Synthesis of New 4(3H)-Quinazolinone Derivatives Using 5(4H)-Oxazolones
Abstract
:Introduction
Results and Discussion
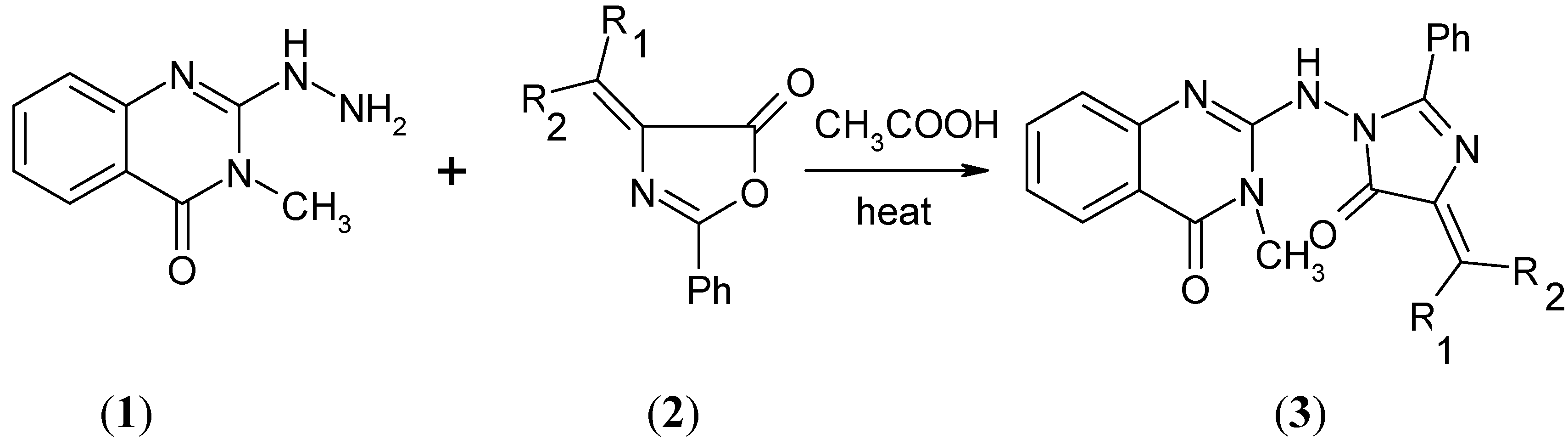
| Compound | R1 | R2 |
|---|---|---|
| 2a | C6H5 | H |
| 2b | 4-ClC6H4 | H |
| 2c | 4-CH3OC6H4 | H |
| 2d | Cyclohexyl | |
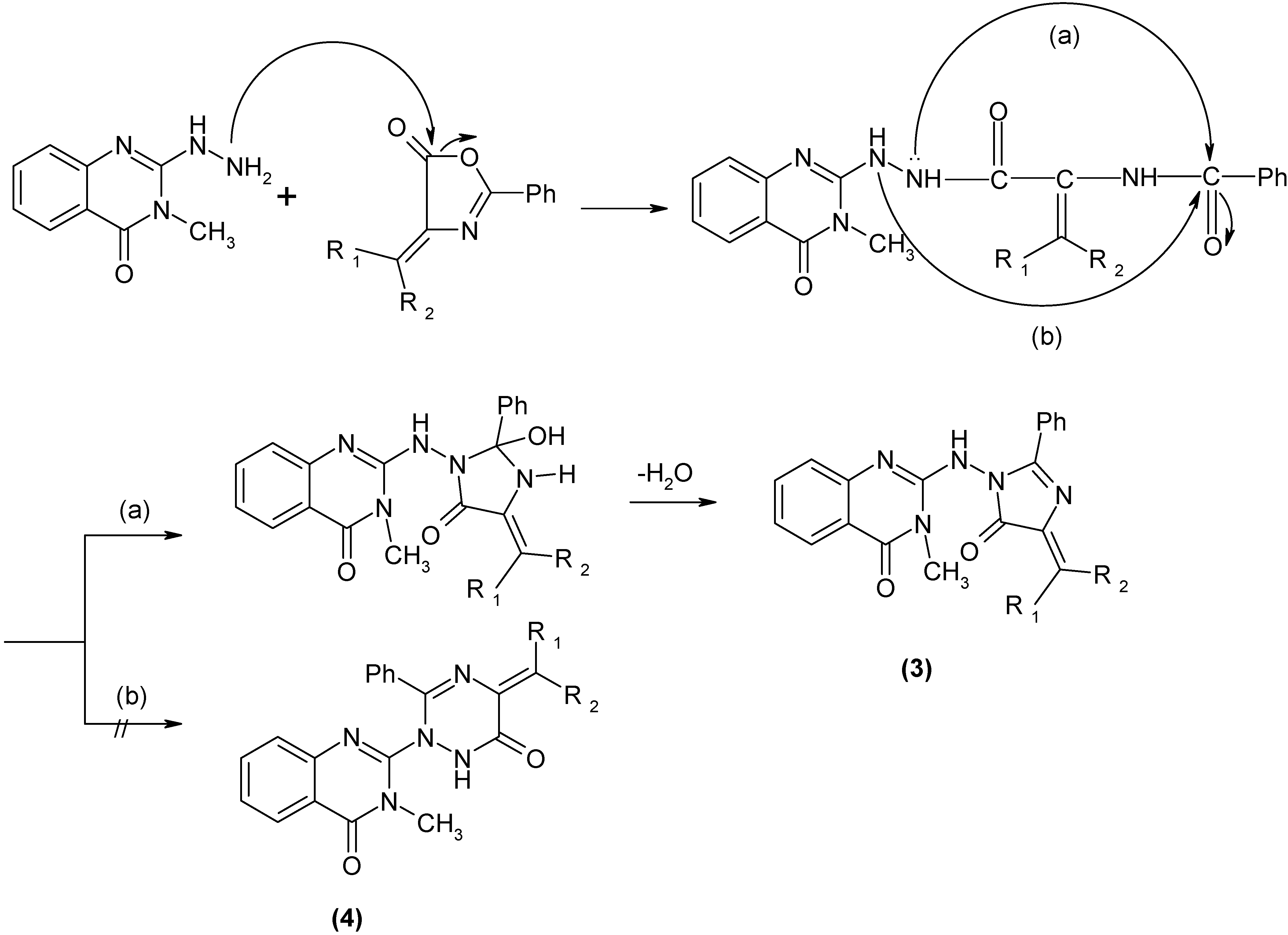
Conclusions
Acknowledgments
Experimental
General
Preparation of 2-mercapto-3-methyl-3,4-dihydro-4-quinazolinone [8].
Preparation of 2-hydrazino-3-methyl-3,4-dihydro-4-quinazolinone (3) [9].
Preparation of 5(4H)-oxazolone derivatives (2a-d) [10]
General procedure for the preparation of compounds (3a-d).
