Synthesis, NMR and Crystallographic Studies of 2-Substituted Dihydroquinazolinones Derived from (S)-Phenylethylamine
Abstract
:Introduction
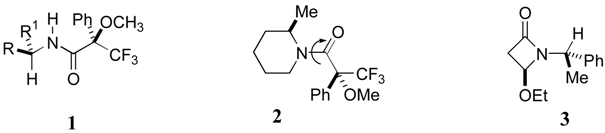
Results and Discussion
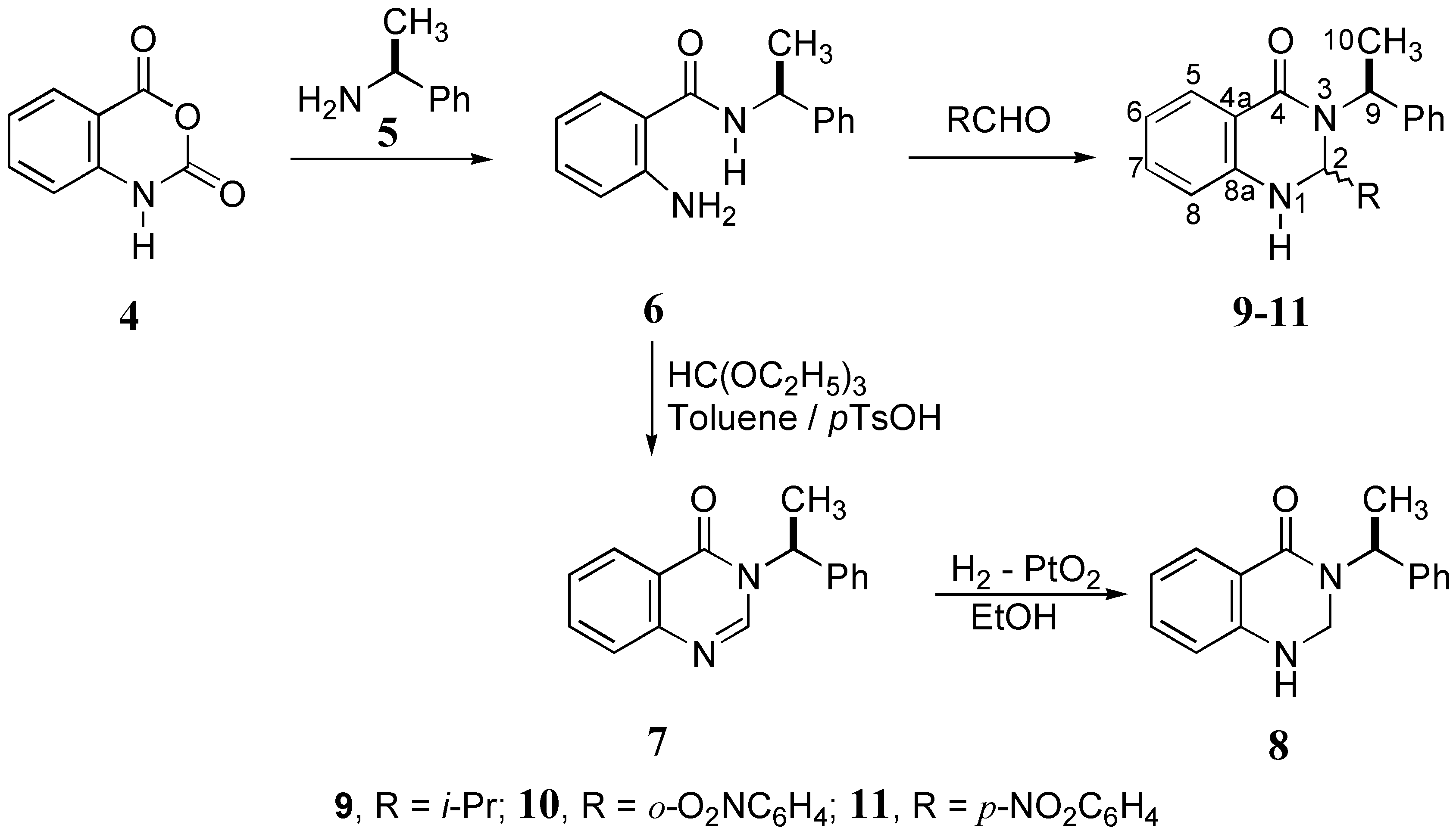
Synthesis of dihydroquinazolinones
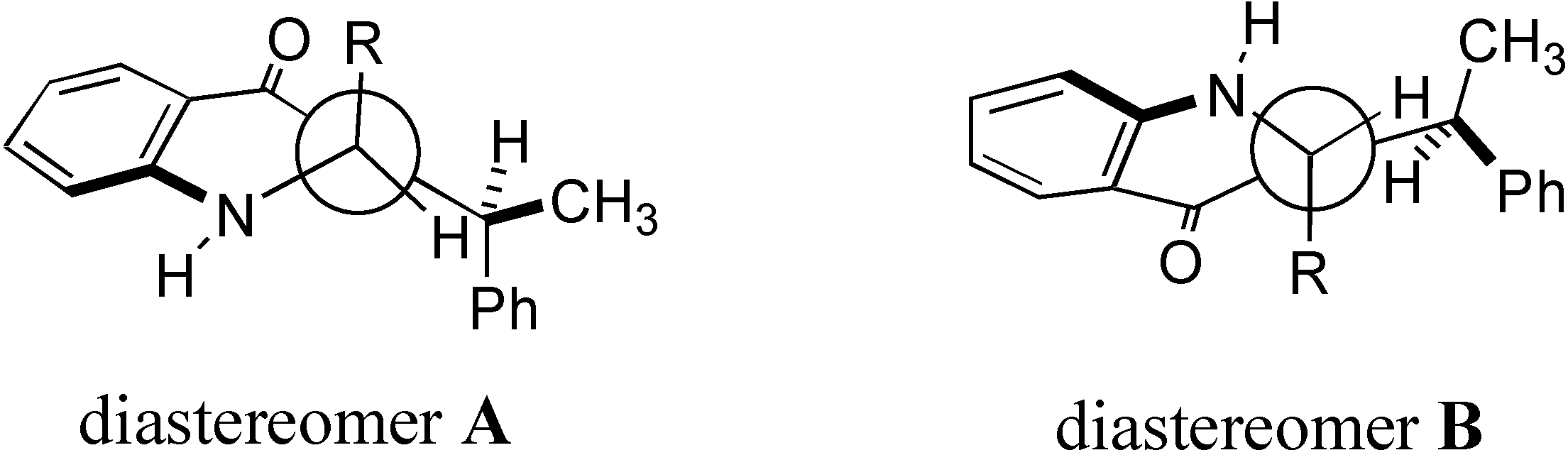
Structure and chemical shift correlations
| Compound | δ diastereomer A | δ diastereomer B | Δδ (ppb) |
| 8 | 4.174, 4.463 | − | − |
| 9 | 4.19 | 4.535 | -340 |
| 10 | 6.035 | 6.138 | -103 |
| 11 | 5.481 | 5.674 | -193 |
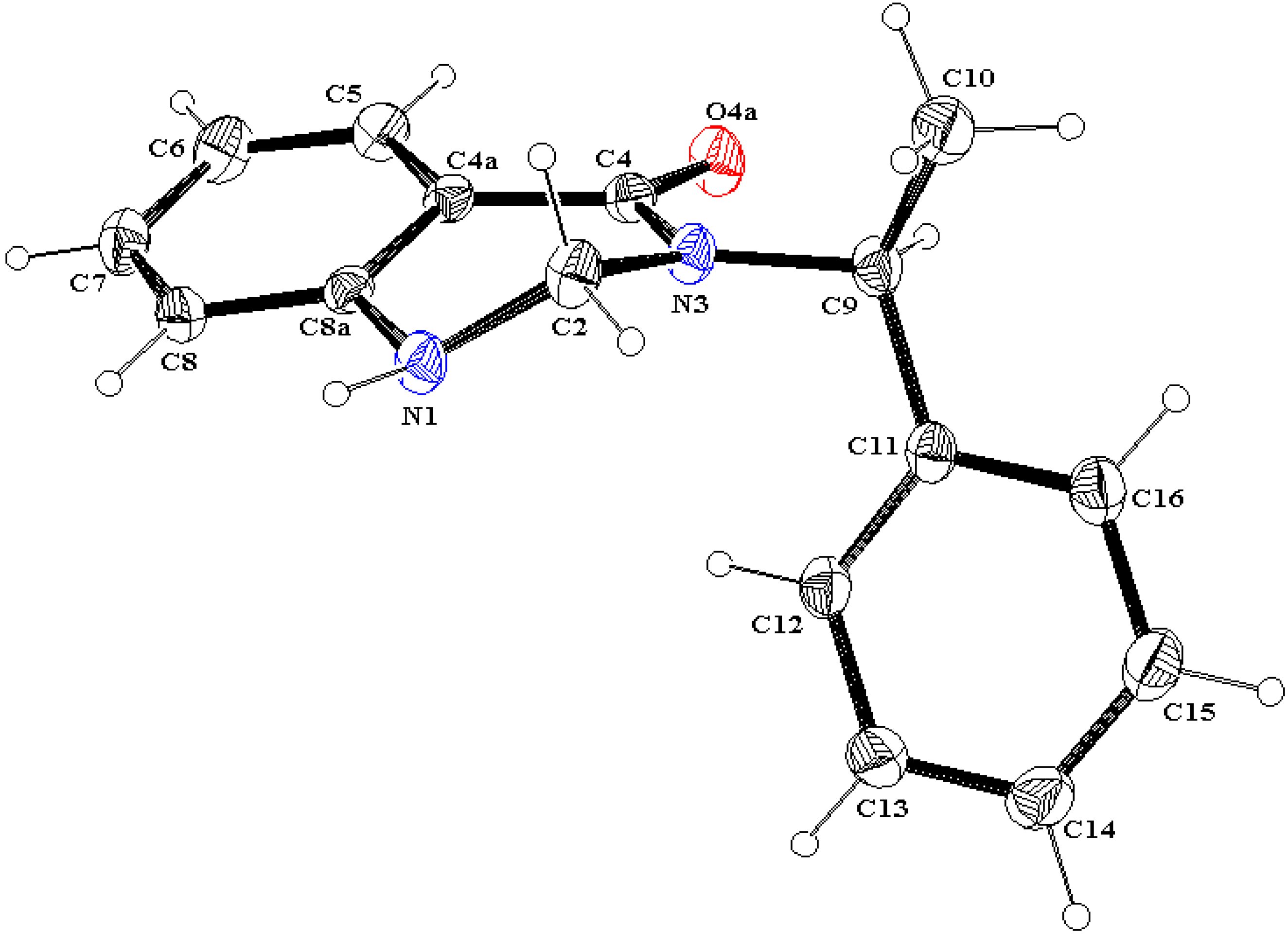
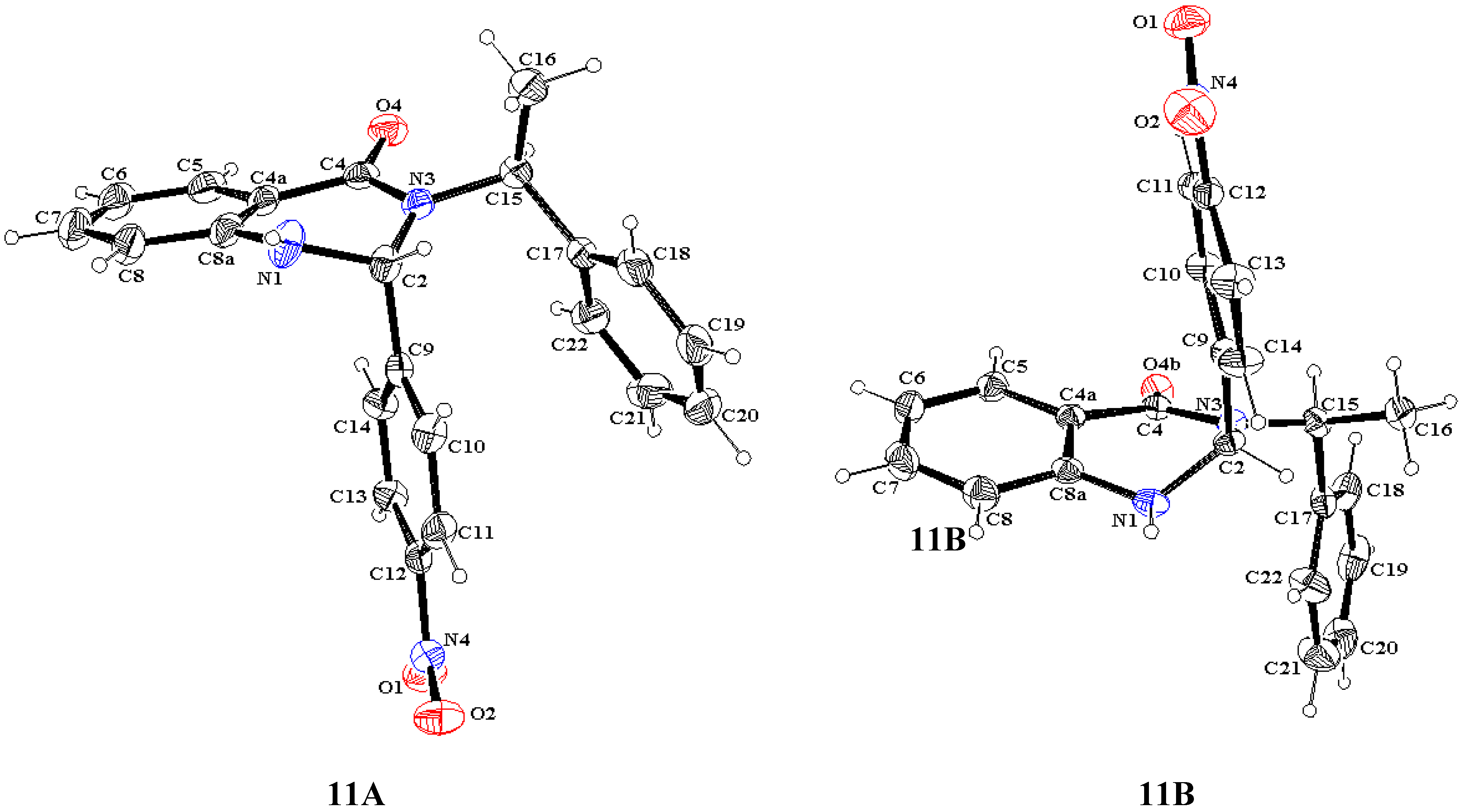
X ray diffraction
Conclusions
Experimental
General
Synthesis of dihydroquinazolinones
2,3-Dihydro-3-[(S)-1-phenethyl]-4(1H)-quinazolinone (8)
2,3-Dihydro-(2S)- and 2,3-dihydro-(2R)-isopropyl-3-[(S)-1-phenethyl]-4(1H)-quinazolinone (9A and 9B, respectively)
