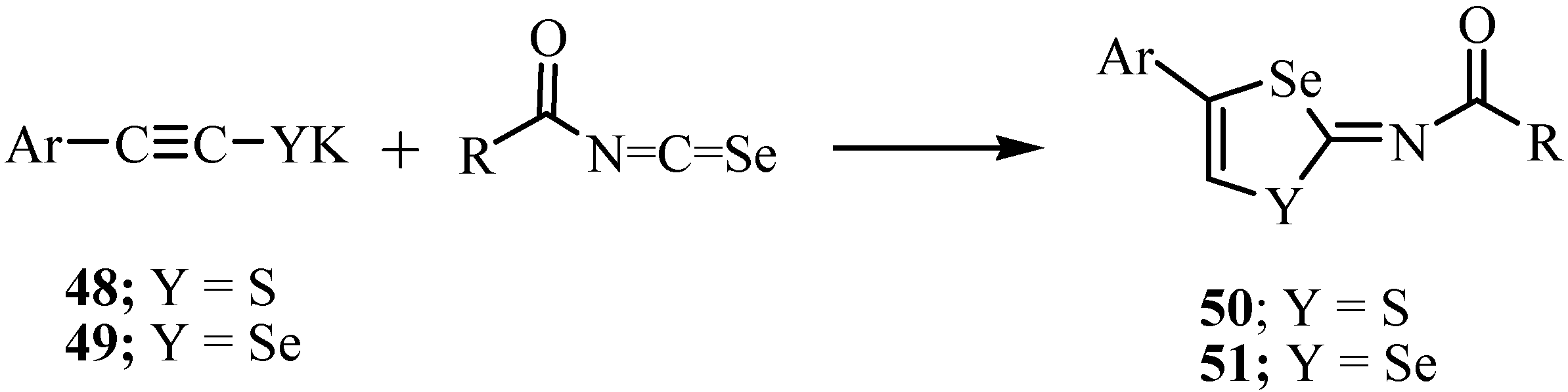
Potassium 2-aryl- and 2-alkylethyneselenolates 53, obtained by the decomposition of 4-substituted-1,2,3-selenadiazoles, participate in a cyclization reaction with isoselenocyanates to afford 2-aryl alkylimino-1,3-diselenoles 55 [54,55], whereas the reaction of potassium 2-aryl- and 2-alkylethynethiolates 52 with isoselenocyanates yielded 2-arylimino-1,3-thiaselenole, 54 (Scheme 30) [55].
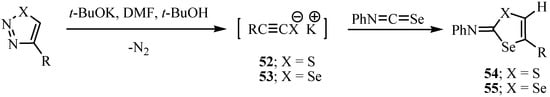
Scheme 30.
Scheme 30.
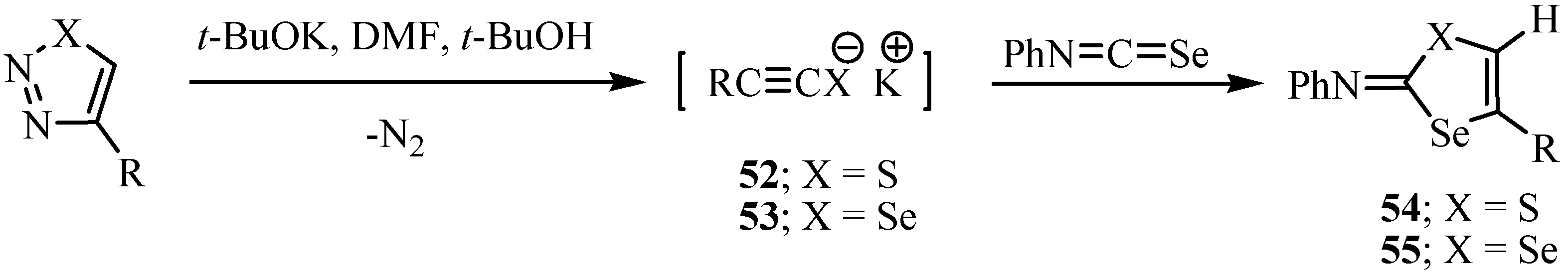
The reaction of allenyl isoselenocyanate with seleno-containing nucleophiles gave selenazoles 56 (Scheme 31) [27].
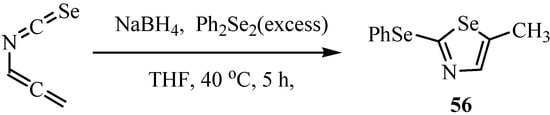
Scheme 31.
Scheme 31.
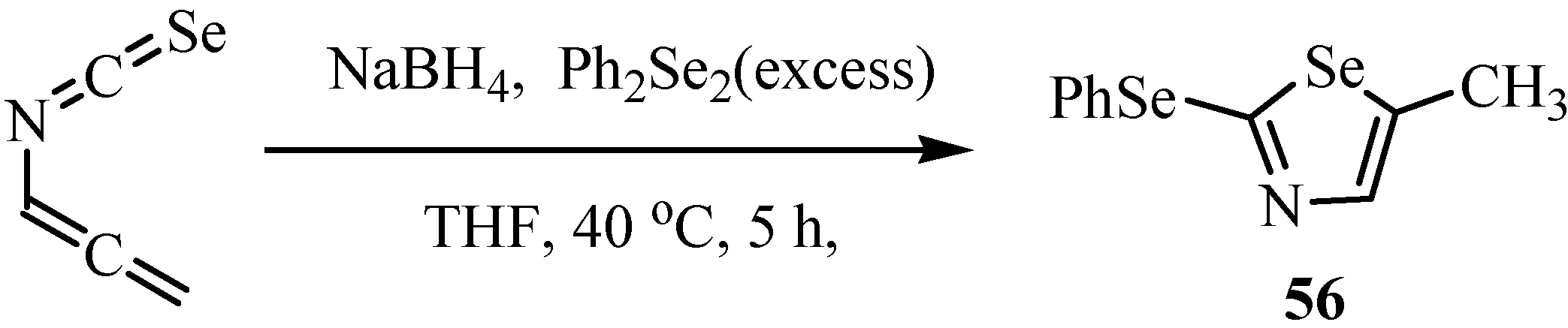
The reaction of 20 and 21 with sodium hydroselenide afforded the respective 2-selenoxo-4-oxopyrido-1,3-selenazines 57 and 58 (Scheme 32) [35].
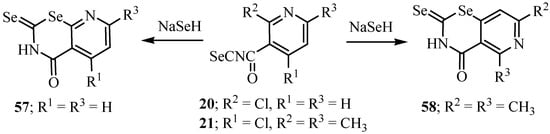
Scheme 32.
Scheme 32.
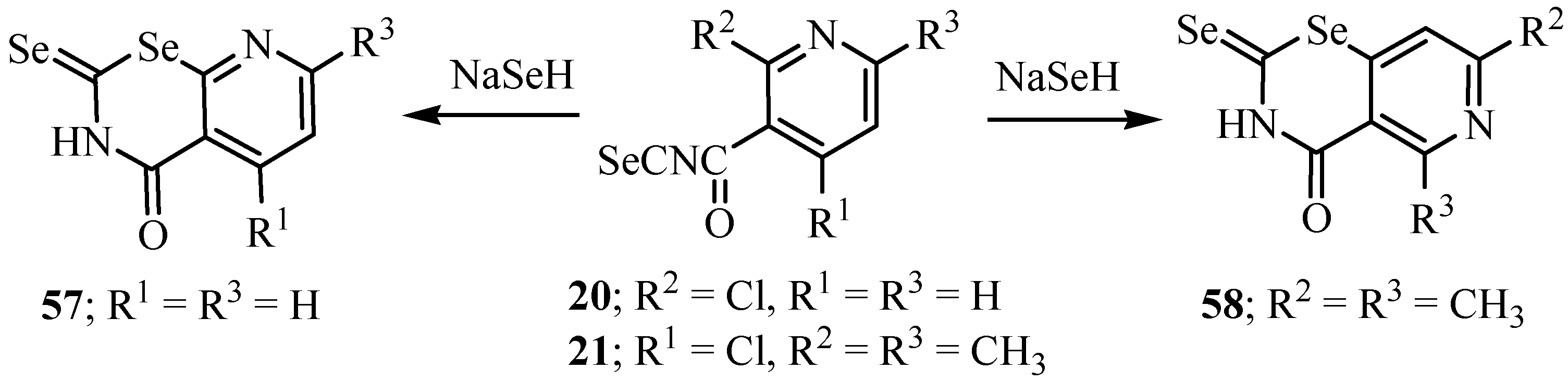
1,3-Selenazines and 1,3-selenzoles have been prepared from isoselenocyanates via diseleno-carbamate intermediates [56]. The acryloyl isoselenocyanates were generated in situ by reaction of an α,β-unsaturated acyl chloride with potassium selenocyanate. The treatment of substituted acryloyl isoselenocyanate with sodium hydroselenide gave 2-selenoxoperhydro-1,3-selenazin-4-ones 59 (Scheme 33) [56].
Scheme 33.
Scheme 33.
Selenium-nitrogen-containing five membered heterocycles were similarly synthesized. An intermediate N-alkyl diselenocarbamate reacted with bromoacetyl bromides to afford 3-alkyl- or 3-aryl-2-selenoxo-1,3-selenazolidine-4-ones 60 in 10-37% yields. The corresponding diselenides were also obtained (Scheme 34) [56].
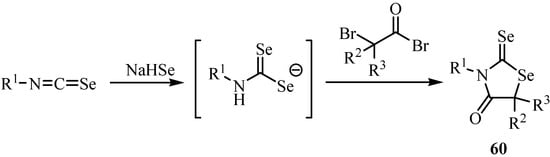
Scheme 34.
Scheme 34.
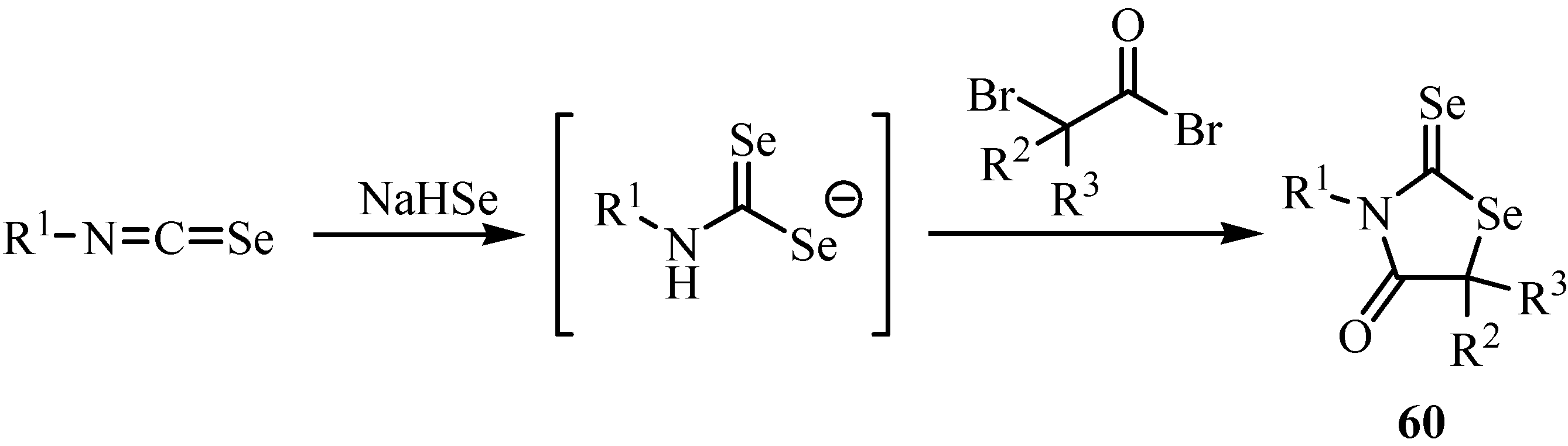
Phenyl isoselenocyanates undergo nucleophilic addition of sodium hydrogen selenide in the presence of a salt of a primary amine and formaldehyde to form 3,5-disubstituted tetrahydro-1,3,5-selenodiazine-2-selenones 61 (Scheme 35) [57]. The best yields were obtained in the case when phenyl isoselenocyanates containing an electron-accepting substituent were used for the addition-cyclization reaction.
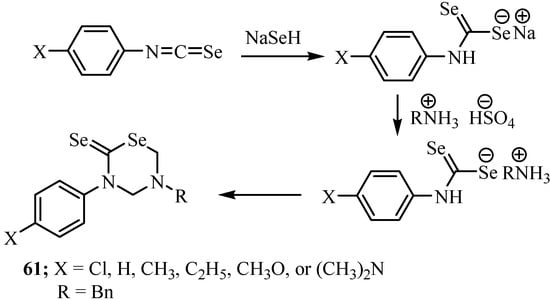
Scheme 35.
Scheme 35.
6. Reactions with carbanions
The carbanion obtained from malononitrile (62a) and triethylamine was reacted in DMF with isoselenocyanates to give an intermediate of type 63. The latter reacted with 1,2-dibromoethane (64) to give another intermediate 65, which cyclized to yield 1,3-selenazolidine derivatives of type 66. Similar reactions were performed starting with ethyl cyanoacetate (62b). Only one isomer was obtained in the case of the cyanoacetates (Scheme 36) [58].
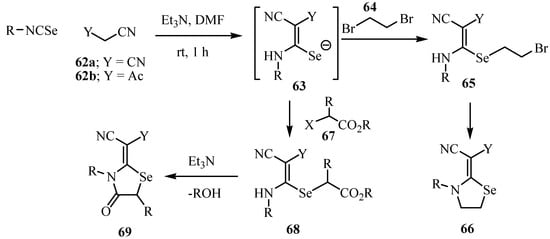
Scheme 36.
Scheme 36.
The analogous reaction of isoselenocyanates, 62a, and methyl 2-chloroacetate (67) gave the intermediate 68, which on subsequent condensation by elimination of alcohol yields 2-(4-oxo-1,3-selenazolidin-2-ylidene)malononitriles 69.
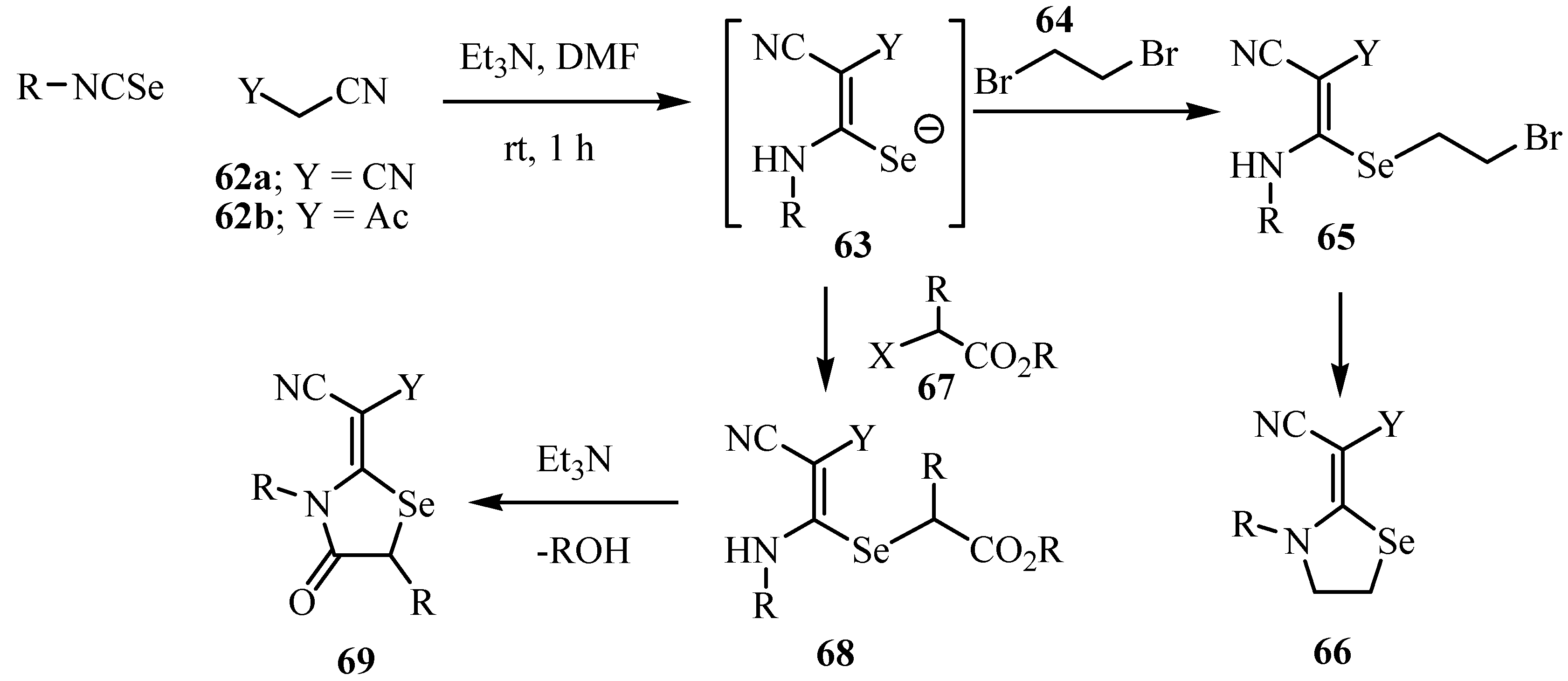
Treatment of the intermediates 63 with bromoacetyl bromide (70) led to the formation of a single product, 4-oxo-1,3-selenazolidine (73). As the reaction between thioureas and acyl halides is known to give S-acylated isothioureas [59], the formation of the 4-oxo-1,3-selenazolidine derivatives 73 in the reactions with 2-bromoacetyl bromide 70 can be explained by the reaction mechanism shown in Scheme 37.
