Dopamine-Imprinted Polymers: Template-Monomer Interactions, Analysis of Template Removal and Application to Solid Phase Extraction
Abstract
:Introduction
Results and Discussion
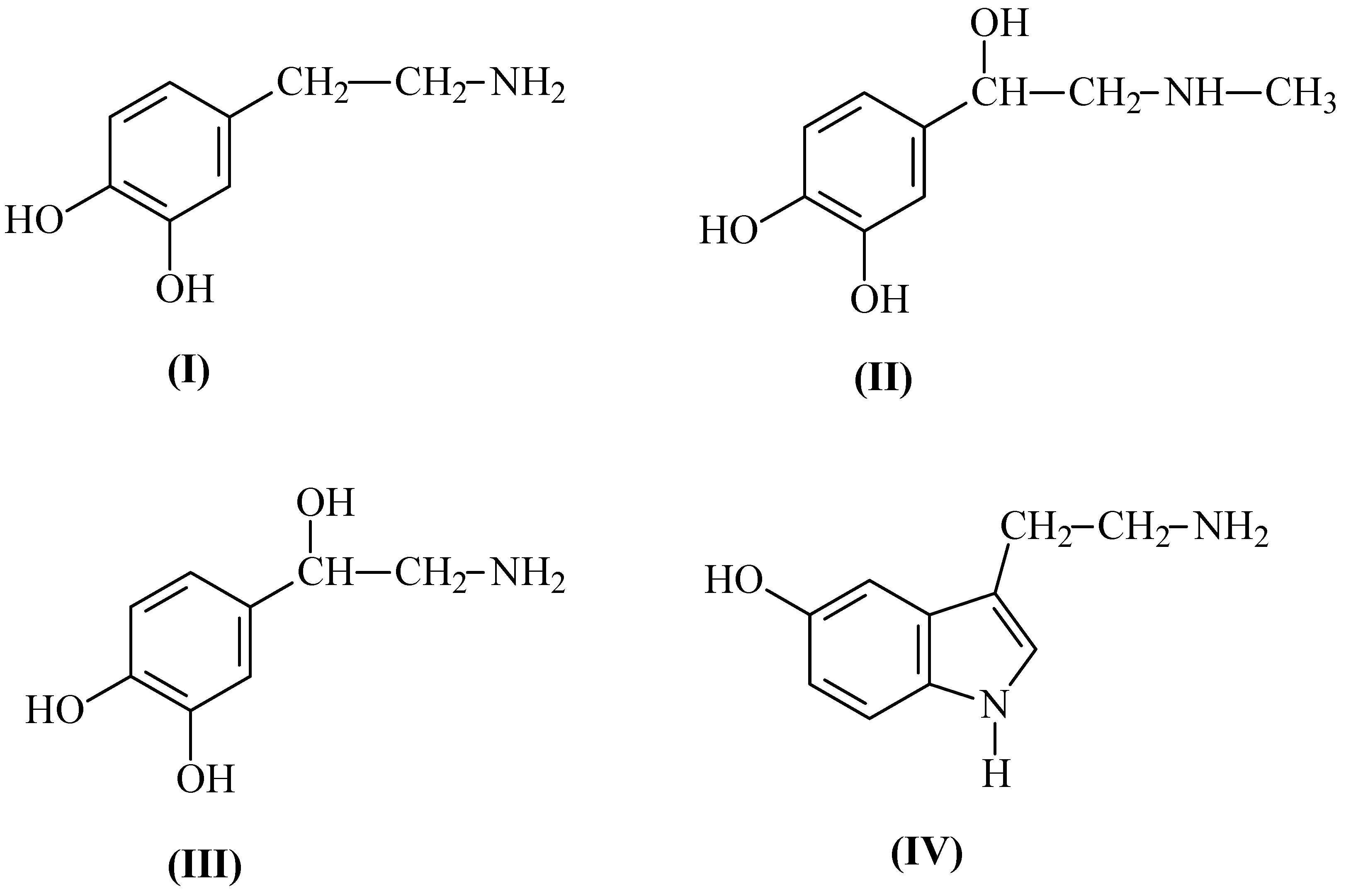
Molecular modeling of complexes
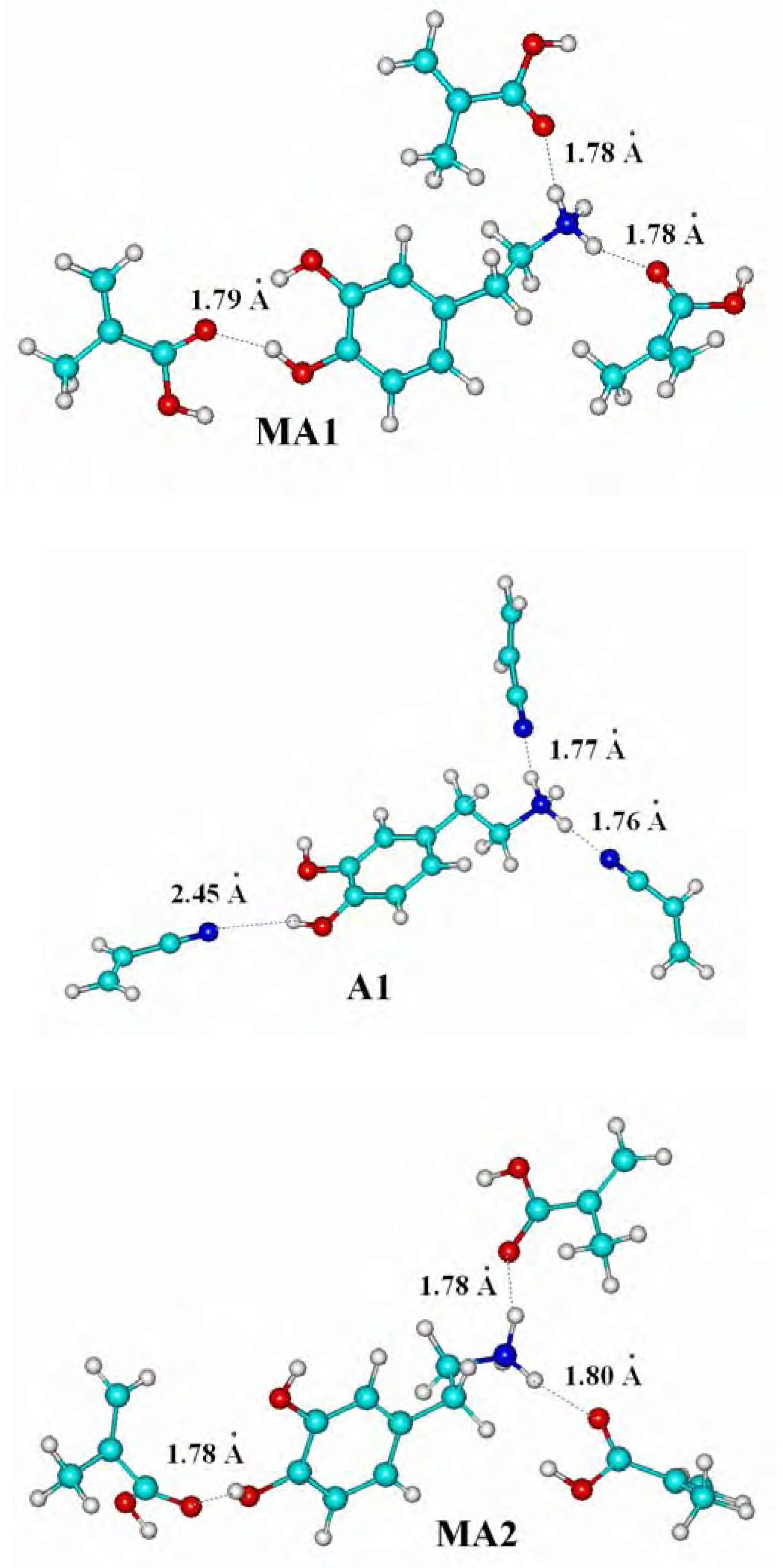
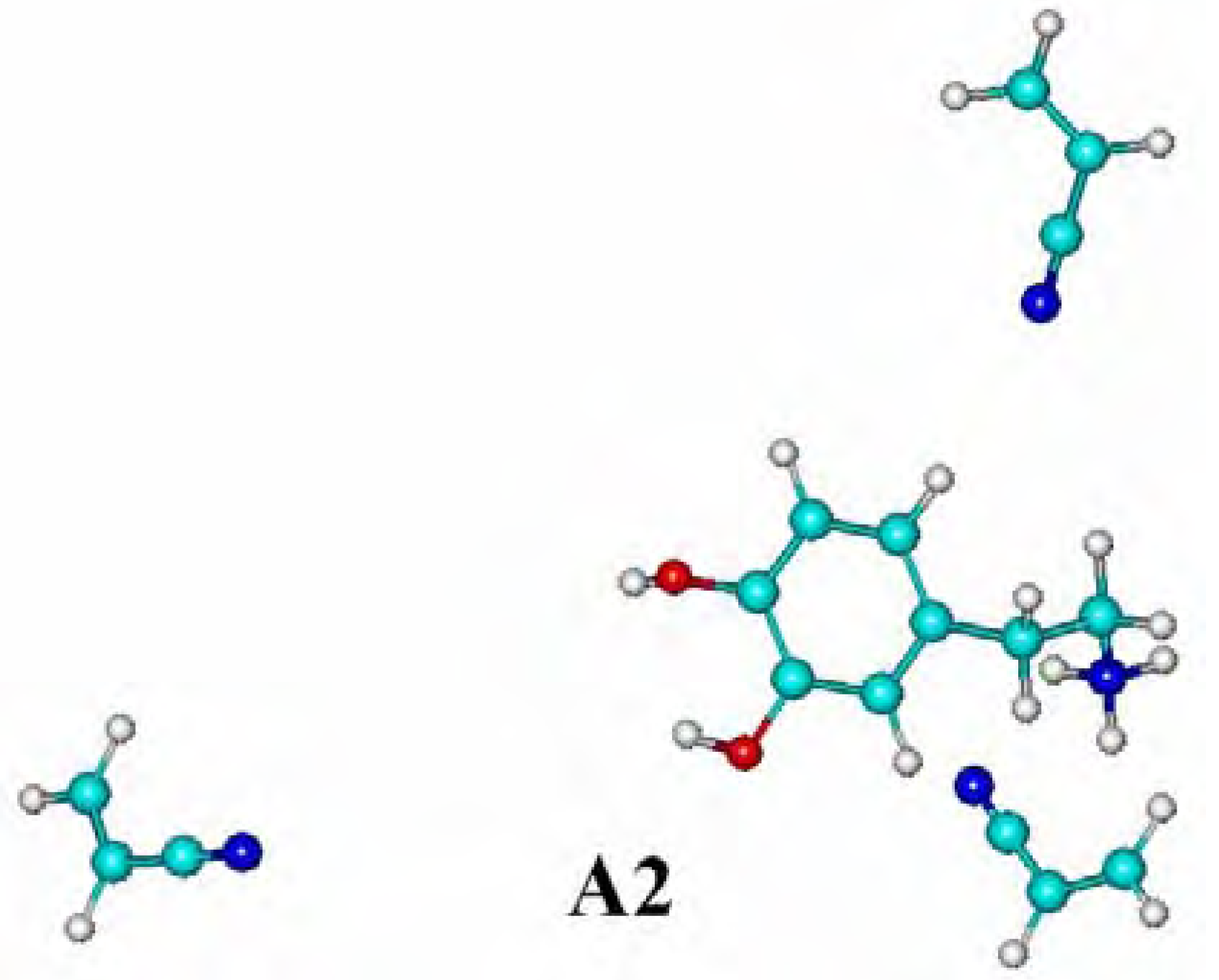
| Complexes | Energy ΔE [kJ.mol-1] |
|---|---|
| MA1 | -175.49 |
| MA2 (in water) | -151.18 |
| A1 | -155.29 |