2,3-Dihydro-(2S)- and 2,3-dihydro-(2R)-o-nitrophenyl-3-[(S)-1-phenethyl]-4(1H)-quinazolinone (10A and 10B, respectively)
2,3-Dihydro-(2S)- and 2,3-dihydro-(2R)-p-nitrophenyl-3-[(S)-1-phenethyl]-4(1H)-quinazolinone (11A and 11B, respectively)
Acknowledgments
References and Notes
- Seco, J. M.; Quiñoá, E.; Riguera, R. The Assignment of Absolute Configuration by NMR. Chem. Rev. 2004, 104, 17–118. [Google Scholar] [CrossRef]
- Hoye, T. R.; Renner, M. K. Applications of MTPA (Mosher) Amides of Secondary Amines: Assignment of Absolute Configuration in Chiral Cyclic Amines. J. Org. Chem. 1996, 61, 8489–8495. [Google Scholar] [CrossRef]
- Rauk, A.; Tavares, D. F.; Khan, M. A.; Borkent, A. J.; Olson, J. F. Conformational analysis of chiral hindered amides. Can. J. Chem. 1983, 61, 2572–2580. [Google Scholar] [CrossRef]
- García-Martínez, C.; Taguchi, Y.; Oishi, A.; Hayamizu, K. Configurational 1H NMR study of optically active 7-(1-phenylethyl)-2-oxa-7-azabicyclo[3.2.0]heptan-6-one derivatives using Pirkle's alcohols and a chiral shift reagent. Magn. Res. Chem. 1998, 36, 429–435. [Google Scholar] [CrossRef]