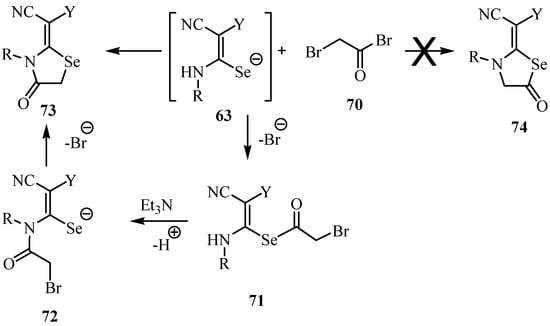
Scheme 37.
Scheme 37.

The intermediate 71, which is formed by the nucleophilic substitution of the acyl bromide of 70 by the Se-atom of 63 undergoes a base catalyzed 1,3-acyl shift to give the rearranged intermediate 72. Similar S/N migrations of the acetyl group are known and have been studied in depth kinetically [60] and were described recently by Pihlaja and coworkers [61]. Finally, the Se-atom attacks the α-carbon atom of the amide group and forms the 1,3-selenazolidinone ring by displacing the bromide ion to give 73.
An analogous cyclization was observed when 75 were reacted with the Na salt of diethyl malonate in EtOH at room temperature to yield the eight-membered selenaheterocycles 76 (Scheme 38). The crystal structure of the 76 revealed the presence of a co-crystal comprising two compounds [32].
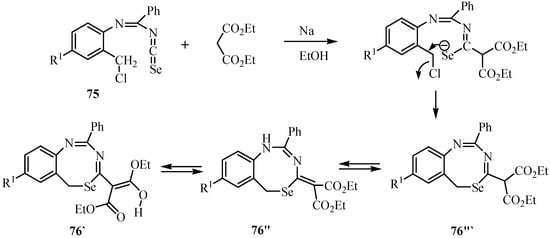
Scheme 38.
Scheme 38.
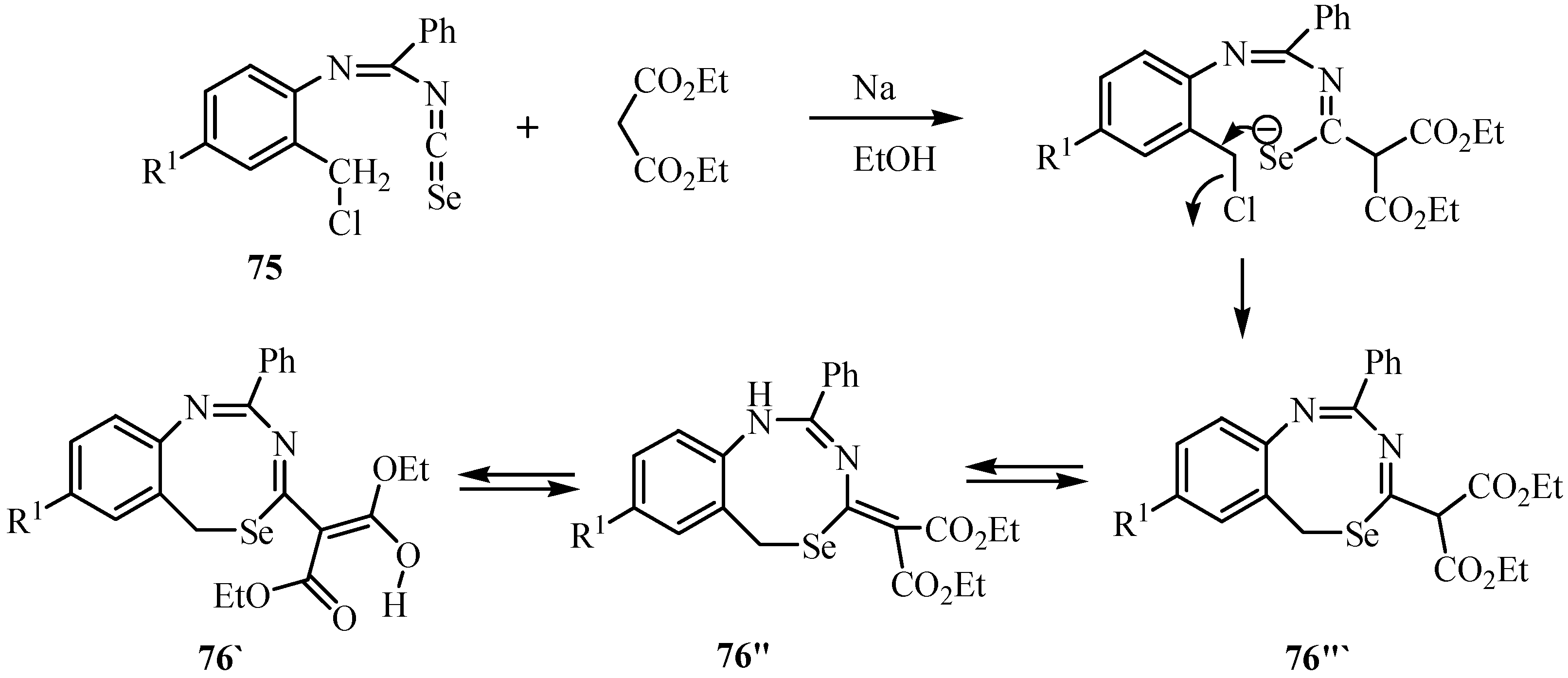
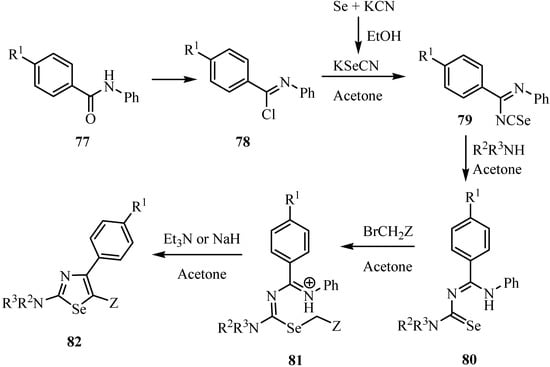
The reaction of N-phenylbenzamides 77 with excess SOCl2 under reflux gave N-phenylbenimidoyl chlorides 78, which on treatment with KeSeCN in acetone yielded imidoyl isoselenocyanates of type 79. These were transformed into selenourea derivatives 80 by the reaction with NH3, primary or secondary amines. In acetone at room temperature, 80 reacted with activated bromomethylene compounds such as 2-bromoacetates, acetamides, and acetonitriles, as well as phenacyl bromides and 4-cyanobenzyl bromides, to give 1,3-selenazol-2-amines of type 82 (Scheme 39) [62]. A reaction mechanism via alkylation of Se-atom of 80, followed by ring closure and elimination of anilines, is most likely [63].
Scheme 39.
Scheme 39.
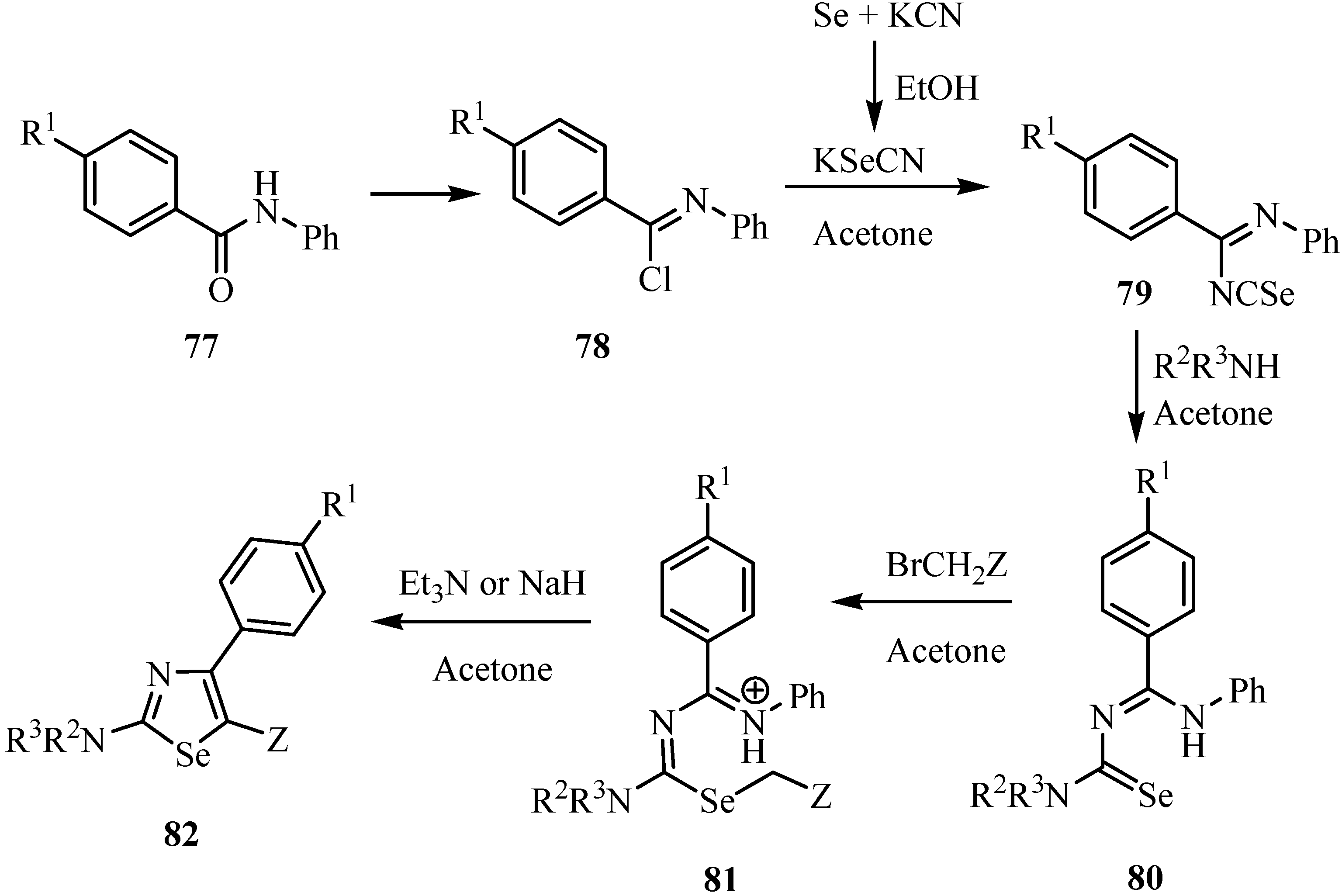
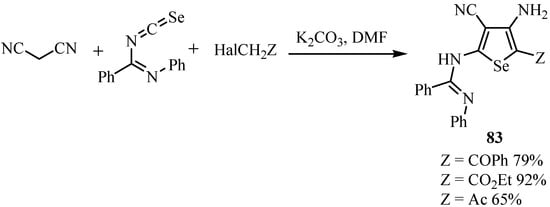
The condensation of benzoyliminoisoselenocyanate in basic media with malononitrile and halides allowed the preparation of aminoselenophenes 83 (Scheme 40) [64].
Scheme 40.
Scheme 40.
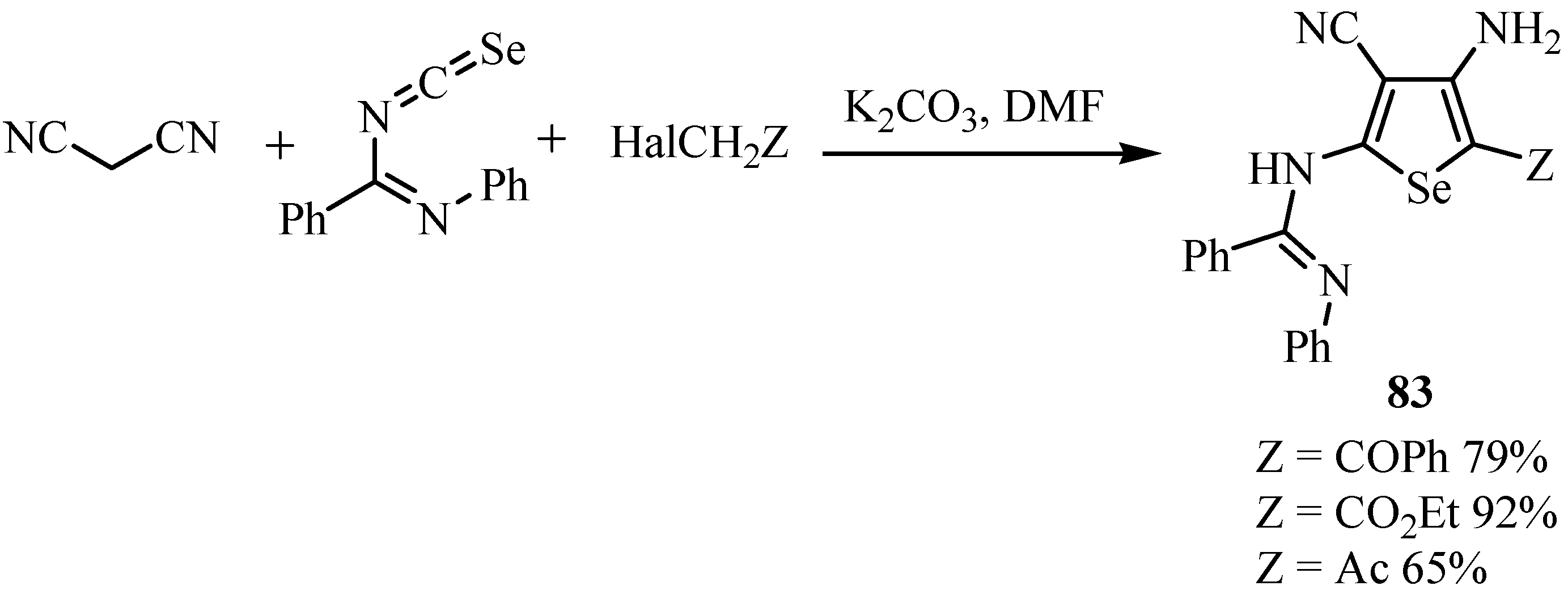
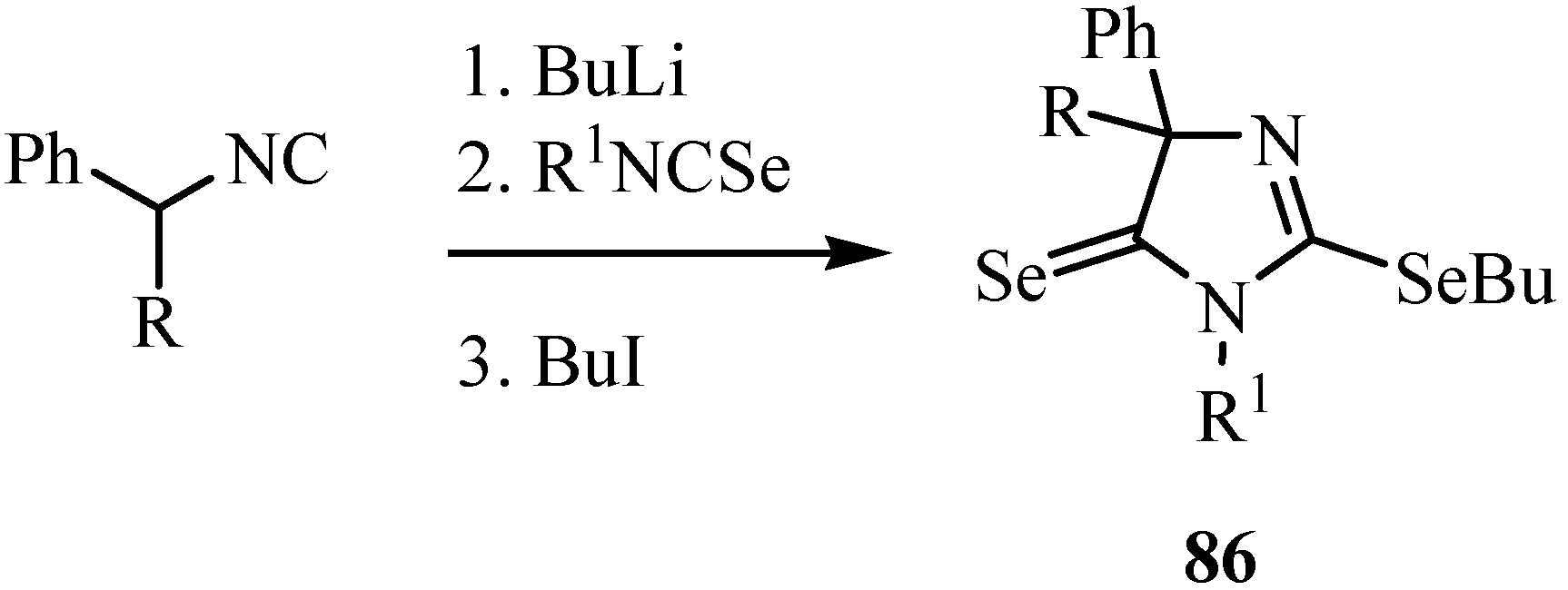
1,3-Selenazoles and 2-imidazolin-5-selones were synthesized by the reaction of isoselenocyanates with α-lithiated isocyanides 84 [65]. Isocyanides having only one substituent on the α-carbon, such as ethyl isocyanoacetate and benzyl isocyanide, gave 1,3-selenazoles 85 in good yields (Scheme 41). On the other hand, α,α-disubstituted isocyanides such as α-methylbenzyl isocyanide and diphenylmethyl isocyanide afforded 2-butylseleno-2-imidazolin-5-selones 86 after trapping with butyl iodide. The latter products were formed from one molecule of isocyanide and two molecules of isoselenocyanate (Scheme 42) [65].