References
- (a) Wolfe, J. F.; Rathman, T. L.; Sleevi, M. C.; Campbell, J. A.; Greenwood, T. D. Synthesis and anticonvulsant activity of some new 2-substituted 3-aryl-4(3H)-quinazolinones. J. Med. Chem. 1990, 33, 161–166. [Google Scholar] (b) Padia, J. K.; Field, M.; Hinton, J.; Meecham, K.; Pablo, J.; Pinnock, R.; Roth, B. D.; Singh, L.; Suman-Chauhan, N.; Trivedi, B. K.; Webdale, L. Novel Nonpeptide CCK-B Antagonists: Design and Development of Quinazolinone Derivatives as Potent, Selective, and Orally Active CCK-B Antagonists. J. Med. Chem. 1998, 41, 1042–1049. [Google Scholar]
- Xia, Y.; Yang, Z. Y.; Hour, M. J.; Kuo, S. C.; Xia, P.; Bastow, K. F.; Nakanishi, Y.; Nampoothiri, P.; Hackl, T.; Hamel, E.; Lee, K. H. Antitumor Agents. Part 204: Synthesis and Biological Evaluation of Substituted 2-Aryl Quinazolinones. Bioorg. Med. Chem. Lett. 2001, 11, 1193–1196. [Google Scholar] [CrossRef]
- Kenichi, O.; Yoshihisa, Y.; Toyonari, O.; Toru, I.; Yoshio, I. Studies on 4(1H)-Quinazolinones. 5. Synthesis and Antiinflammatory Activity of 4(1H)-Quinazolinone Derivatives. J. Med. Chem. 1985, 28, 568–576. [Google Scholar]
- Buchanan, J. G.; Sable, H. Z. Selective Organic Transformations; Thyagarajan B., S., Ed.; Wiley-Interscience: New York, 1972; Vol. 2, pp. 1–95. [Google Scholar]
- Lyle, F. R. U.S. Patent 5,973,257, 1985. [Chem. Abstr. 1985, 65, 2870].
- Segarra, V.; Crespo, M. I.; Pujol, F.; Belata, J.; Domenech, T.; Miralpeix, M.; Palacios, J. M.; Castro, A.; Martinez, A. Phosphodiesterase inhibitory properties of losartan. design and synthesis of new lead compounds. Bioorg. Med. Chem. Lett. 1998, 8, 505–510. [Google Scholar]
- Akazome, M.; Yamamoto, J.; Kondo, T.; watanabe, Y. Palladium complex-catalyzed intermolecular reductive N-heterocyclization: novel synthesis of quinazoline derivatives from 2-nitrobenzaldehyde or 2-nitrophenyl ketones with formamide. J. Organomet. Chem. 1995, 494, 229–233. [Google Scholar]
- Butler, K.; Partridge, M. W. Cyclic Amidines. Part VIII. Derivatives of 12H-6:7:12a-Triazabenz[a]anthracene and 5aH-5:6:11a-Triazanaphthacene. J. Chem. Soc. 1959, 1512–1520. [Google Scholar]
- Yesilada, A.; Koyunoglu, S.; Saygili, N.; Kupeli, E.; Yesilada, E.; Bedir, E.; Khan, I. Synthesis, Anti-inflamatory and Analgesic Activity Screening of some New 4(3-H)-Quinazolinone Derivatives. Arch. Pharm. Pharm. Med. Chem. 2004, 337, 96–104. [Google Scholar]
- Crawford, M.; Little, W.T. The Erlenmeyer Reaction with Aliphatic Aldehydes, 2-Phenyloxazol-5-one Being Used Instead of Hippuric Acid. J. Chem. Soc. 1959, 729–731. [Google Scholar] [CrossRef]
- Sample availability: available from the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Hamidian, H.; Tikdari, A.M.; Khabazzadeh, H. Synthesis of New 4(3H)-Quinazolinone Derivatives Using 5(4H)-Oxazolones. Molecules 2006, 11, 377-382. https://doi.org/10.3390/11050377
Hamidian H, Tikdari AM, Khabazzadeh H. Synthesis of New 4(3H)-Quinazolinone Derivatives Using 5(4H)-Oxazolones. Molecules. 2006; 11(5):377-382. https://doi.org/10.3390/11050377
Chicago/Turabian StyleHamidian, Hooshang, Ahmad Momeni Tikdari, and Hojatollah Khabazzadeh. 2006. "Synthesis of New 4(3H)-Quinazolinone Derivatives Using 5(4H)-Oxazolones" Molecules 11, no. 5: 377-382. https://doi.org/10.3390/11050377
APA StyleHamidian, H., Tikdari, A. M., & Khabazzadeh, H. (2006). Synthesis of New 4(3H)-Quinazolinone Derivatives Using 5(4H)-Oxazolones. Molecules, 11(5), 377-382. https://doi.org/10.3390/11050377