| A2 (in water) | -49.51 |
Preparation of molecularly imprinted polymers
| Binding (%)a) | |||||
|---|---|---|---|---|---|
| Porogen system | water-acetonitrile (15:85, v/v) | water-methanol (12.5:87.5, v/v) | |||
| Conditioning | pH 5 | pH 8 | pH 5 | pH 8 | |
| Load | MIP | 71.4 | 100.0 | 84.1 | 98.3 |
| NIP | 59.2 | 100.0 | 29.1 | 91.7 | |
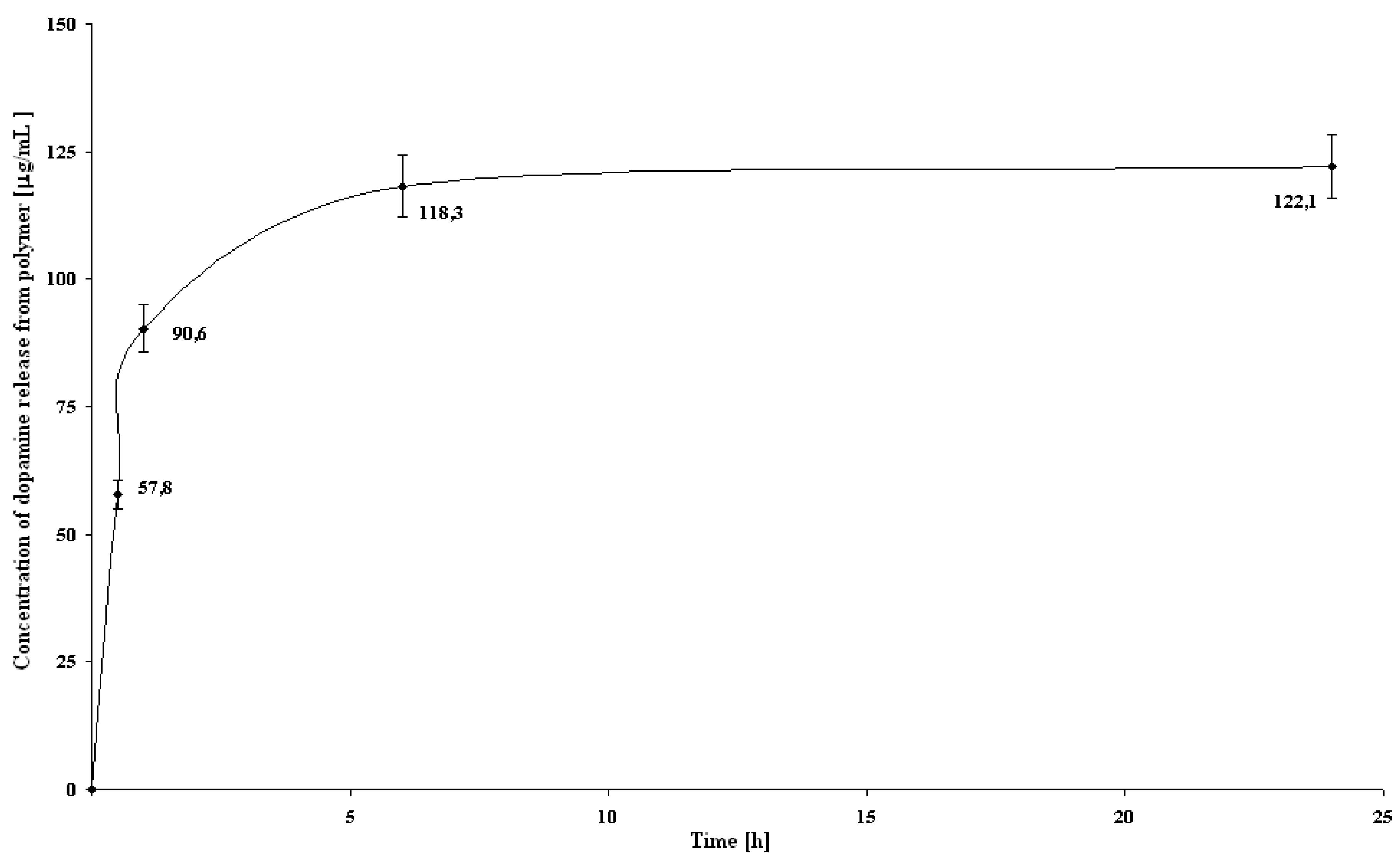
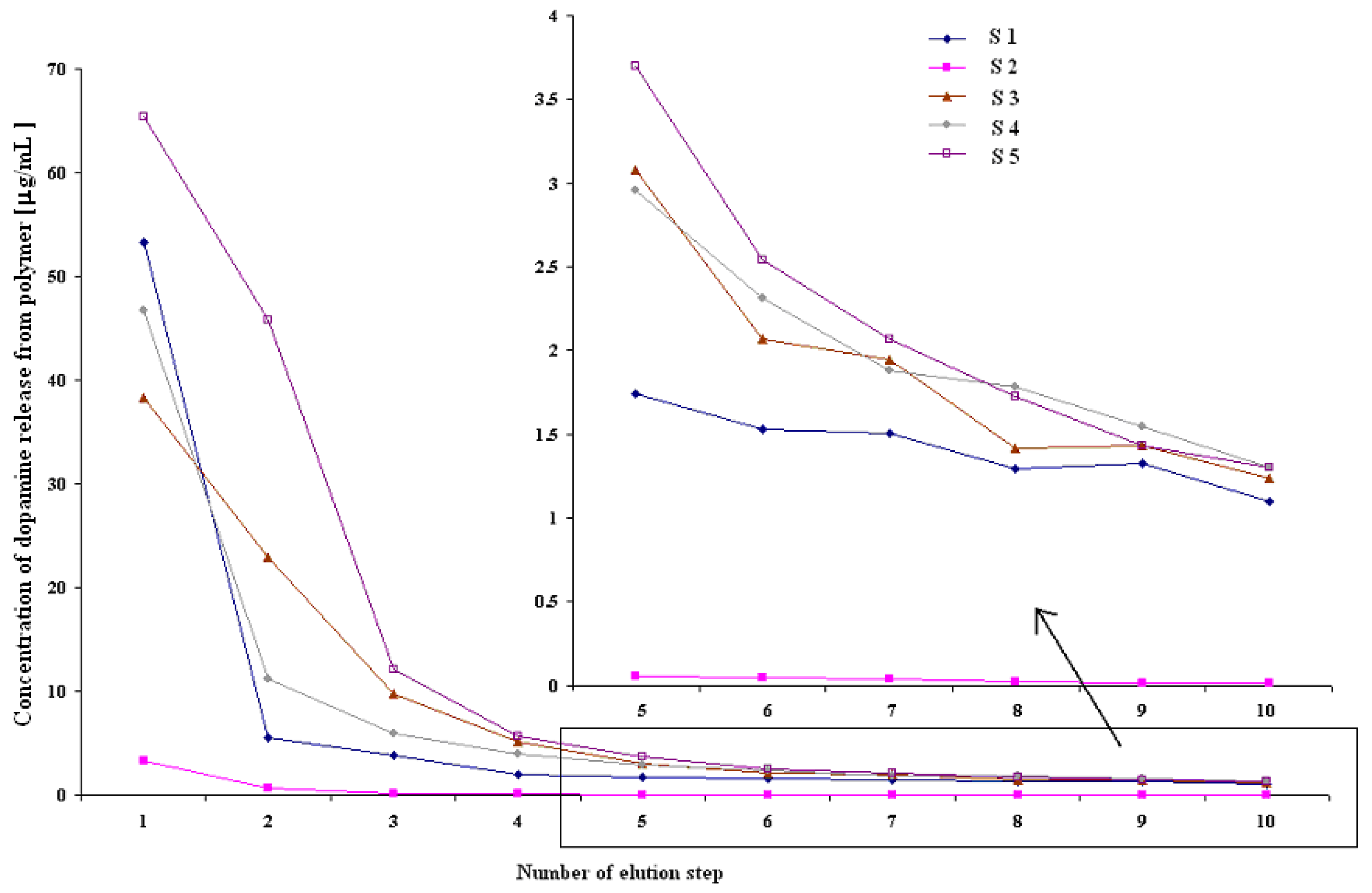
Template removal