- Bongini, A.; Cardillo, G.; Orena, M.; Porz, G.; Sandri, S. A new synthesis of both the enantiomers of 4-amino-3-hydroxybutanoic acid (gabob) and mm2 calculations for rotamers of the intermediate oxazolidin-2-one. Tetrahedron 1987, 43, 4377–4383. [Google Scholar] [CrossRef]García-Martínez, C.; Cervantes-Cuevas, H.; Escalante-García, J. Synthesis and NMR Configurational Analysis of 1,3-Imidazolidin-4-ones Derived from (-)-(S)-Phenylethylamine. Chirality 2003, 15, S74–S81. [Google Scholar] [CrossRef]
- Juaristi, E.; Rizo, B.; Natal, V.; Escalante, J.; Regla, I. Alternative method for the resolution of 1-benzoyl-2-tert-butyl-3-methyl-1,3-imidazolidin-4-one. Tetrahedron: Asymmetr. 1991, 2, 821–826. [Google Scholar] [CrossRef]Hazelard, D.; Fadel, A.; Girard, C. Stereoselective synthesis of (1R,2R)-1-amino-2-hydroxycyclobutanecarboxylic acid—serine derivative—, from racemic or optically active 2-benzyloxycyclobutanone. Tetrahedron: Asymmetr. 2006, 17, 1457–1464. [Google Scholar] [CrossRef]
- Escalante, J.; Flores, P.; Priego, J. M. Synthesis of 1,2-dihydroquinazolin-4(1H)-ones. Heterocycles 2004, 63, 2019–2032. [Google Scholar] [CrossRef]
- Connolly, D. J.; Cusack, D.; O’Sullivan, T. P.; Guiry, P. J. Synthesis of quinazolinones and quinazolines. Tetrahedron 2005, 61, 10153–10202. [Google Scholar] [CrossRef]
- Cardillo, G.; Tomasini, C. Enantioselective synthesis of β-aminoacids; Juaristi, E., Ed.; JW Wiley: N.Y., 1997; pp. 211–248. [Google Scholar]
- Still, W. C.; Kahn, M.; Mitra, A. Rapid chromatographic technique for preparative separations with moderate resolution. J. Org. Chem. 1978, 43, 2923–2925. [Google Scholar] [CrossRef]
- CS-Chem3D Pro 3.5.1/Chem Office 4.0, CambridgeSoft: Cambridge, MA, 1997.