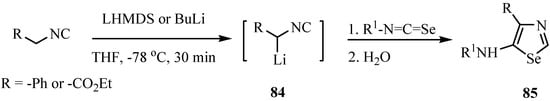
Scheme 41.
Scheme 41.
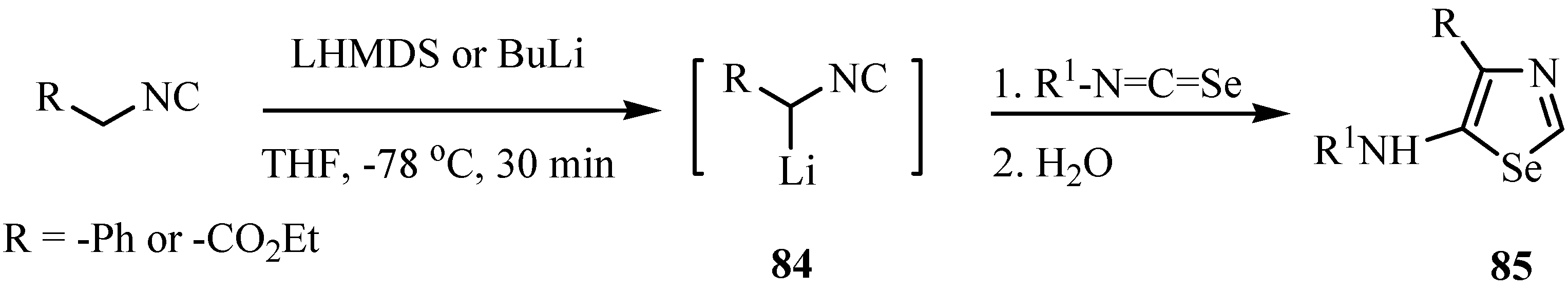
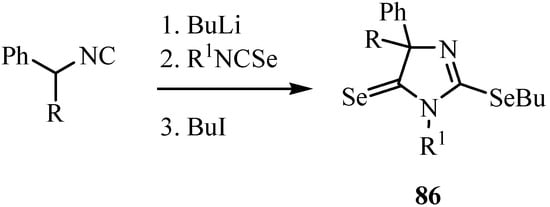
Scheme 42.
Scheme 42.
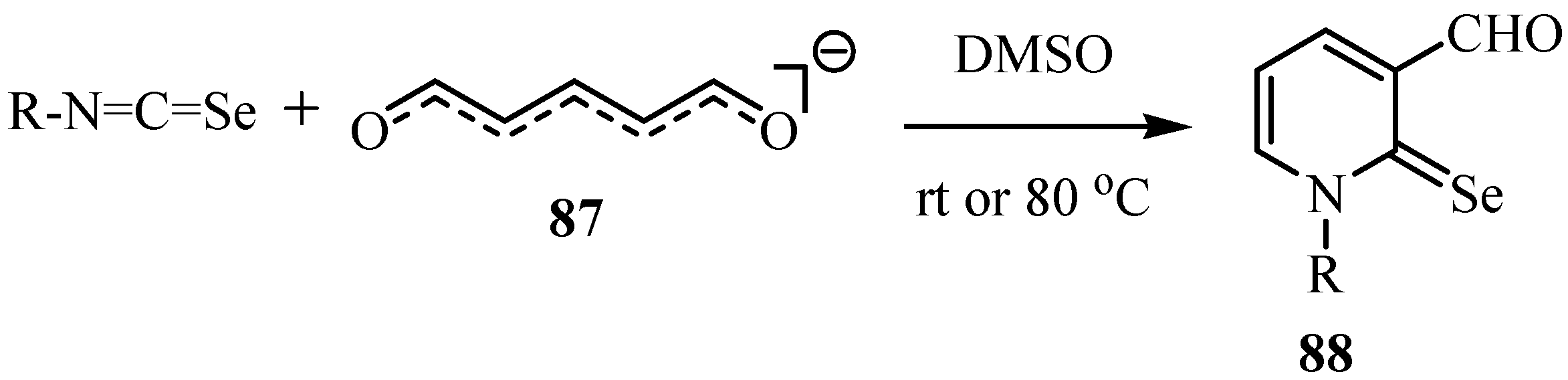
Isoselenocyanates reacts with glutacondialdehyde anion (87) to give 1-substituted-3-formyl-2(1H)-pyridineselones 88. The aryl isoselenocyanates reacted readily with 87 at room temperature, whereas the reaction with alkyl isoselenocyanates required an elevated temperature (80 °C) (Scheme 43) [66].
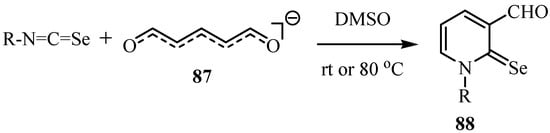
Scheme 43.
Scheme 43.
7. Reactions with azodicaboxylates and diazomethanes
The reaction of isoselenocyanates with diethyl azodicarboxylate (89) and triphenylphosphine under Mitsunobu conditions was reported by Heimgartner et al. [67]. A mixture of an azodicarboxylate and triphenylphosphine in dichloromethane reacted with arylisoselenocyanates at room temperature to give 4,5-dihydro-5-selenoxo-1H-1,2,4-triazole-1-carboxylates 90 in a one-pot reaction in good to excellent yields (Scheme 44) [67].
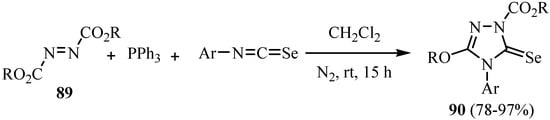
Scheme 44.
Scheme 44.
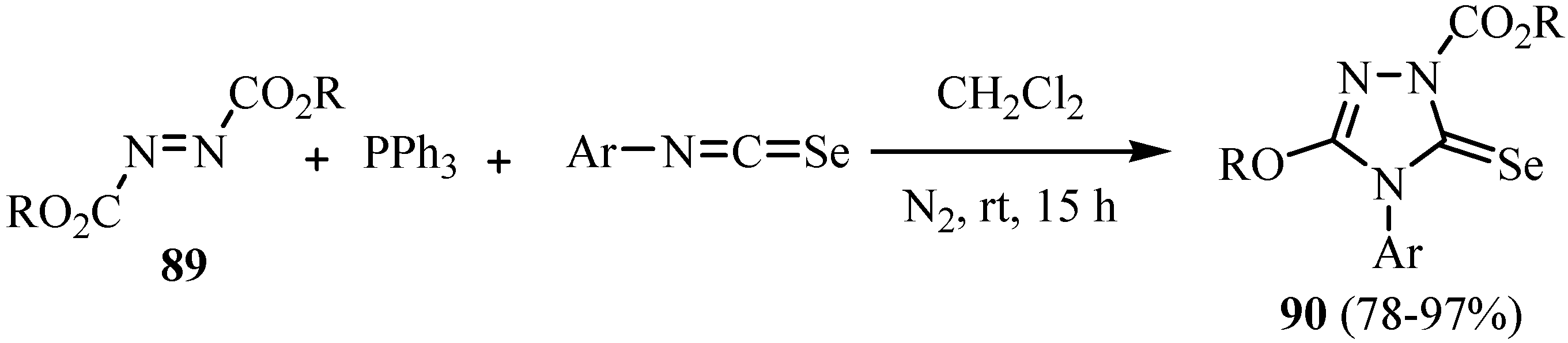
The reaction mechanism for the formation of 90 is shown in Scheme 45. The addition of Ph3P to the azodicarboxylate 89 generates the zwitterion 91, which, as a nucleophile, attacks the isoseleno-cyanate to give 92. Ring closure by nucleophilic addition of the N-atom at the ester group leads to 93, and elimination of Ph3PO via the intermediate 94 yields the product 90. The use of the diethyl azodicarboxylate (oxidant)/Ph3P (reducing agent) system is well established [68] and is known as the Mitsunobu reaction [69] when the reactant is an alcohol. The betaine 91 is the initially formed intermediate in all cases and it reacts with the alcohol. In the present case, this intermediate reacts as a nucleophile with the strongly electrophilic isoselenocyanate.
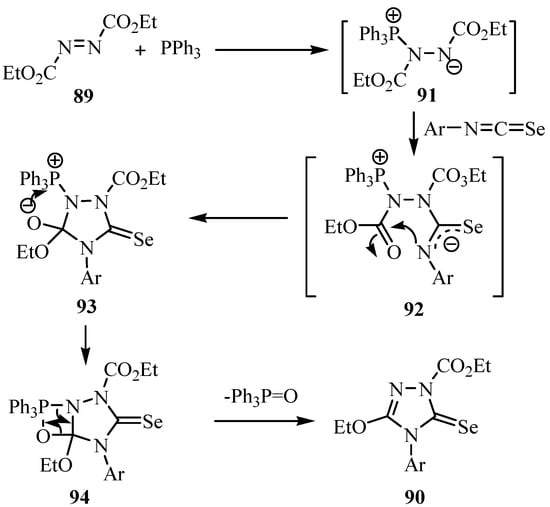
Scheme 45.
Scheme 45.
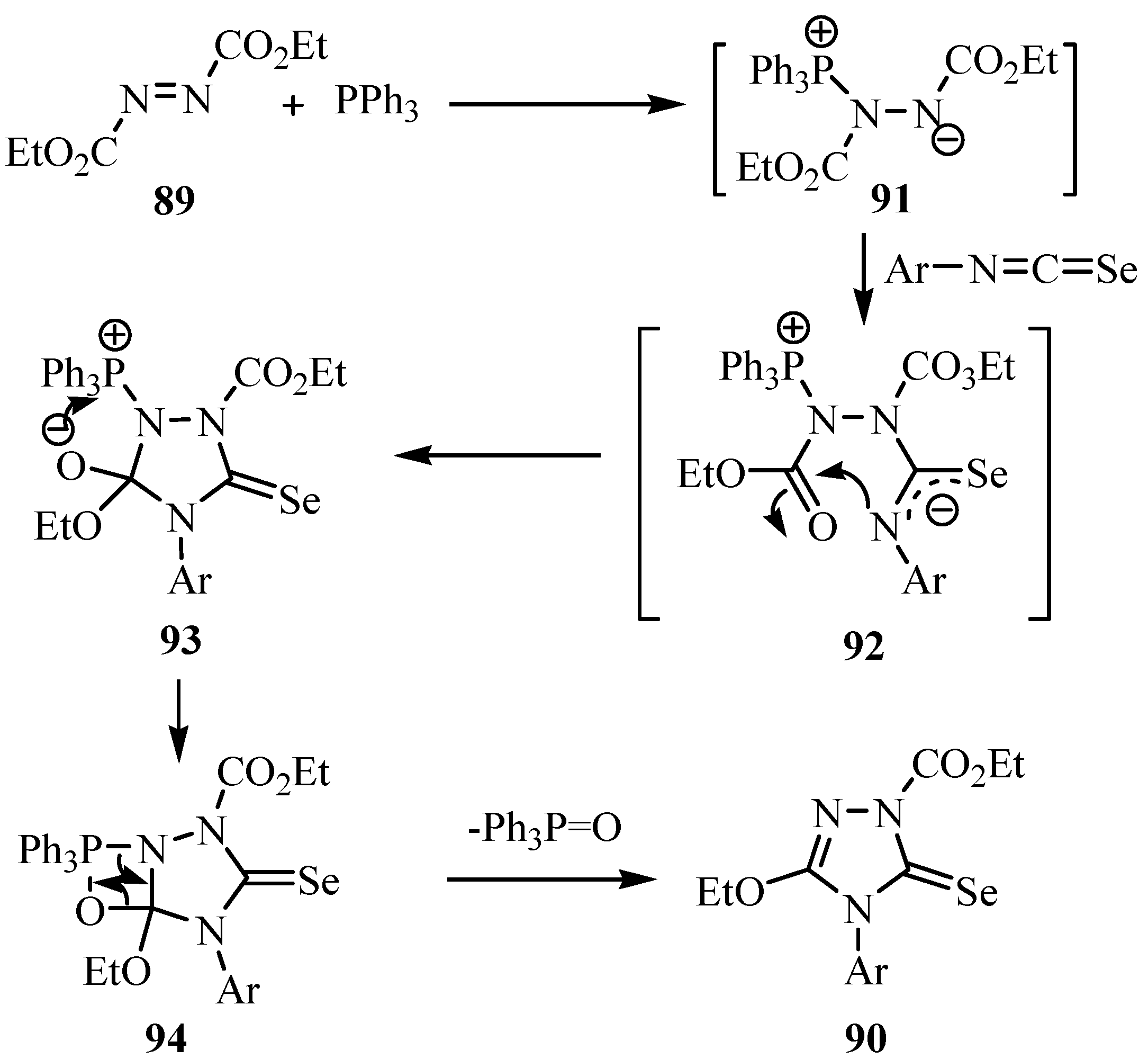
On treatment of the crude acylisoselenocyanates 2a, prepared in situ by the reaction of benzoyl chloride (1a) and KSeCN, with diphenyldiazomethane, L´Abbé et al. obtained benzoselenophene 95 in 27% yield [70]. A mechanism for its formation via cycloadduct A, elimination of N2 to give zwitterion B (or the corresponding biradical), ring closure and aromatization to C, and tautomerization to yield 95 is shown in Scheme 46.
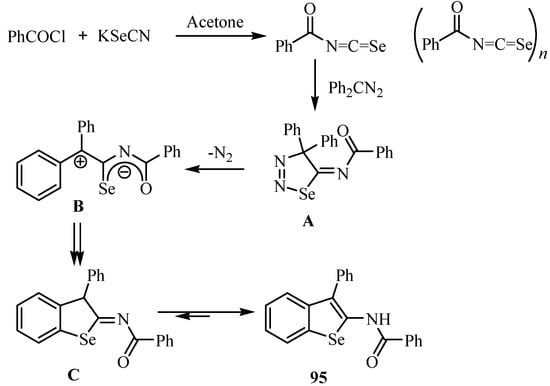
Scheme 46.
Scheme 46.