| Number of protocol/column | Volume of eluant [mL]a) | Interval between elution steps [min] | Total time of ten elution steps [min] | Drying |
|---|---|---|---|---|
| S 1 | 1 | 5 | 60 | No special treatment |
| S 2 | 25 | 5 | 300 | No special treatment |
| S 3 | 1 | 30 | 310 | No special treatment |
| S 4 | 1 | 5 | 60 | Nitrogen stream |
| S 5 | 1 | 5 | 60 | Hot air stream |

Solid-phase extraction
| Step | Recovery (± S.D., n = 5, %) | |
|---|---|---|
| MIP | NIP | |
| Loading | 15.9 ± 3.7 | 70.8 ± 2.4 |
| Washing | 17.3 ± 1.6 | 10.6 ± 2.2 |
| Elution 1 | 62.3 ± 3.1 | 10.6 ± 2.2 |
| Elution 2 | 6.9 ± 1.3 | 3.3 ± 0.9 |
| Step | Recovery (± S.D., n = 5, %) | ||
|---|---|---|---|
| Epinephrine | Norepinephrine | 5-Hydroxytryptamine | |
| Loading | 76.8 ± 5.0 | 86.5 ± 4.3 | 67.5 ± 1.0 |
| Washing | 5.2 ± 2.4 | 7.8 ± 0.9 | 12.0 ± 0.4 |
| Elution 1 | 2.9 ± 1.4 | 4.9 ± 2.2 | 9.3 ± 5.1 |
| Elution 2 | 0.2 ± 0.1 | 0.3 ± 0.1 | 3.7 ± 0.6 |
Conclusions
Experimental
General
Stock solutions of catecholamines and 5-hydroxytryptamine
Template removal
- i)
- Continuous extraction using a Soxhlet apparatus (CE): MIP particles (439 mg) were placed in a Soxhlet apparatus and continuously extracted (30 cycles, 80 mL, methanol - water, 1:1 v/v). Before further analysis, particles were dried at 50oC in an oven at atmospheric pressure.