- Crystallographic data of 8, 9A, 10B, 11A and 11B are deposited with the Cambridge Crystallographic Data Center (CCDC # 627346, 264374, 264375, 264376, and 264377, respectively). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- Sheldrick, G. M. SHELX97, Programs for Crystal Structure Analysis, release 97-2; Germany, 1998. [Google Scholar]
- Seebach, D.; Lamatsch, B.; Amstutz, R.; Beck, A.; Dobler, M.; Egli, M.; Fitzi, R.; Gutschi, M.; Herradón, B.; Hidber, P.; Irwin, J.; Locher, R.; Maestro, M.; Maetzke, T.; Mouriño, A.; Pfammatter, E.; Plattner, D.; Schickli, C.; Schweizer, W.; Seiler, P.; Stucky, G.; Petter, W.; Escalante, J.; Juaristi, E.; Quintana, D.; Miravitlles, C.; Molins. Structure and Reactivity of Five- and Six-Ring N,N-,O-, and O,O-Acetals: A Lesson in Allylic 1,3-Strain (A1,3 Strain). E. Helv. Chim. Acta 1992, 75, 913–934. [Google Scholar] [CrossRef]
- The interatomic distance, in angstroms, between the methine hydrogen at C9 or C15 and the oxygen at C4 is as follows: 9A, 2.275; 10B, 2.280; 11A, 2.486; 11B, 2.316.
- Mei, X.; Wolf, C. Highly Congested Nondistorted Diheteroarylnaphthalenes: Model Compounds for the Investigation of Intramolecular π-Stacking Interactions. J. Org. Chem. 2005, 70, 2299–2305. [Google Scholar] [CrossRef]
- Priego, J.; Flores, P.; Ortiz-Nava, C.; Escalante, J. Synthesis of enantiopure cis- and trans-2-aminocyclohexane-1-carboxylic acid from octahydroquinazolin-4-ones. Tetrahedron: Asymmetr. 2004, 15, 3545–3549. [Google Scholar] [CrossRef]
- Sample Availability: Small samples (a few milligrams) of 8, 9, 10 and 11 are available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Escalante, J.; Ortíz-Nava, C.; Flores, P.; Priego, J.M.; García-Martínez, C. Synthesis, NMR and Crystallographic Studies of 2-Substituted Dihydroquinazolinones Derived from (S)-Phenylethylamine. Molecules 2007, 12, 173-182. https://doi.org/10.3390/12020173
Escalante J, Ortíz-Nava C, Flores P, Priego JM, García-Martínez C. Synthesis, NMR and Crystallographic Studies of 2-Substituted Dihydroquinazolinones Derived from (S)-Phenylethylamine. Molecules. 2007; 12(2):173-182. https://doi.org/10.3390/12020173
Chicago/Turabian StyleEscalante, Jaime, Claudia Ortíz-Nava, Patricia Flores, Jaime M. Priego, and Cirilo García-Martínez. 2007. "Synthesis, NMR and Crystallographic Studies of 2-Substituted Dihydroquinazolinones Derived from (S)-Phenylethylamine" Molecules 12, no. 2: 173-182. https://doi.org/10.3390/12020173
APA StyleEscalante, J., Ortíz-Nava, C., Flores, P., Priego, J. M., & García-Martínez, C. (2007). Synthesis, NMR and Crystallographic Studies of 2-Substituted Dihydroquinazolinones Derived from (S)-Phenylethylamine. Molecules, 12(2), 173-182. https://doi.org/10.3390/12020173