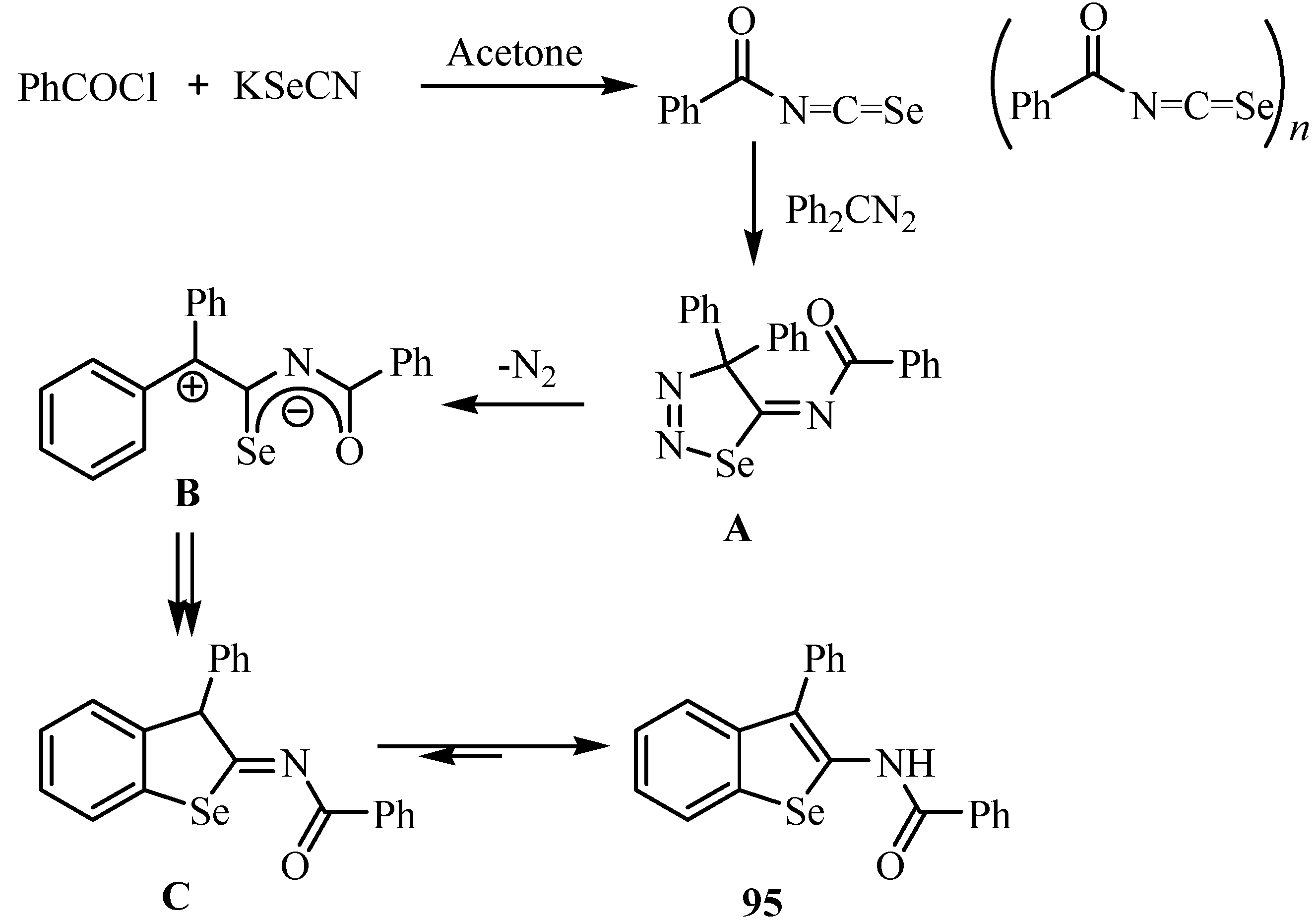
A second example of a reaction between an isoselenocyanate and a diazo compound is shown in Scheme 47 [71]. In this case, the primarily formed cycloadduct 96 has been isolated. It should be noted that the 1,3-dipolar cycloaddition occurs regioselectively to give the 1,2,3-selenadiazole derivative 96.
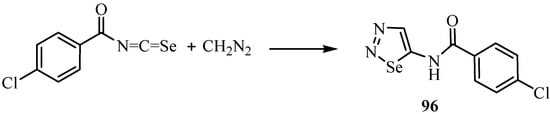
Scheme 47.
Scheme 47.
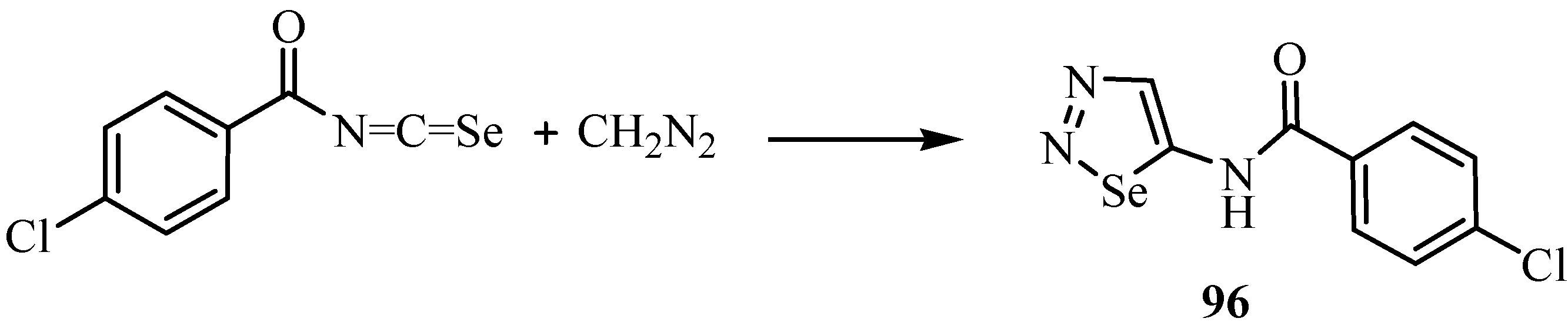
The reaction of aroyl chlorides with KSeCN and ethyl diazoacetate (97) in acetone at room temperature yields ethyl 2-aroyl-5-(aroylimino)-2,5-dihydro-1,2,3-selenadiazole-4-carboxylates 98 by a 1,3-dipolar cycloaddition (Scheme 48) [72].
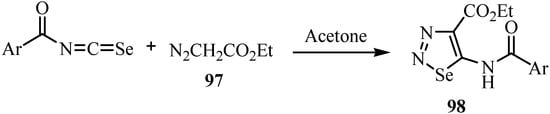
Scheme 48.
Scheme 48.
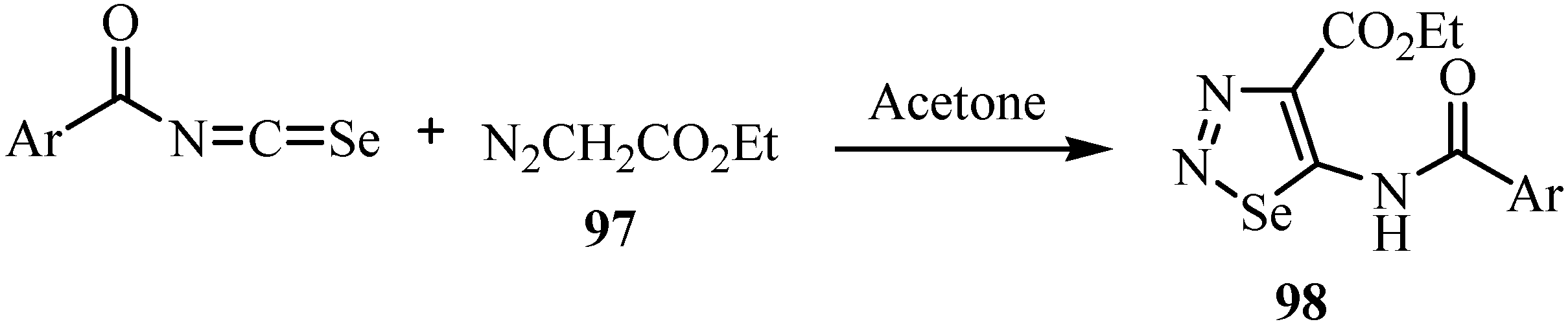
8. Cycloaddition reactions
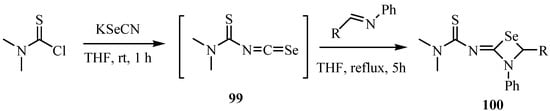
Thiocarbamoyl isoselenocyanates 99 were prepared by reactions of thiocarbamoyl chloride with KSeCN. Reaction of thiocarbamoyl isoselenocyanates 99 with imines at reflux in THF for 5 h gave 1,3-selenzetidines 100, formal [2+2] cycloadducts (Scheme 49) [73].
Scheme 49.
Scheme 49.
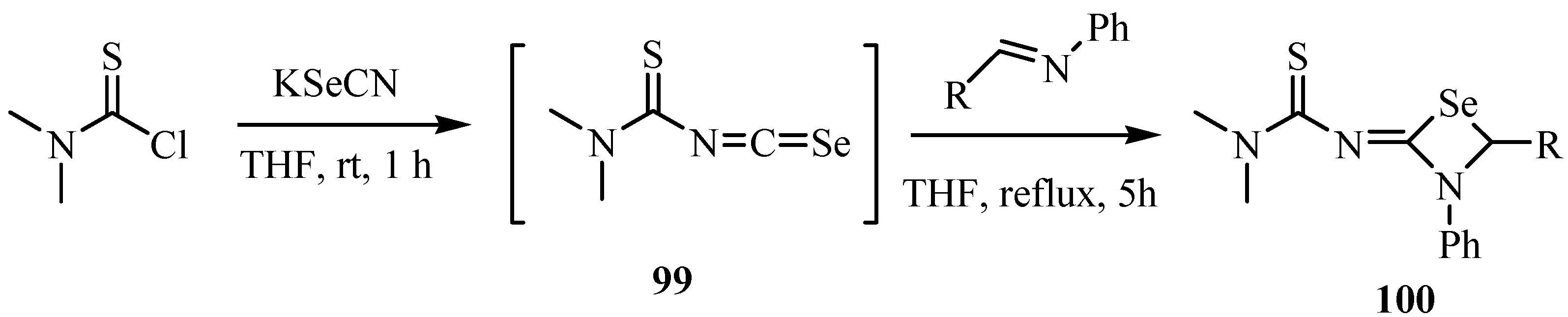
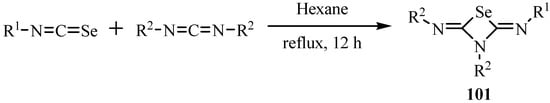
The reaction of isoselenocyanate with cabodiimides in refluxing hexane afforded 1,3-selenazetidine-2,4-diimides 101 in moderate to good yields by a [2+2] cycloaddition (Scheme 50) [74]. Both the imino groups of 101 are (Z) configured and were confirmed by its X-ray crystal structure [74].
Scheme 50.
Scheme 50.
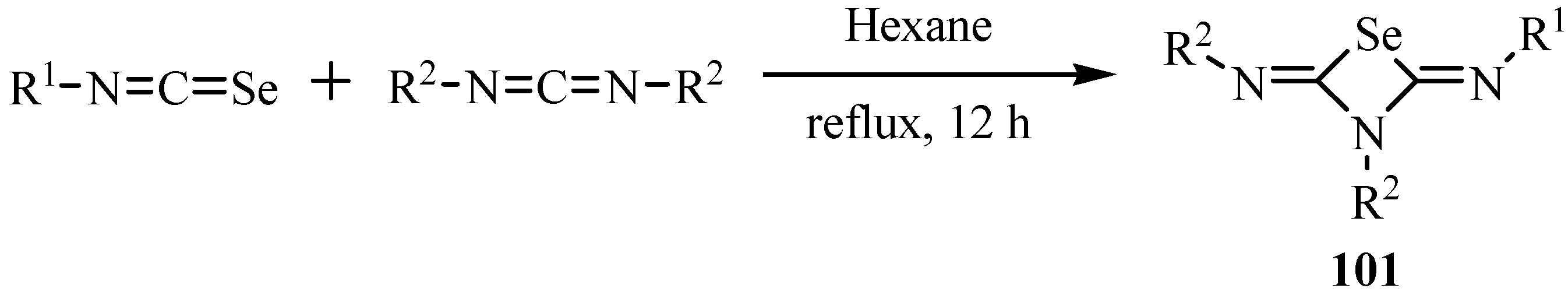
The reaction of acylisoselenocyanates with carbodiimides at room temperature gave formal [2+2] cycloadduct 1,3-selenazetidines 102, as the major products, and 4-selenoxo-3,4-dihydro-2H-1,3,5-oxadiazines 103, formal [4+2] cycloadducts, as minor products, respectively (Scheme 51) [75].
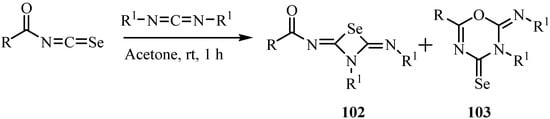
Scheme 51.
Scheme 51.
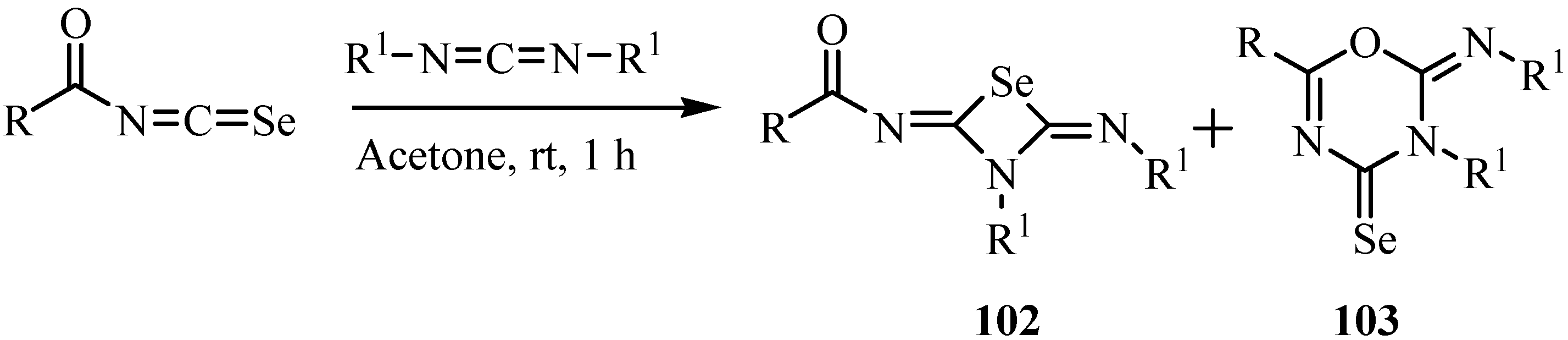
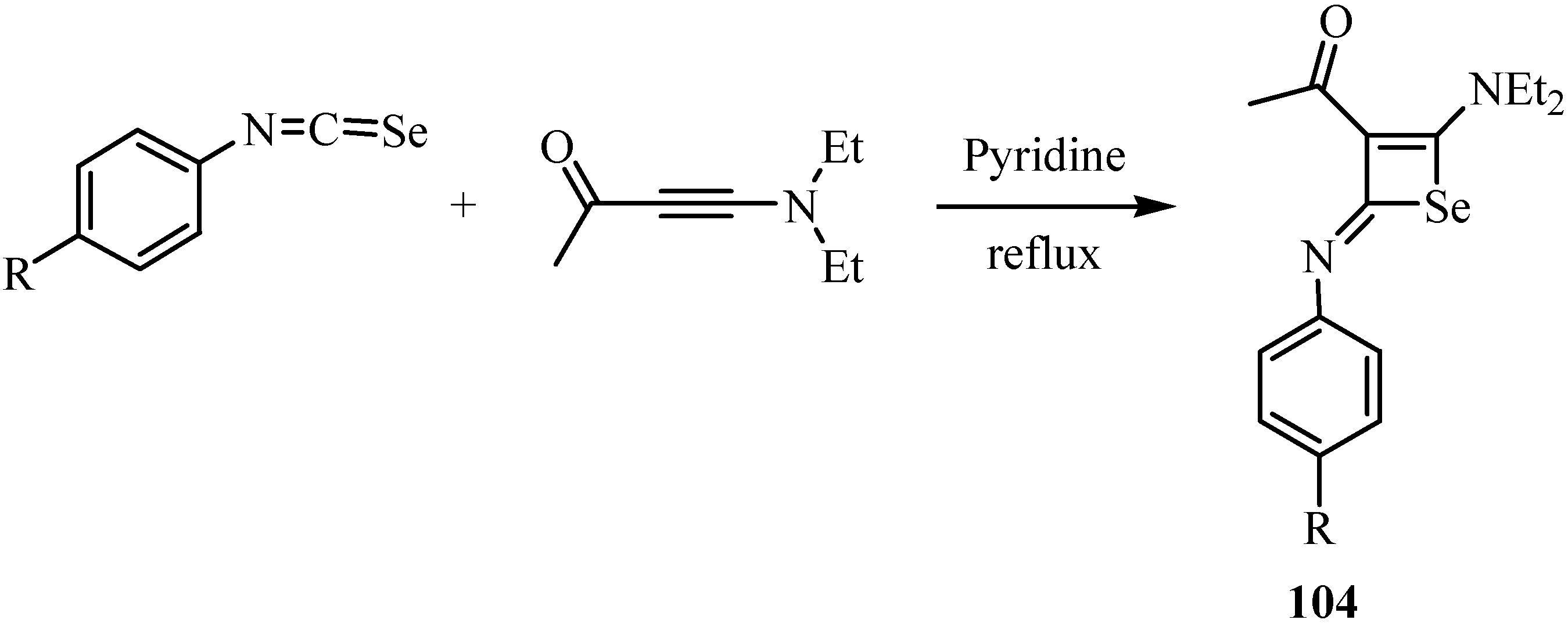
A formal [2+2] cycloaddition of arylisoselenocyanates with 4-diethylamino-3-butyn-2-one in refluxing tetrahydrofuran afforded N-arylselenet-2(2H)-imines 104 [76] in moderate yields (Scheme 52), in analogy to the reaction involving isothiocyanates, which leads to the corresponding thiet-2(2H)-imines [77].