- ii)
- Microwave-assisted extraction (ME): MIP particles (415 mg) were put into a microwave vessel, and methanol - water (1:1 v/v, 10 mL) was added; the mixture was refluxed with internal stirring for 2 min, then cooled for 2 min. This step was repeated twice, then the particles were filtered off and transferred to the vessel. The procedure was repeated twice with a fresh portion of the solvent mixture, then the solvent system was changed to isopropanol and once more to methanol - water, 1:1 v/v, and the same procedure was followed. Finally, the vessel was allowed to reach room temperature and its contents were collected and dried at 50oC in an oven at atmospheric pressure. To compare the influence of microwave radiation on polymer network structure, a sample of non-imprinted polymer (NIP) was also treated once with each solvent system according to the given procedure. To determine the level of dopamine bleeding from resin after treatments i and ii, we proceeded as follows: dry MIP samples (56.2 mg) were put into 1 mL glass SPE columns and eluted with pH 3 ammonium formate, - methanol solution (3:1 v/v, 1 mL). An aliquot of solvent (400 μL) was used to analyze the amount of bleeding DA by fluorimetry.
- iii)
- Stationary extraction (SE): MIP particles (56.2 mg) were placed in Eppendorf vials and pH 3 ammonium formate - methanol solution (3:1 v/v, 1 mL) was added to each sample. Vials were put into a shaker, and shaken out over various periods of time ranging from half an hour to 24 h. Then the samples were centrifuged (2500 rpm, 5 min). An aliquot of solvent (400 μL) was used to analyze the amount of desorbed DA by fluorimetry.
- iv)
- Permanent elution (PE): Five kinds of 1 mL glass SPE columns with dry packed MIP (56.2 mg) were eluted with pH 3 ammonium formate - methanol solution (3:1 v/v, flow rate 1 mL/min) in different ways S1 - S5, as shown in Table 3. An aliquot of solvent (400 μL) was used to detect template concentration by the fluorescence spectroscopic method. Each experiment was performed in triplicate. To study the template removal for a long period of time, PE protocol of S1 (see Table 1) was repeated three times: after 3 h, 72 h and 75 h (between the experiments sample S1 was closed and stored in a refrigerator).
Binding energy calculations
Solid-phase extraction
- Prewashing the particles with water (1 mL).
- Conditioning with pH 8 phosphate buffered saline (2 mL).
- Loading with 1 μg/mL concentration of dopamine hydrochloride (1 mL).
- Washing with water (1 mL).
- Elution stages (1 and 2) were performed using 0.05 M aqueous ammonium formate, pH 3 - methanol solution (75:25 v/v, 0.5 mL).
- Prewashing the particles with water (1 mL).
- Conditioning with water (2 mL) adjusted to pH 5 with 0.04 M perchloric acid.
- Loading with 1 μg/mL concentration of dopamine hydrochloride (1 mL).
- Washing with water (1 mL).
- Elution stages (1 and 2) were performed using 0.05 M aqueous ammonium formate, pH 3 - methanol solution (75:25 v/v, 0.5 mL).
References
- Jaber, M.; Robinson, S. W.; Missale, C.; Caron, M. G. Dopamine receptors and brain function. Neuropharmacology 1996, 35, 1503–1519. [Google Scholar] [CrossRef]
- Wilson, J. M.; Sanyal, S.; Van Tol, H. H. M. Dopamine D2 and D4 receptor ligands: relation to antipsychotic action. Eur. J. Pharmacol. 1998, 351, 273–286. [Google Scholar] [CrossRef]
- Wong, A. H. C.; Buckle, C. E.; Van Tol, H. H. M. Polymorphisms in dopamine receptors: what do they tell us? Eur. J. Pharmacol. 2000, 410, 183–203. [Google Scholar]
- Wang, H. Y.; Sun, Y.; Tang, B. Study on fluorescence property of dopamine and determination of dopamine by fluorimetry. Talanta 2002, 57, 899–907. [Google Scholar] [CrossRef]
- Wang, H. Y.; Hui, Q. S.; Xu, L. X.; Jiang, J. G.; Sun, Y. Fluorimetric determination of dopamine in pharmaceutical products and urine using ethylene diamine as the fluorigenic agent. Anal. Chim. Acta 2003, 497, 93–99. [Google Scholar]
- Mamiński, M.; Olejniczak, M.; Chudy, M.; Dybko; Brzózka, Z. Spectrophotometric determination of dopamine in microliter scale using microfluidic system based on polymeric technology. Anal. Chim. Acta 2005, 540, 153–157. [Google Scholar]
- Wang, H.-S.; Li, T.-H.; Jia, W.-L.; Xu, H.-Y. Highly selective and sensitive determination of dopamine using a Nafion/carbon nanotubes coated poly(3-methylthiophene) modified electrodes. Biosens. Bioelectron. 2006, 22, 664–669. [Google Scholar] [CrossRef]
- Komiyama, M.; Takeuchi, T.; Mukawa, T.; Asanuma, H. (Eds.) Molecular Imprinting: From Fundamentals to Applications; Wiley-VCH: Weinheim, 2003.
- Haupt, K. Molecularly imprinted polymers and their use in biomimetics sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef]
- Wuff, G. Enzyme-like catalysis by molecularly imprinted polymers. Chem. Rev. 2002, 102, 1–28. [Google Scholar]
- Rick, J.; Chou, T. C. Amperometric protein sensor – fabricated as a polypyrrole, poly-aminophenylboronic acid bilayer. Biosens. Bioelectron. 2006, 22, 329–335. [Google Scholar] [CrossRef]
- Haupt, K. Imprinted polymers – tailor-made mimics of antibodies and receptors. Chem. Commun. 2003, 171–178. [Google Scholar]
- Alexander, C.; Davidson, L.; Hayes, W. Imprinted polymers: artificial molecular recognition materials with applications in synthesis and catalysis. Tetrahedron 2003, 59, 2025–2057. [Google Scholar] [CrossRef]
- He, C. Y.; Long, Y. Y.; Pan, J. L.; Li, K.; Lui, F. Application of molecularly imprinted polymers to solid-phase extraction of analytes from real samples. J. Biochem. Biophys. Meth. 2007, 70, 133–150. [Google Scholar] [CrossRef]
- Qiao, F. X.; Sun, H. W.; Yan, H. Y.; Row, K. H. Molecularly imprinted polymers for solid phase extraction. Chromatographia 2006, 64, 625–634. [Google Scholar] [CrossRef]
- Yang, J.; Hu, Y.; Cai, J.-B.; Zhu, X.-L.; Su, Q.-D. A new molecularly imprinted polymer for selective extraction of cotinine from urine samples by solid phase extraction. Anal. Bioanal. Chem. 2006, 384, 761–768. [Google Scholar]
- Svenson, J.; Nicholls, I. A. On the thermal and chemical stability of molecularly imprinted polymers. Anal. Chim. Acta 2001, 435, 19–24. [Google Scholar] [CrossRef]
- Owens, P. K.; Karlsson, L. Molecular imprinting for bio- and pharmaceutical analysis. Trends Anal. Chem. 1999, 18, 146–154. [Google Scholar] [CrossRef]
- Andersson, L. I. Molecular imprinting for drug bioanalysis. A review on the application of imprinted polymers to solid-phase extracton and binding assay. J. Chromatogr. B 2000, 739, 163–173. [Google Scholar] [CrossRef]
- Makote, R.; Collinson, M. M. Dopamine recognition in templated silicate films. Chem. Commun. 1998, 425–426. [Google Scholar] [CrossRef]
- Ling, T.-R.; Syu, Y. Z.; Tasi, Y.-C.; Chou, T.-C.; Liu, C.-C. Size-selective recognition of catecholoamines by molecular imprinting on silica-alumina gel. Biosens. Bioelectron. 2005, 21, 901–907. [Google Scholar] [CrossRef]
- Takeuchi, T.; Murase, N.; Maki, H.; Mukawa, T.; Shinmori, H. Dopamine selective molecularly imprinted polymers via post-imprinting modification. Org. Biomol. Chem. 2006, 4, 565–568. [Google Scholar]