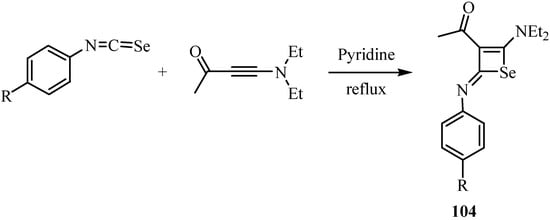
Scheme 52.
Scheme 52.
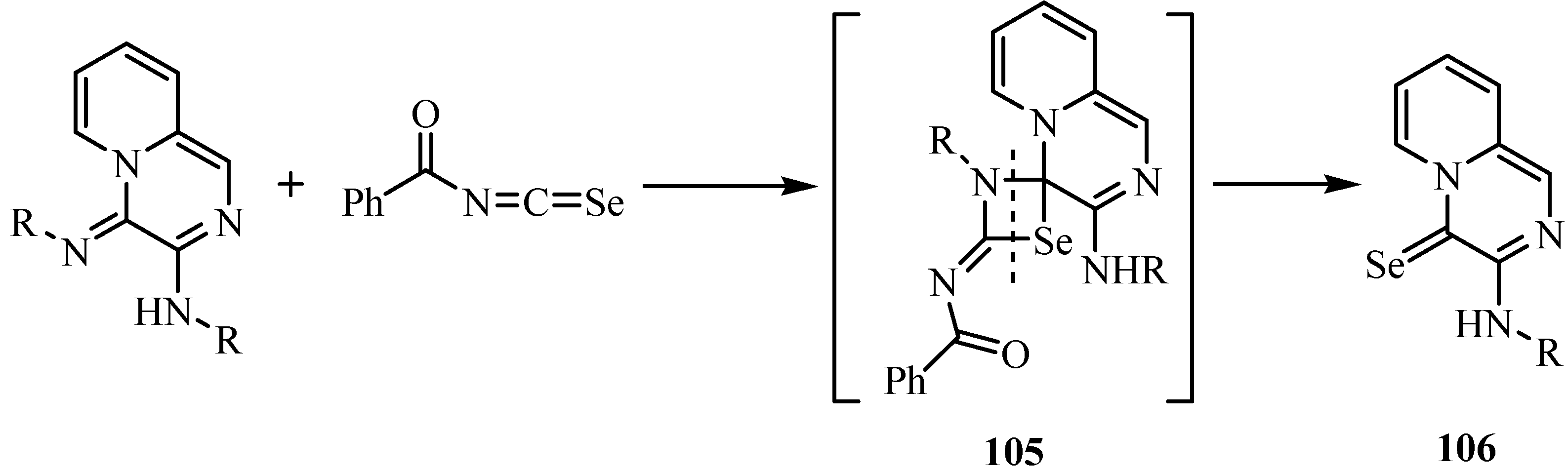
In the course of a hetero-metathesis the new aryl-(4-selono-4H-pyrido[1,2-a] pyrazin-3-yl)amines 106 were formed by the reaction of acylisoselenocyanates with exocyclic imino functions (Scheme 53) [78]. In this reaction the exocyclic imino function was attacked exclusively by the acyl isoselenocyanate to form intermediate 105, whose further reaction resulted in the formation of compound 106.
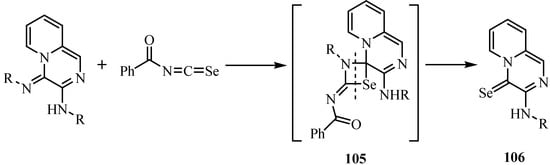
Scheme 53.
Scheme 53.
9. Iodo- and acid-catalyzed cyclization reactions
The regiochemistry of intramolecular addition of N-allylselenoureas 107 leading to 2-imino-5-methyl-1,3-selenazolidines 108 or 2-amino-5-iodo-4H-5,6-dihydro-1,3-selenazines 109 depends on the treatment of hydrogen chloride or iodine (Scheme 54) [79]. N-Allylselenoureas 107 were prepared by reactions of isoselenocyanates with allylamine. Treatment of N-allyl-selenoureas with hydrogen chloride affords 2-imino-5-methyl-1,3-selenazolidines 108 preferentially, through 5-endo closure. The driving force, in this case, is the formation of the more stable carbonium ion [79]. This behavior is in agreement with the cyclization of imidates, amides, and carbonates, which afford five-membered heterocyclic rings exclusively [80].
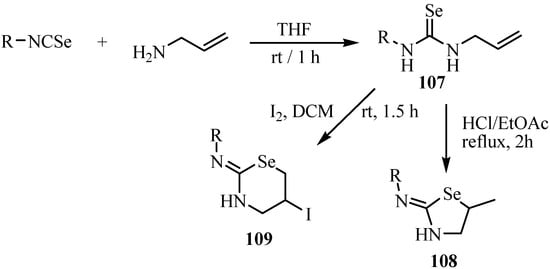
Scheme 54.
Scheme 54.
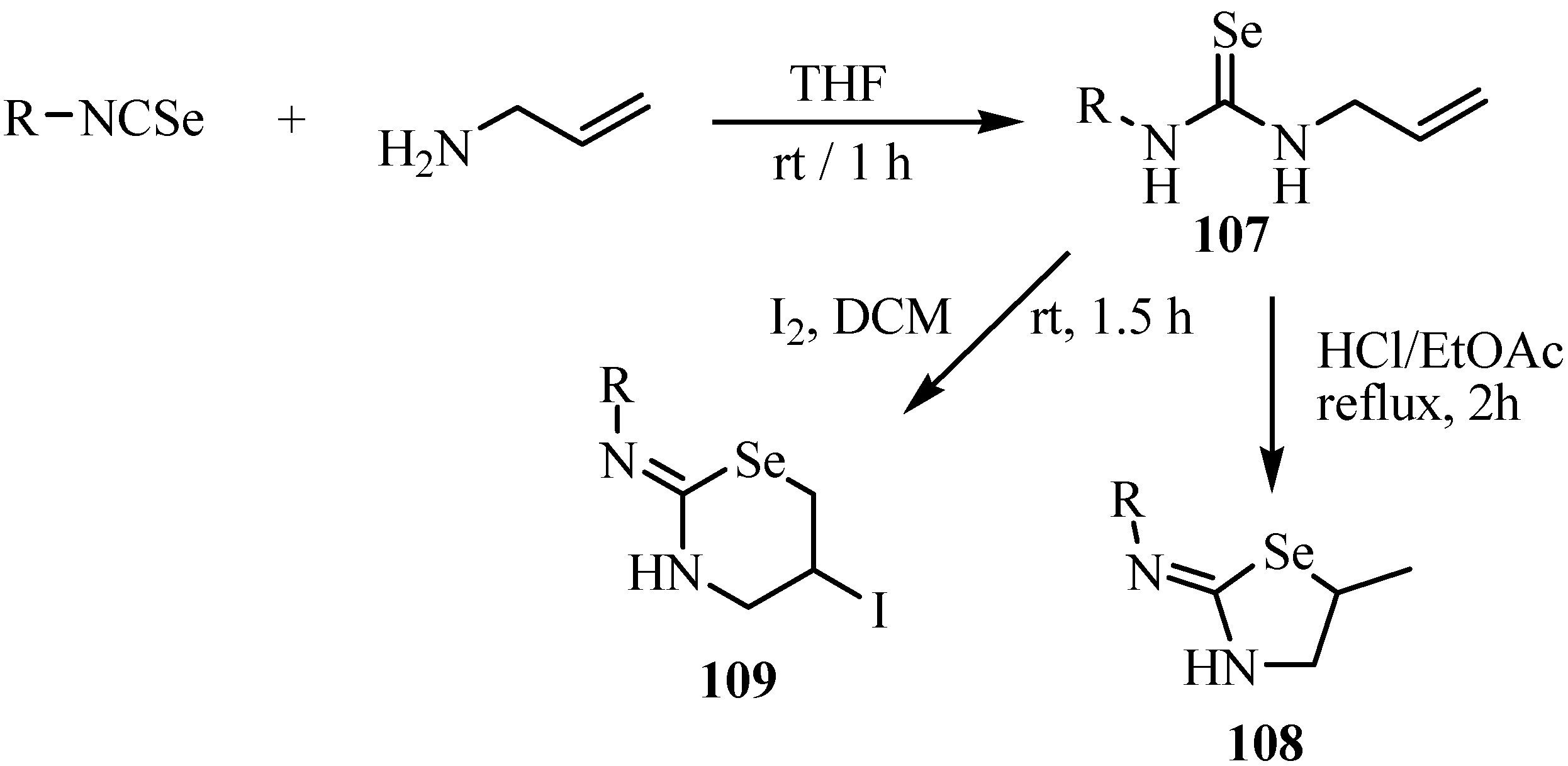
On the other hand, treatment of N-allylselenoureas with iodine at room temperature affords preferentially 2-amino-5-iodo-4H-5,6-dihydro-1,3-selenazines 109 through 6-exo closure. Reaction at −40ºC also resulted in the formation of only six-membered rings [79].
10. Synthesis of pentalenes
The one pot reactions of thiocarbamoyl isoselenocyanates 99, obtained from thiocabamoyl chloride and potassium selenocyanate, with the carbanions of β-diketones 110 afford the corresponding 1-thia-6-oxa-6aλ4-seleno-3-azapentalene skeletons containing a hypervalent coordinate selenium atom 111 as the major product. This is the first example of a heterocyclic compound containing a C-O-Se-S-C=N moiety in this order. 3-Diacylmethylidene-5-dimethylamino-3H-1,2,4-dithiazole 112 and thio-carbamate thioanhydride 113 were obtained as by-products (Scheme 55) [81].
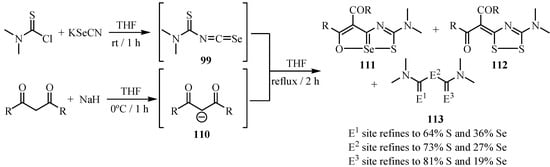
Scheme 55.
Scheme 55.
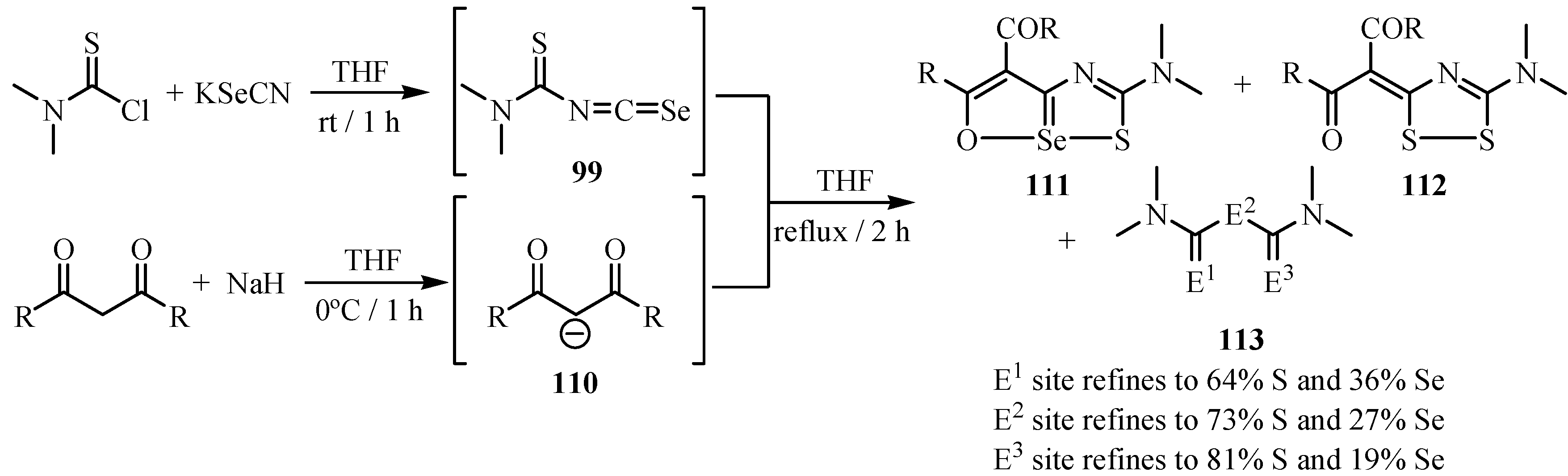
The mechanism for the formation of 111 involves initiation by nucleophilic addition of the carbon of the carbanion 110 to the central carbon of the isoselenocyanate 99, yielding 111 via intermediate 114 (Scheme 56). Intermolecular exchange of Se for S in intermediate 114 under reflux conditions would yield compound 112. All the products (111, 112 and 113) were confirmed by X-ray.
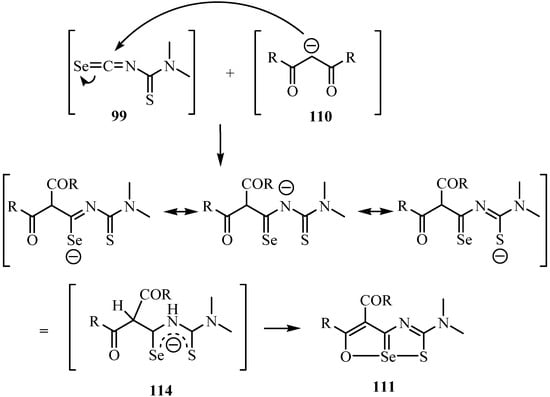
Scheme 56.
Scheme 56.
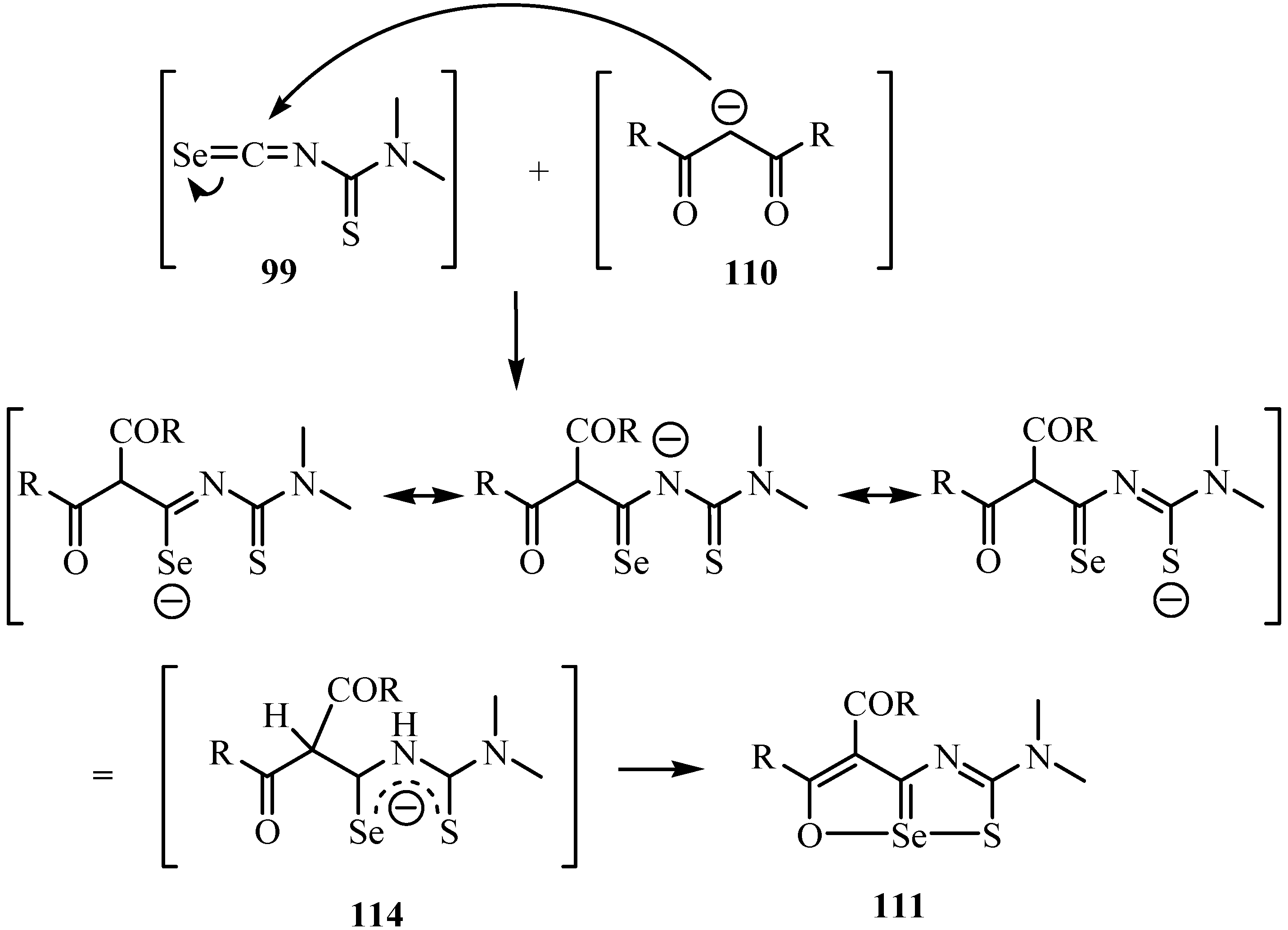
The 5-imino-2,5-dihydro-1,2,4-thiadiazole hydrochlorides 115 were converted into a variety of 2,3-dihydro-6aλ4-thiapolyheterapentalenes 116 in nearly quantitative yields by the reaction with isoselenocyanates using triethylamine as a base, using addition of heterocumulenes (Scheme 57) [82]. Structures 116 were supported by the 13C-NMR data, in agreement with values for related polyheterapentalenes [83,84].