- Suedee, R.; Seechamnanturakit, V.; Canyuk, B.; Ovatlarnporn, C.; Martin, G. P. Temperature sensitive dopamine-imprinted (N,N-methylene-bis-acrylamide cross-linked) polymer and its potential application to the selective extraction of adrenergic drugs from urine. J. Chromatogr. A 2006, 1114, 239–249. [Google Scholar] [CrossRef]
- Liu, K.; Wie, W. Z.; Zeng, J. X.; Liu, X. Y.; Gao, Y. P. Application of novel electrosynthesized polydopamine – imprinted film to the capacitive sensing of nicotine. Anal. Bioanal. Chem. 2006, 385, 724–729. [Google Scholar]
- Liu, Y.; Wang, F.; Tan, T.; Lei, M. Study of the properties of molecularly imprinted polymers by computational and conformational analysis. Anal. Chim. Acta 2007, 581, 137–146. [Google Scholar]
- Dineiro, Y.; Menendez, I.; Blanco-Lopez, M. C.; Lobo-Castanon, M. J.; Miranda-Ordieres, A. J.; unon-Blanco, P. Computational predictions and experimental affinity distributions for a homovanillic acid amolecularly imprinted polymer. Biosens. Bioelectron 2006, 22, 364–371. [Google Scholar] [CrossRef]
- Piletska, E. V.; Turner, N. W.; Turner, A. P. F.; Piletsky, S. A. Controlled release of the herbicide simazine from computationally designed molecularly imprinted polymers. J. Control. Release 2005, 108, 132–139. [Google Scholar] [CrossRef]
- Wie, S.; Jakusch, M.; Mizaikoff, B. Capturing molecules with templated materials – Analysis and rational design of molecularly imprinted polymers. Anal. Chim. Acta 2006, 578, 50–58. [Google Scholar]
- Cormack, P. A. G.; Elorza, A. Z. Molecularly imprinted polymers: synthesis and characterisation. J. Chromatogr. B 2004, 804, 173–182. [Google Scholar] [CrossRef]
- Luliński, P.; Maciejewska, D.; Bamburowicz-Klimkowska, M.; Szutowski, M. Preliminary evaluation of molecularly imprinted polymer synthesized with dopamine hydrochloride as a templat. Ninth Electronic Conference on Synthetic Organic Chemistry, ECSOC-9 2005, B-001. [Google Scholar]
- Ellwagner, A.; Berggren, C.; Bayoudh, S.; Crecenzi, C.; Karlsson, L.; Owens, P. K.; Ensing, K.; Cormack, P.; Sherrington, D.; Sellergren, B. Evaluation of methods aimed at complete removal of template from molecularly imprinted polymers. Analyst 2001, 126, 784–792. [Google Scholar] [CrossRef]
- Lakshmana, M. K.; Raju, T. R. An isocratic assay for norepinephrine, dopamine, and 5-hydroxytryptamine using their native fluorescence by high-performance liquid chromatography with fluorescence detection in discrete brain areas of rat. Anal. Biochem. 1997, 246, 166–170. [Google Scholar] [CrossRef]
- Sigma - Aldrich Co. Report. Eur. 2003, 9, 2.
- HyperChem 7.02 program. HyperCube Inc: Waterloo, ON, Canada, 2002.
- Sample Availability: Available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Luliński, P.; Maciejewska, D.; Bamburowicz-Klimkowska, M.; Szutowski, M. Dopamine-Imprinted Polymers: Template-Monomer Interactions, Analysis of Template Removal and Application to Solid Phase Extraction. Molecules 2007, 12, 2434-2449. https://doi.org/10.3390/12112434
Luliński P, Maciejewska D, Bamburowicz-Klimkowska M, Szutowski M. Dopamine-Imprinted Polymers: Template-Monomer Interactions, Analysis of Template Removal and Application to Solid Phase Extraction. Molecules. 2007; 12(11):2434-2449. https://doi.org/10.3390/12112434
Chicago/Turabian StyleLuliński, Piotr, Dorota Maciejewska, Magdalena Bamburowicz-Klimkowska, and Mirosław Szutowski. 2007. "Dopamine-Imprinted Polymers: Template-Monomer Interactions, Analysis of Template Removal and Application to Solid Phase Extraction" Molecules 12, no. 11: 2434-2449. https://doi.org/10.3390/12112434
APA StyleLuliński, P., Maciejewska, D., Bamburowicz-Klimkowska, M., & Szutowski, M. (2007). Dopamine-Imprinted Polymers: Template-Monomer Interactions, Analysis of Template Removal and Application to Solid Phase Extraction. Molecules, 12(11), 2434-2449. https://doi.org/10.3390/12112434