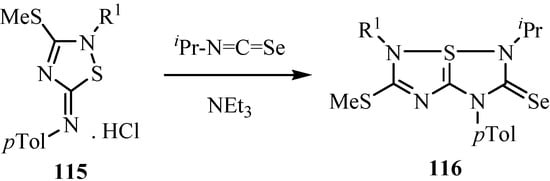
Scheme 57.
Scheme 57.
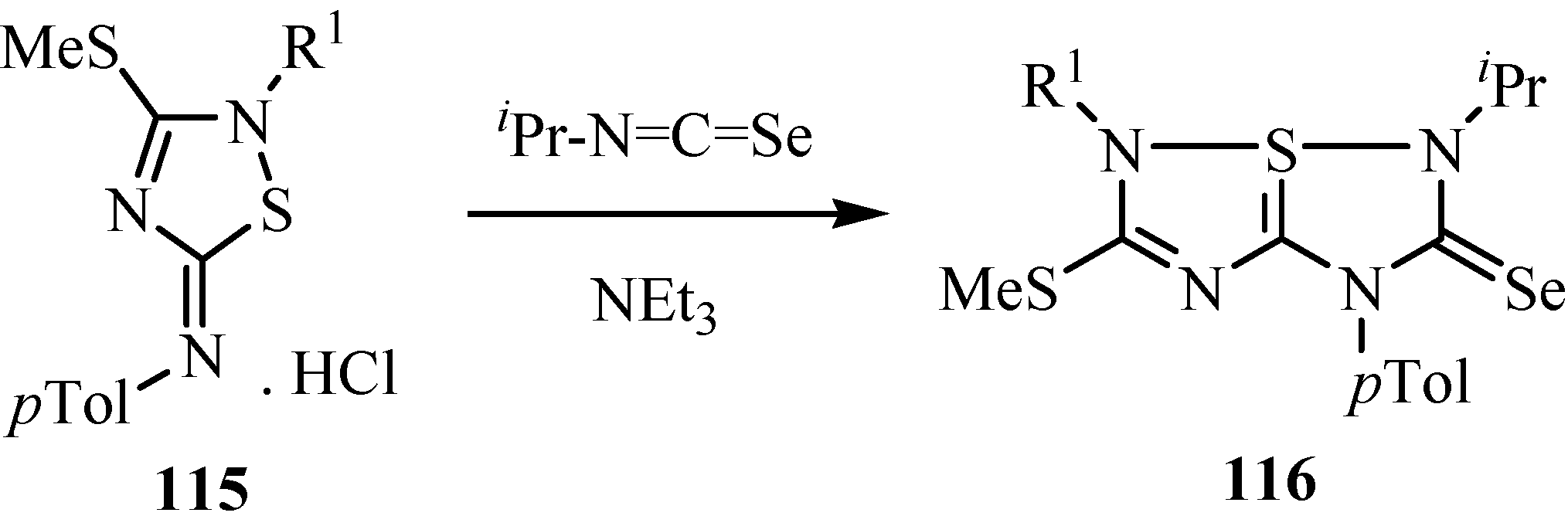
The dithioles 117 underwent thermal [2+3] cycloaddition reaction with isoselenocyanates to give 2-(substituted amino)-5-aryl-3-phenyl-6,6aλ4-dithia-1-selena- pentalenes 118 (Scheme 58) [85].
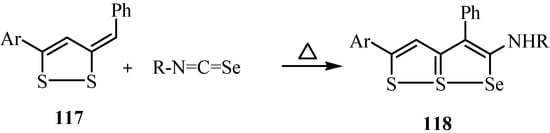
Scheme 58.
Scheme 58.
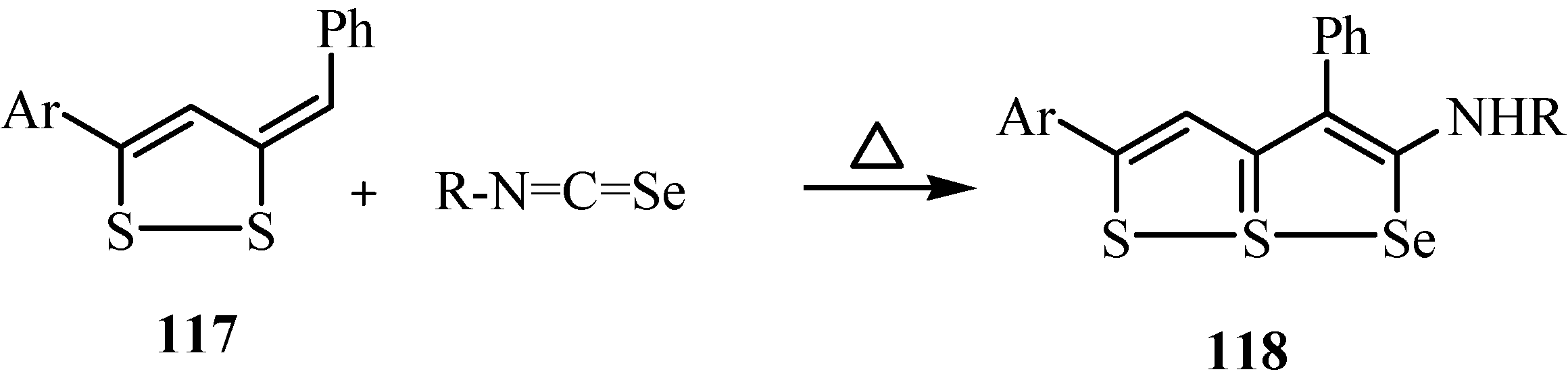
The cycloaddition reaction takes place as shown in Scheme 59, in which a zwitterionic addition product 119 is first formed. Conversion of 119 into the triheterapentalene 118 takes place by path (a) by a successive proton-transfer and ring closure sequence 119 → 120 → 118, or by path (b) involving a 2,3-dihydrotriheterapentalene intermediate 121 that tautomerizes to give 118.
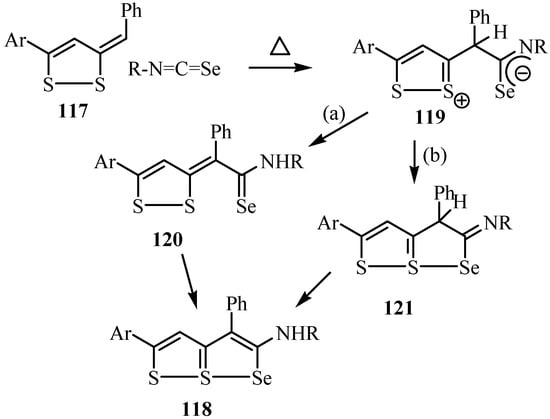
Scheme 59.
Scheme 59.
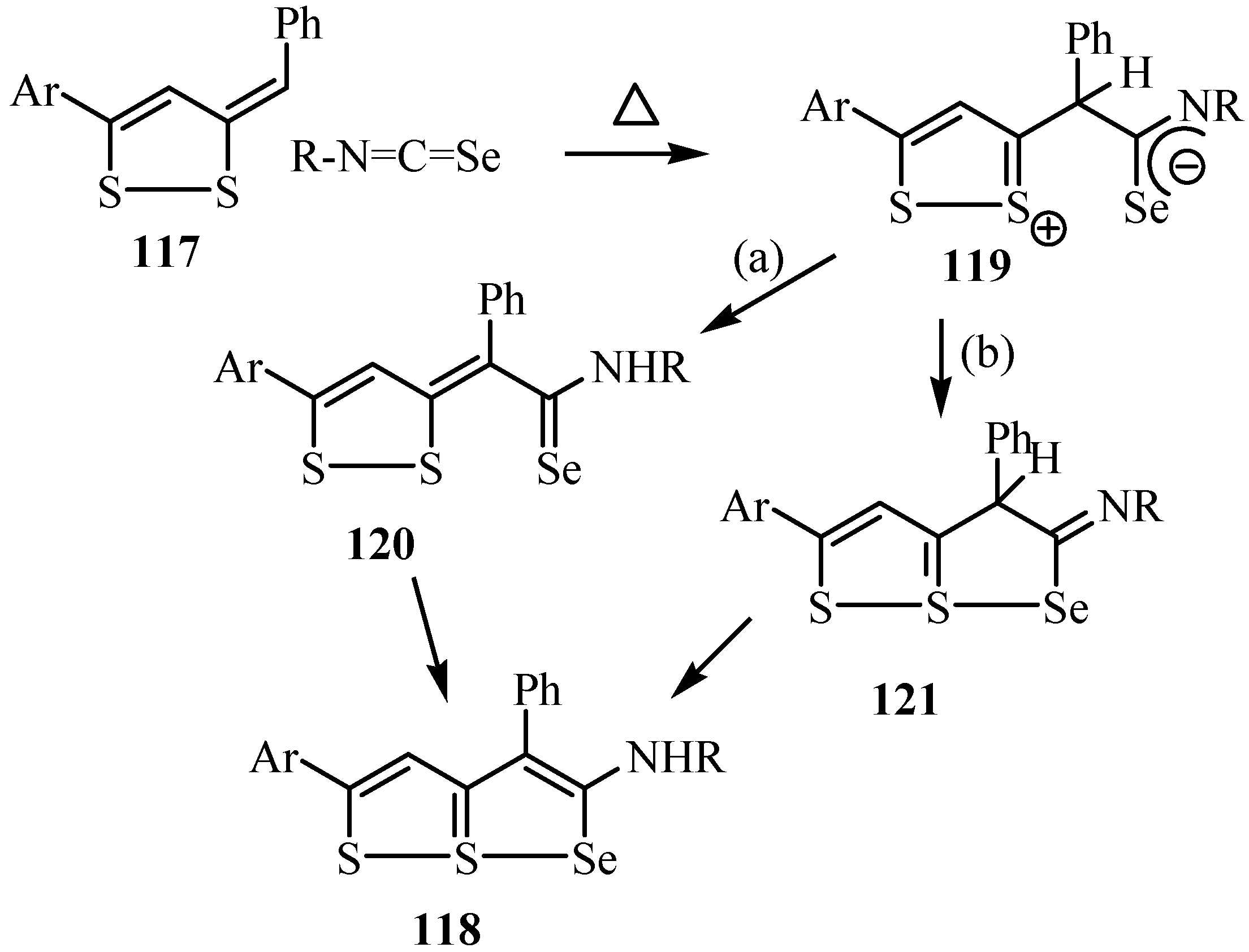
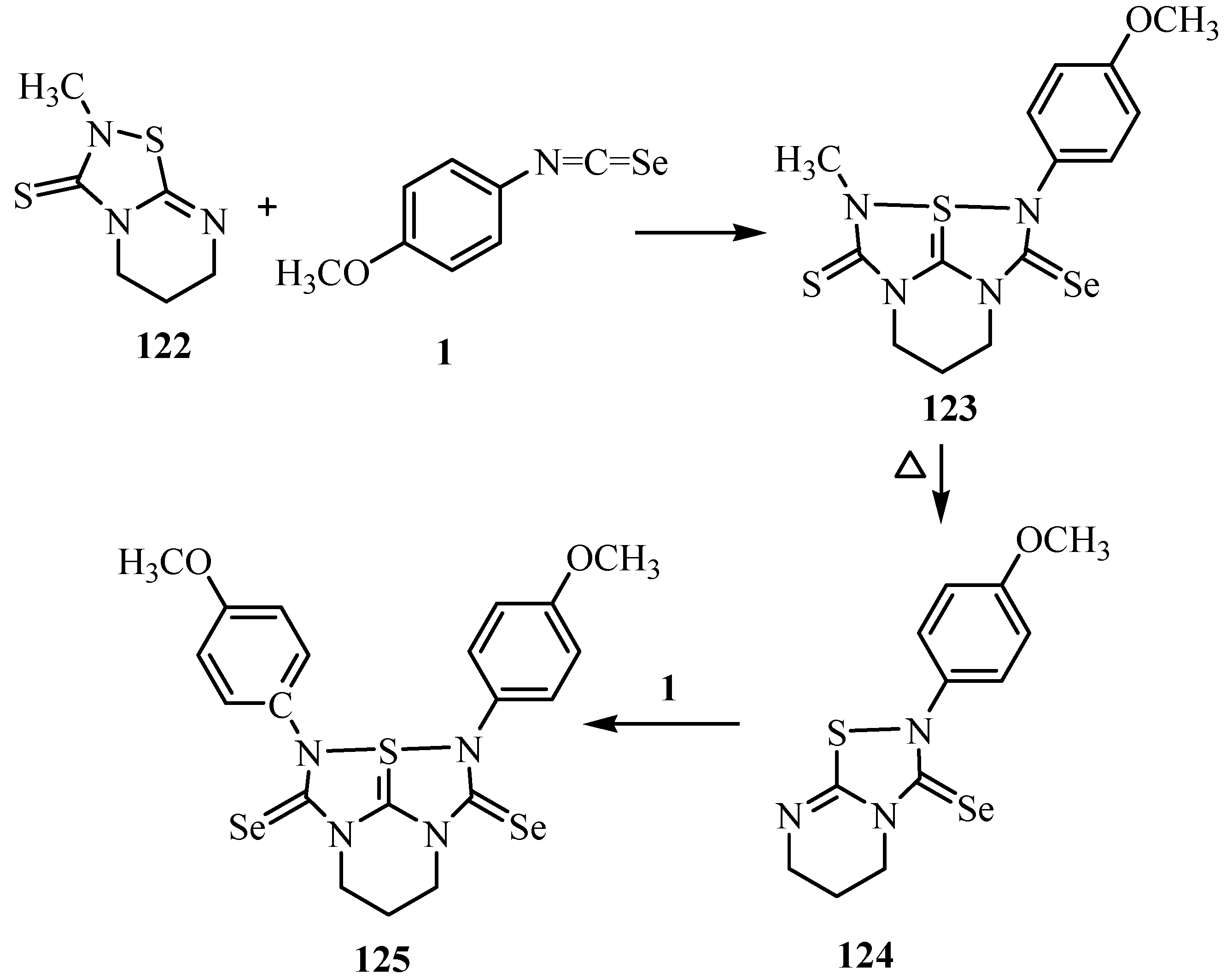
The tetraazathiapentalene derivative 123 was prepared by the reaction of 122 with isoseleno-cyanates 1. The removal of the methylisothiocyanate was observed when the tetraazathiapentalene derivative 123 was subjected to heating. Furthermore, thiadiazole derivative 124 undergoes a 1,3-dipolar cycloaddition with isoselenocyanates 1 to give tetraazathiapentalene 125 in good yield. The tetraazathiapentalene derivative 125 is stable in air in the solid state, but decomposes slowly in solution (Scheme 60) [86].
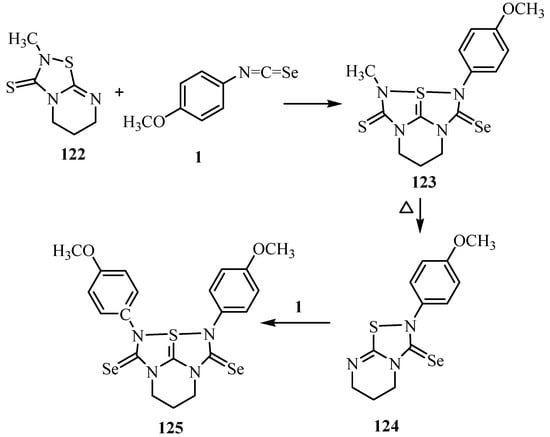
Scheme 60.
Scheme 60.
11. Conclusions
In summary, isoselenocyanates have been emerged as a powerful tool for the synthesis of selenium-containing heterocycles. This review provides a comprehensive survey of the progress in the various reactions of isoselenocyanates, their application in the preparation of various types of selenium-containing heterocycles.
Acknowledgments
This work was supported by a Grant-in-Aid for Science Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 15550030 and 17550099) to which we are grateful.
References and Notes
- Berzelius, J. J. Afhandl. Fys. Kemi Mineral. 1818, 6, 42.
- Wöhler, F.; Siemens, C. Ueber das Selenmercaptan. Ann. Chem. 1847, 61, 360–362. [Google Scholar] [CrossRef]
- Srivastava, P. C.; Robins, R. K. Synthesis and antitumor activity of 2-β-D-ribofuranosylselenazole-4-carboxamide and related derivatives. J. Med. Chem. 1983, 26, 445–448. [Google Scholar] [CrossRef] Wu, W.; Murakami, K.; Koketsu, M.; Yamada, Y.; Saiki, I. Induction of apoptosis in human gastric adenocarcinoma cells by two novel organoselenium compounds and TS-2 and TS-6. Anticancer Res. 1999, 19, 5375–5382. [Google Scholar]
- El-Bayoumy, K.; Sinha, R. Mechanisms of mammary cancer chemopre prevention by organoselenium compounds. Mutat. Res. 2004, 551, 181–197. [Google Scholar] [CrossRef] Patrick, L. Selenium biochemistry and cancer: a review of the literature. Altern. Med. Chem. 2004, 9, 239–258. [Google Scholar] Block, E. Recent results in the organosulfur and organoselenium chemistry of genus Allium and Brassica plants. Relevance for cancer prevention. Adv. Exp. Med. Biol. 1996, 401, 155–169. [Google Scholar] [CrossRef] Koketsu, M.; Ishihara, H.; Wu, W.; Murakami, K.; Saiki, I. 1,3-Selenazine derivatives induce cytotoxicity and DNA fragmentation in human HT-1080 fibrosarcoma cells. Eur. J. Pharm. Sci. 1999, 9, 157–161. [Google Scholar]
- Block, E.; Bird, S.; Tyson, J. F.; Uden, P. C.; Zhang, X.; Denoyer, E. The search for anticarcinogenic organoselenium compounds from natural sources. Phosphorus Sulfur Silicon Relat. Elem. 1998, 136, 1–10. [Google Scholar] [Green Version]
- May, S. W. Selenium-based pharmacological agents: an update. Exp. Opin. Invest. Drugs 2002, 11, 1261–1269. [Google Scholar] [CrossRef]
- Parnham, M. J.; Graf, E. Pharmacology of synthetic organic selenium compounds. Prog. Drug. Res. 1991, 36, 9–47. [Google Scholar]
- Koketsu, M.; Ishihara, H.; Hatsu, M. Novel compounds, 1,3-selenazine derivatives, as antibacterial agents against Escherichia coli and Staphylococcus aureus. Res. Commun. Mol. Pathol. Pharmacol. 1998, 101, 179–186. [Google Scholar]
- May, S. W.; Wang, L.; Gill-Woznichak, M. M.; Browner, R. F.; Ogonowski, A. A.; Smith, J. B.; Pollock, S. H. An orally active selenium-based antihypertensive agent with restricted CNS permeability. J. Pharm. Exp. Ther. 1997, 283, 470–477. [Google Scholar]
- Göbel, T.; Gsell, L.; Hüter, O. F.; Maienfisch, P.; Naef, R.; O’Sullivan, A. C.; Pitterna, T.; Rapold, T.; Seifert, G.; Sern, M.; Szczepanski, H.; Wadsworth, D. J. Synthetic approaches towards CGA 293'343: a novel broad-spectrum insecticide. Pestic. Sci. 1999, 55, 355–357. [Google Scholar] [CrossRef] El-Desoky, S. I.; Bondock, S. B.; Etman, H. A.; Fadda, A. A.; Metwally, M. A. Synthesis of some new thiazole derivatives of pharmaceutical interest. Sulfur Lett. 2003, 26, 127–135. [Google Scholar] [CrossRef] Lamberth, C. Sulfur chemistry in crop protection. J. Sulfur Chem. 2004, 25, 39–62. [Google Scholar] [CrossRef] Kedar, R. M.; Vidhale, N. N.; Chincholkar, M. M. Synthesis of new heterocycles from simple chalcones and their antimicrobial study. Orient. J. Chem. 1996, 12, 301–304. [Google Scholar] Metwally, M. A.; Abdel-Latif, E.; Amer, F. A.; Kaupp, G. Versatile 2-amino-4-substituted-1,3-thiazoles: synthesis and reactions. J. Sulfur Chem. 2004, 25, 63–85. [Google Scholar] Erol, D. D.; Aytemir, M. D.; Yulug, N. Synthesis and antibacterial and antifungal properties of thiazolinoethyl-2(3H)-benzoxazolone derivatives. II. Eur. J. Med. Chem. 1996, 31, 731–734. [Google Scholar] [CrossRef]
- Bulka, E.; Ahlers, K.-D.; Tućek, E. Synthese und IR-spektren von aryl-isoselenocyanaten. Chem Ber. 1967, 100, 1367–1372. [Google Scholar] Bulka, E. The Chemistry of Cyanates and Thiocyanates; Patai, S., Ed.; Wiley & Sons: New York, 1972. [Google Scholar]
- Barton, D. H. R.; Parekh, S. I.; Tajbakhsh, M.; Theodorakis, E. A.; Tse, C.-L. A convenient and high yielding procedure for the preparation of isoselenocyanates. Synthesis and reactivity of O-alkylselenocarbamates. Tetrahedron 1994, 50, 639–654. [Google Scholar]
- Bakhsh, M. T.; Behshtiha, Y. S.; Heravi, M. M. A facile method for the preparation of isoselenocyanates. J. Chem. Soc. Pak. 1996, 18, 159. [Google Scholar] Su, W. K.; Liang, X. R. An efficient and convenient route to some isoselenocyanates via reaction of formamides with bis(trichloromethyl) carbonate and selenium. J. Indian Chem. Soc. 2003, 80, 645–647. [Google Scholar]
- Fernández-Bolaños, J. G.; López, Ó.; Ulgar, V.; Maya, I.; Fuentes, J. Synthesis of O-unprotected glycosyl selenoureas. A new access to bicyclic sugar isoureas. Tetrahedron Lett. 2004, 45, 4081–4084. [Google Scholar] [CrossRef]
- Henriksen, L.; Ehrbar, U. One-step synthesis of alkyl and aryl isoselenocyanates from primary amines. Synthesis 1976, 519. [Google Scholar] [CrossRef]
- Tarantelli, T.; Pecile, C. Triphenylmethyl isoselenocyanate. Ann. Chim. (Rome) 1962, 52, 75–79. [Google Scholar] Pederson, C. T. Preparation of some 4-substituted selenosemicarbazides. Acta. Chem. Scan. 1963, 17, 1459–1461. [Google Scholar] [CrossRef]
- Stolte, H. Ber, Dtsch. Chem. Ges. 1886, 19, 2350. [CrossRef] Collard-Charon, C.; Renson, M. Synthesis of substituted selenosemicarbazides. I. Synthesis of isoselenocyanic esters. Bull. Soc. Chim. Belg. 1962, 71, 531–540. [Google Scholar] [CrossRef]
- Suzuki, H.; Usuki, M.; Hanafusa, T. A Photochemical route to some substituted benzyl isoselenocyanates. Synthesis 1979, 705–707. [Google Scholar] [CrossRef]
- Koketsu, M.; Suzuki, N.; Ishihara, H. Preparation of isoselenocyanate and synthesis of carbodiimide by oxidation of selenourea. J. Org. Chem. 1999, 64, 6473–6475. [Google Scholar] [CrossRef]
- Douglas, I. B. Acylselenoureas. J. Am. Chem. Soc. 1937, 59, 740–742. [Google Scholar] [CrossRef]
- Koketsu, M.; Yamamura, Y.; Aoki, H.; Ishihara, H. The preparation of acylselenourea and selenocarbamate using isoselenocyanate. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 2699–2708. [Google Scholar] [CrossRef]
- Hashim Nizar, P. N.; Parkash, S.; Chauhan, S. M. S. Synthesis of 3-cyclohexyl-1-alkylselenoureas and 2-(N-nitrosocyclohexylamino)-2-selenazoline. J. Indian Chem. Soc. 1997, 74, 161–162. [Google Scholar]
- Sommen, G. L.; Linden, A.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: Synthesis of 1,3-selenazolidine and perhydro-1,3-selenazine derivatives. Eur. J. Org. Chem. 2005, 3128–3137. [Google Scholar]
- Sommen, G. L.; Linden, A.; Heimgartner, H. First synthesis of a selenazepane. Tetrahedron Lett. 2005, 46, 6723–6725. [Google Scholar]
- Azerbaev, I. N.; Tsoi, L. A.; Salimbaeva, A. D.; Cholpankulova, S. T.; Ryskieva, G. A.; Kalkabaeva, L. T.; Aitkhozhaeva, M. Zh. Reactions of acetylene amines with isocyanates, isothiocyanates, and isoselenocyanates. Trudy Instit. Khim. Nauk Akad. Nauk Kazak.SSR 1980, 52, 128–146. [Google Scholar]
- Koketsu, M.; Sakai, T.; Kiyokuni, T.; Garud, D. R.; Ando, H.; Ishihara, H. One-pot synthesis of 2-imino-1,3-selenazolidines by reaction of isoselenocyanates with propargylamine. Heterocycles 2006, 68, 1607–1615. [Google Scholar] [CrossRef]
- Banert, K.; Toth, C. Synthesis and reactions of vinyl isoselenocyanates. Angew. Chem. Int. Ed. Engl. 1995, 34, 1627–1629. [Google Scholar] [CrossRef]
- Banert, K.; Hückstädt, H.; Vrobel, K. Synthesis and reactions of isothiocyanate-substituted allenes and 1,3-butadienes. Angew. Chem. Int. Ed. Engl. 1992, 31, 90–92. [Google Scholar] [CrossRef]
- Ueda, S.; Terauchi, H.; Suzuki, K.; Yano, A.; Matsumoto, M.; Kubo, T.; Minato, H.; Arai, Y.; Tsuji, J.; Watanabe, N. Novel and orally bioavailable inducible nitric oxide synthase inhibitors: synthesis and evaluation of optically active 4,5-dialkyl-2-iminoselenazolidine derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 1361–1366. [Google Scholar] [CrossRef]
- Ueda, S.; Terauchi, H.; Yano, A.; Ido, M.; Matsumoto, M.; Kawasaki, M. 4,5-Disubstituted-1,3-oxazolidin-2-imine derivatives: a new class of orally bioavailable nitric oxide synthase inhibitor. Bioorg. Med. Chem. Lett. 2004, 14, 313–316. [Google Scholar] [CrossRef]
- Ueda, S.; Terauchi, H.; Yano, A.; Matsumoto, M.; Kubo, T.; Kyoya, Y.; Suzuki, K.; Ido, M.; Kawasaki, M. 4,5-Dialkylsubstituted 2-imino-1,3-thiazolidine derivatives as potent inducible nitric oxide synthase inhibitors. Bioorg. Med. Chem. 2004, 12, 4101–4116. [Google Scholar] [CrossRef]
- Atanassov, P. K.; Linden, A.; Heimgartner, H. Derivatives from isoselenocyanates: Synthesis of 2-phenyl-6H-[5,1,3]benzoselenadiazocine. Helv. Chim. Acta 2004, 87, 1452–1466. [Google Scholar] [CrossRef]
- Koketsu, M.; Takahashi, A.; Ishihara, H. A facile preparation of selenohydantoins using isoselenocyanate. J. Heterocycl. Chem. 2007, 44, 79–81. [Google Scholar] [CrossRef]
- Atanassov, P. K.; Linden, A.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: Synthesis of 2-arylaminoselenazolo[5,4-b]pyridines. Heterocycles 2003, 61, 569–579. [Google Scholar] [CrossRef]
- Kristian, P.; Koščik, D.; Gonda, J. Heterocycles with pyrido[3,2-e]-1,3-selenazine and pyrido[3,4-e]-1,3-selenazine ring systems. Collect. Czech. Chem. Commu. 1983, 48, 3567–3574. [Google Scholar] [CrossRef]
- Atanassov, P. K.; Linden, A.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: Synthesis of 1H-5-selena-1,3,6-triazaaceanthrylene derivatives. Helv. Chim. Acta 2003, 86, 3235–3243. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hara, S.; Nagata, N.; Ikeda, K.; Mizuno, Y. Synthesis of new 1-chalcogenapurines by the reaction of 5-aminoimidazole-4-carbonitrile with isochalcogenocyanates. Heterocycles 1995, 41, 47–56. [Google Scholar] [CrossRef]
- Atanassov, P. K.; Linden, A.; Heimgartner, H. Synthesis of 4-(phenylamino)quinazoline-2(1H)-selones and diselenides from isoselenocyanates: Dimroth rearrangement of an intermediate. Helv. Chim. Acta 2004, 87, 1873–1887. [Google Scholar] [CrossRef]
- EI Ashry, E. S. H.; EI Kilany, Y.; Rashed, N.; Assafir, H. Dimroth rearrangement: Translocation of heteroatoms in heterocyclic rings and its role in ring transformations of heterocycles. Adv. Heterocycl. Chem. 1999, 75, 79–167. [Google Scholar] [CrossRef] Fujii, T.; Itaya, T. The Dimroth rearrangement in the adenine series: A review updated. Heterocycles 1998, 48, 359–390. [Google Scholar] [CrossRef] Itaya, T.; Ito, N.; Kanai, T.; Fujii, T. Purines. LXXV. Dimroth rearrangement, hydrolytic deamination, and pyrimidine-ring breakdown of 7-alkylated 1-alkoxyadenines: N(1)-C(2) versus N(1)-C(6) bond fission. Chem. Pharm. Bull. 1997, 45, 832–841. [Google Scholar] [CrossRef]
- Taylor, E. C.; Ravindranathan, R. V. Reaction of anthranilonitrile and N-methylanthranilonitrile with phenyl isocyanate and phenyl isothiocyanate. J. Org. Chem. 1962, 27, 2622–2627. [Google Scholar] [CrossRef]
- Koketsu, M.; Yamamura, Y.; Ishihara, H. Synthesis of selenosemicarbazides and 1,2,4-triazoles. Heterocycles 2006, 68, 1191–1200. [Google Scholar] [CrossRef]
- Sommen, G. L.; Linden, A.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: Use of hydrazine for the synthesis of 1,3,4-selenadiazine derivatives. Helv. Chim. Acta 2006, 89, 1322–1329. [Google Scholar] [CrossRef]
- Athayde-Filho, P. F. D.; Simas, A. M.; Goncalves, S. M. C.; Miller, J. Synthesis and characterization of mesoionic 1,3,4-triazolium-2-selenolates. Phosphorus Sulfur Silicon Relat. Elem. 2000, 161, 115–121. [Google Scholar] [CrossRef]
- Stefaniak, L.; Jazwiuski, J. Chem. Heterocycl. Compd. 1995, 31, 1027. [CrossRef]
- Bocian, W.; Jazwinski, J.; Stefaniak, L. Multinuclear 77Se, 15N, 14N, 13C and 1H NMR study of mesoionic 1,3,4-triazolium-5-selenolates and related compounds. Polish J. Chem. 1995, 69, 85–89. [Google Scholar]
- Bartels-Keith, J.R.; Burgess, M.; Stevenson, J. M. Carbon-13 nuclear magnetic resonance studies of heterocycles bearing carbon-sulfur and carbon-selenium bonds: 1,3,4-thiadiazole, 1,3,4-selenadiazole, and tetrazole derivatives. J. Org. Chem. 1977, 42, 3725–3731. [Google Scholar] [CrossRef]
- Duarte, H.C. Master’s Thesis, Universidade Estadual de Campinas, 1979.
- Asanuma, Y.; Fujiwara, S.; Shin-ike, T.; Kambe, N. Selenoimidoylation of alcohols with selenium and isocyanides and its application to the synthesis of selenium-containing heterocycles. J. Org. Chem. 2004, 69, 4845–4848. [Google Scholar]
- Fujiwara, S.-i; Shikano, Y.; Shin-ike, T.; Kambe, N.; Sonoda, N. Stereoselective synthesis of new selenium-containing heterocycles by cyclocarbonylation of aminoalkynes with carbon monoxide and selenium. J. Org. Chem. 2002, 67, 6275–6278. [Google Scholar]
- Mizuno, T.; Nakamura, F.; Ishino, Y.; Nishiguchi, I.; Hirashima, T.; Ogawa, A.; Kambe, N.; Sonoda, N. Facile stereoselective synthesis of 4-alkylidene-2-oxo-1,3-oxathiolanes from 2-alkyn-1-ols, carbon monoxide, and sulfur. Synthesis 1989, 770–771. [Google Scholar]
- Silks, L. A.; Peng, J.; Odom, J. D.; Dunlap, R. B. Synthesis of (4S,5R)-(–)-4-methyl-5-phenyloxazolidine-2-selone: a chiral auxiliary reagent capable of detecting the enantiomers of (R,S)-lipoic acid by 77Se nuclear magnetic resonance spectroscopy. J. Chem. Soc., Perkin Trans. 1 1991, 2495–2498. [Google Scholar]
- Sommen, G. L.; Linden, A.; Heimgartner, H. Synthesis of 2-selenoxo-1,3-thiazolidin-4-ones and 2-selenoxo-1,3-thiazinan-4-ones from isoselenocyanates. Heterocycles 2005, 65, 1903–1915. [Google Scholar] [CrossRef]
- Shafiee, A.; Fanaii, G. A facile synthesis of N-(4-aryl-1,3-dithiol-2-ylidene)-amides, N-(4- or 5-aryl-1,3-thiaselenol-2-ylidene)-amides, and N-(4-aryl-1,3-diselenol-2-ylidene)-amides. Synthesis 1984, 512–514. [Google Scholar] [CrossRef]
- Zmitrovich, N. I.; Petrov, M. L.; Potekhin, K. A.; Balashova, E.V. Synthesis, crystal and molecular structure of unsubstituted 2-phenylimino-1,3-diselenol. Zh. Obshchei Khim. 1996, 66, 1684–1687. [Google Scholar]
- Zmitrovich, N. I.; Petrov, M. L. α,β-Unsaturated thiolates and their analogs in cycloaddition reactions. XXIII. A convenient one-pot synthesis of 2-phenylimino-1,3-thiaselenoles and -1,3-diselenoles. Zh. Org. Khim. 1996, 32, 1870–1874. [Google Scholar]
- Koketsu, M.; Yamamura, Y.; Ishihara, H. Synthesis of 2-selenoxoperhydro-1,3-selenazin-4-ones and 2-selenoxo-1,3-selenazolidin-4-ones via diselenocarbamate intermediates. Synthesis 2006, 2738–2742. [Google Scholar] [CrossRef]
- Suchár, G.; Štefko, R. Synthesis of tetrahydro-1,3,5-selenodiazine-2-selenones. Chem. Zvesti 1982, 36, 419–422. [Google Scholar]
- Sommen, G. L.; Linden, A.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: synthesis of 2-methylidene-1,3-selenazolidine derivatives. Tetrahedron 2006, 62, 3344–3354. [Google Scholar] [Green Version]
- Murai, T. Thio-, seleno-, telluro-amides. Top. Curr. Chem. 2005, 251, 247–272. [Google Scholar] Baskakov, Yu. A.; Volovnik, L. L.; Vasil’ev, A. F.; Aryutkina, N. L.; Tibanov, P. V.; Negrebetskii, V. V. Herbicidal derivatives of hydroxylamine. XXXIV. Reaction of haloacetic acid halides with hydroxylamine derivatives of thiourea. Khim. Geterotsikl. Soedin. 1971, 3, 104–107. [Google Scholar] Velkov, Z. Thioamides - some properties and preparations. Bulg. Chem. Commun. 2003, 35, 227–230. [Google Scholar] Jagodzinski, T. S. Thioamides as useful synthons in the synthesis of heterocycles. Chem. Rev. 2003, 103, 197–228. [Google Scholar] Mitchell, S. C.; Steventon, G. B. Thiourea and its biological interactions. Sulfur Rep. 1994, 16, 117–137. [Google Scholar] [CrossRef] Bobbitt, J. M.; Bourque, A. J. Synthesis of heterocycles using aminoacetals. Heterocycles 1987, 25, 601–616. [Google Scholar] [CrossRef]
- Kaválek, J.; Jirman, J.; Štĕrba, J. V. Kinetics and mechanism of rearrangement and methanolysis of acylphenylthioureas. Collect. Czech. Chem. Commun. 1985, 50, 766–778. [Google Scholar] [CrossRef] Kaválek, J.; Novak, J.; Štĕrba, V. Kinetics of hydrolysis and rearrangements of S-acylthiouronium salts. Collect. Czech. Chem. Commun. 1982, 47, 2702–2710. [Google Scholar] [CrossRef] Pratt, R. F.; Bruice, T. C. Reactions of S-acylisothioureas. II. Effects of structure and stereochemistry on the rates of hydrolysis, thiol elimination, and S to N acyl migration in acylic systems. J. Am. Chem. Soc. 1972, 94, 2823–2837. [Google Scholar] [CrossRef] Bruice, T. C.; Pratt, R. F. Reactions S-acylisothioureas. I. S- to N-acyl migrations in S-benzoylisothiobiotin and analogs. Biochemistry 1971, 10, 3178–3185. [Google Scholar] [CrossRef]
- Klika, K. D.; Janovec, L.; Imrich, J.; Suchár, G.; Kristian, P.; Sillanpää, R.; Pihlaja, K. Regioselective synthesis of 2-imino-1,3-thiazolidin-4-ones by treatment of N-(anthracen-9-yl)-N`-ethylthiourea with bromoacetic acid derivatives. Eur. J. Org. Chem. 2002, 1248–1255. [Google Scholar]
- Zhou, Y.; Linden, A.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: Synthesis of 1,3-selenazoles from N-phenylimidoyl isoselenocyanates. Helv. Chim. Acta 2000, 83, 1576–1598. [Google Scholar]
- Teller, J.; Dehne, H.; Zimmermann, T.; Fischer, G.W.; Olk, B. Novel synthesis of 2-dialkylamino-4-arylthiazoles. Z. Chem. 1989, 29, 255. [Google Scholar] Teller, J.; Dehne, H.; Zimmermann, T.; Fischer, G.W.; Olk, B. Substituierte 2-amino-thiazole aus α-thiocyanato-acetophenonen und dialkylaminen. J. Prakt. Chem. 1990, 332, 453–460. [Google Scholar] [CrossRef] Zimmermann, T.; Fischer, G.W.; Teller, J.; Dehne, H.; Olk, B. Substituierte N,N`-bis(thiazol-2-yl)-diaminoalkane aus α-thiocyanato-acetophenonen und N,N`-dialkyl-diamino-alkanen. J. Prakt. Chem. 1990, 332, 723–730. [Google Scholar] [CrossRef]
- Sommen, G.; Comel, A.; Kirsch, G. New synthesis of selenophenes and condensed ring systems from ketene dithioacetals. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 939–943. [Google Scholar] [CrossRef]
- Maeda, H.; Kambe, N.; Sonoda, N.; Fujiwara, S.; Shin-ike, T. Synthesis of 1,3-selenazoles and 2-imidazolin-5-selones from isoselenocyanates and isocyanides. Tetrahedron 1997, 53, 13667–13680. [Google Scholar]
- Becher, J.; Frandsen, E. G.; Dreier, C.; Henriksen, L. Derivatives and reactions of glutacondialdehyde. VII. Reaction of the glutacondialdehyde anion with heterocumulenes. Acta Chem. Scand. B 1977, 31, 843–847. [Google Scholar]
- Favero, F.; Sommen, G. L.; Linden, A.; Heimgartner, H. Synthesis of 5-selenoxo-1,2,4-triazole-1-carboxylates from isoselenocyanates and azodicarboxylates. Heterocycles 2006, 67, 749–762. [Google Scholar] [CrossRef]
- Bittner, S.; Assaf, Y.; Krief, A.; Pomerantz, M.; Ziemnicka, B. T.; Smith, C. G. Synthesis of N-acyl-, N-sulfonyl-, and N-phosphinylphospha(PV)azenes by a redox-condensation reaction using amides, triphenylphosphine, and diethyl azodicarboxylate. J. Org. Chem. 1985, 50, 1712–1718. [Google Scholar] [CrossRef] Mukaiyama, T. Oxidation-reduction condensation. Angew. Chem., Int. Ed. Engl. 1976, 15, 94–103. [Google Scholar] [CrossRef] Mitsunobu, O. The use of diethyl azodicarboxylate and triphenylphosphine in synthesis and transformation of natural products. Synthesis 1981, 1–28. [Google Scholar] [CrossRef] Fauduet, H.; Burgada, R. Condensation of spirophosphoranes containing phosphorus-hydrogen bond with diethyl azodicarboxylate. C.R. Acad. Sci. Ser. C 1980, 291, 81–83. [Google Scholar] Itzstein, M. V.; Jenkins, I. D. The reaction of diols with triphenylphosphine and di-isopropyl azodicarboxylate. Part 1. Formation of cyclic phosphoranes from 1,3- and 1,4- diols. J. Chem. Soc., Perkin Trans. 1 1986, 437–445. [Google Scholar] [CrossRef] Camp, D.; Jenkins, I. D. The mechanism of the Mitsunobu esterification reaction. Part II. The involvement of (acyloxy)alkoxyphosphoranes. J. Org. Chem. 1989, 54, 3049–3054. [Google Scholar] [CrossRef] Itzstein, M. V.; Jenkins, I. D. The mechanism of the Mitsunobu reaction. II. Dialkoxytriphenylphosphoranes. Aust. J. Chem. 1983, 36, 557–563. [Google Scholar] Niclas, H.-J.; Martin, D. Eine einfache synthese von phosphin-imiden unter verwendung von azodicarbonsäure-dialkylestern. Tetrahedron Lett. 1978, 19, 4031–4032. [Google Scholar] [CrossRef]
- Hughes, D. L. Org. Reactions 1992, 42, 335–656.
- Ľabbé, G.; Dekerk, J.-P.; Martens, C.; Toppet, S. Chemistry of N-sulfonyl-substituted thiiranimines. J. Org. Chem. 1980, 45, 4366–4371. [Google Scholar] [CrossRef]
- Suchár, G.; Kristian, P. Synthesis, properties, and reactions of heterodienes. III. Cycloaddition reactions of isoselenocyanates with enamines and diazomethane. Chem. Zvesti 1975, 29, 244–249. [Google Scholar]
- Zhou, Y.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: Synthesis of 1,2,3-selenadiazole derivatives. Helv. Chim. Acta 2000, 83, 539–553. [Google Scholar] [CrossRef]
- Koketsu, M.; Otsuka, T.; Ishihara, H. Synthesis of 1,3-selenazetidine derivatives from imines and thiocarbamoyl isoselenocyanate. Heterocycles 2006, 68, 2107–2112. [Google Scholar] [CrossRef]
- Sommen, G. L.; Linden, A.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: Cycloaddition of carbodiimides to selenazetidines. Helv. Chim. Acta 2005, 88, 766–773. [Google Scholar] [CrossRef]
- Koketsu, M.; Yamamura, Y.; Ando, H.; Ishihara, H. Synthesis of 1,3-selenazetidines and 4H-1,3,5-oxadiazines using acyl isoselenocyanates. Heterocycles 2006, 68, 1267–1273. [Google Scholar] [CrossRef]
- Atanassov, P.K.; Linden, A.; Heimgartner, H. Synthesis of 3-acetyl-N-aryl-4-diethylaminoselenet-2(2H)-imines from 4-diethylamino-3-butyn-2-one and aryl isoselenocyanates. Heterocycles 2004, 62, 521–533. [Google Scholar]
- Yoo, C. Y.; Choi, E. B.; Pak, C. S. Preparation of 2H-thietimines from the reaction of 4-dialkylamino-3-butyn-2-one with aryl isothiocyanates. Synlett 2001, 361–364. [Google Scholar]
- Billert, T.; Beckert, R.; Döring, M.; Görls, H. Beiträge zur Chemie der pyrido[1,2-a]pyrazine - reaktivität gegenüber heterocumulenen der kohlensäurereihe und ketenen. J. Prakt. Chem. 1999, 341, 332–341. [Google Scholar] [CrossRef]
- Koketsu, M.; Kiyokuni, T.; Sakai, T.; Ando, H.; Ishihara, H. Carbonate extension. Synthesis of 1,3-selenazines and 1,3-selenazolidines via intramolecular addition of N-allylselenoureas. Chem. Lett. 2006, 35, 626–627. [Google Scholar] [CrossRef]
- Tamaru, Y.; Mizutani, M.; Furukawa, Y.; Kawamura, S.; Yoshida, Z.; Yanagi, K.; Minobe, M. 1,3-Asymmetric induction: highly stereoselective synthesis of 2,4-trans-disubstituted γ-butyrolactones and γ-butyrothiolactones. J. Am. Chem. Soc. 1984, 106, 1079–1085. [Google Scholar] Cardillo, G.; Orena, M.; Sandri, S. A new synthetic approach to 3-amino-1,2-diols from allylic alcohols via trichloroacetimidates. J. Chem. Soc., Chem. Commun. 1983, 1489–1490. [Google Scholar] [CrossRef] Bongini, A.; Cardillo, G.; Orena, M.; Porzi, G.; Sandri, S. Regio- and stereocontrolled synthesis of epoxy alcohols and triols from allylic and homoallylic alcohols via iodocarbonates. J. Org. Chem. 1982, 47, 4626–4633. [Google Scholar] Bartlett, P. A.; Meadows, J. D.; Brown, E. G.; Morimoto, A.; Jernstedt, K. K. Carbonate extension. A versatile procedure for functionalization of acyclic homoallylic alcohols with moderate stereocontrol. J. Org. Chem. 1982, 47, 4013–4018. [Google Scholar] [CrossRef]
- Koketsu, M.; Otsuka, T.; Swenson, D.; Ishihara, H. The synthesis of 1-thia-6-oxa-6aλ4-seleno-3-azapentalene and a 3H-1,2,4-dithiazole. Org. Biomol. Chem. 2007, 5, 613–616. [Google Scholar] [CrossRef]
- Morel, G.; Marchand, E.; Sinbandhit, S.; Toupet, L. 5-Chloro-3-methylthio-1,2,4-thiadiazol-2-ium chlorides as useful synthetic precursors to a variety of 6aλ4-thiapentalene systems. Heteroatom. Chem. 2003, 14, 95–105. [Google Scholar] [CrossRef]
- Lai, L.-L.; Reid, D. H.; Nicol, R. H.; Rhodes, J. B. Reactions of fused dihydro-1,2,4-thiadiazoles with isocyanates and isothiocyanates to give 6aλ4-thia-1,3,4,6-tetraazapentalene derivatives. Heteroatom. Chem. 1994, 5, 149–157. [Google Scholar] [CrossRef] Lai, L.-L.; Reid, D. H. Reactions of fused dihydro-1,2,4-thiadiazoles with isoselenocyanates giving 6aλ4-thia-1,3,4,6-tetraazapentalene derivatives and 5,10-dihydro-1,2,4-thiaselenazolo[4,5-b][2,4]benzodiazepines. Heteroat. Chem. 1996, 7, 97–109. [Google Scholar] [CrossRef] Lai, L.-L.; Reid, D. H. Synthesis of 6aλ4-thia-1,6-diselena-3,4-diazapentalenes, 1,6,6aλ4-triselena-3,4-diazapentalenes, 6aλ4-thia-1,3,4,6-tetraazapentalenes, and 6aλ4-Selena-1,3,4,6-tetraazapentalenes from cyclic thioureas and selenoureas. Heteroatom. Chem. 1997, 8, 13–27. [Google Scholar] [CrossRef]
- Goerdeler, J.; Löbach, W. Ringöffnende Cycloadditionen, VI. Reaktionen von 5-imino-∆3-1,2,4-thiadiazolinen mit heterocumulenen (Präparative gesichtspunkte). Chem Ber. 1979, 112, 517–531. [Google Scholar] [CrossRef]
- Ding, Y.; Kong, J.; Reid, D. H. Trithia- and dithiaselenapentalenes from benzylidene-1,2-dithioles and heterocumulenes. Heteroatom. Chem. 1997, 8, 233–244. [Google Scholar] [CrossRef]
- Matsumura, N.; Konishi, T.; Hayashi, H.; Yasui, M.; Iwasaki, F.; Mizuno, K. Synthesis and properties of novel macrocyclic compounds bearing thiourea moieties by use of chemical feature of hypervalent sulfur. J. Heterocyclic Chem. 2002, 39, 189–202. [Google Scholar] [CrossRef]
- Sample Availability: Not applicable.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
