Abstract
Chemical analysis of the secondary metabolite pattern of the sacoglossan mollusc Elysia cf. expansa, collected along South Indian coasts, showed the presence of the typical Caulerpa-derived sesquiterpene caulerpenyne (1) and two new minor co-occurring metabolites, the compounds dihydrocaulerpenyne (4) and expansinol (5). The chemical characterization of these molecules, structurally related to 1, is reported.
Introduction
Sacoglossan molluscs are herbivorous and primarily associated, with a few exceptions, with siphonalean green algae [1]. They are often well camouflaged, as much of their color being derived from the chloroplasts of algae on which they feed [2,3]. The order Sacoglossa includes both shelled and shell-less species, it has been reported that shelled sacoglossans, all belonging to the superfamily Oxynoidea, feed exclusively on the morphologically variable algal genus Caulerpa, while changes in the diet occur in the major groups of shell-less species [4]. The dietary relationship between Caulerpa and its shelled sacoglossan predators has been confirmed by the presence of typical Caulerpa metabolites and/or their derivatives, in the molluscs [5]. On the other hand, shell-less sacoglossans, belonging to the genus Elysia, are able to either accumulate sesquiterpenoids [6], diterpenoids [6] and depsipeptides [7,8] from their algal prey, modify such molecules [6,9] or biosynthesize de novo polypropionates [10,11]. However, in analogy with shelled sacoglossans [12,13,14], a trophic relationship between some Caribbean Elysia species and Caulerpa has also been demonstrated by chemical studies [6], while there is a lack of chemical information about the Indo-Pacific species that seems to have similar alimentary habits.
The ability of Caulerpa-feeder molluscs to transform dietary caulerpenyne (1), the main Caulerpa sesquiterpene [15], into the toxic aldehyde derivatives, oxytoxin-1 (2) and oxytoxin-2 (3), has been also suggested for different oxynoidean [12,13] and elysioidean species [6]. The lipase-mediated conversion of caulerpenyne (1) into oxytoxin-2 (3) has been recently demonstrated in cell-free preparations of Mediterranean sacoglossan Oxynoe olivacea [16].
We report here the first chemical study of a shell-less Indo-Pacific Caulerpa-feeder sacoglossan mollusc, collected along the South Indian coasts and tentatively identified as Elysia cf. expansa, it showed a secondary metabolite pattern dominated by caulerpenyne (1) [15] co-occurring with two novel minor metabolites, dihydrocaulerpenyne (4) and expansinol (5). A small amount of the pigment caulerpin (6), already isolated from several Caulerpa algae [17,18], was also detected in the extract. We report here the chemical characterization of compounds 4 and 5 using a variety of spectroscopic methods as well as by comparison with appropriate synthesized model compounds.
Results and Discussion
TLC analyses in different eluent systems of the ether extracts of the external (obtained by ultrasound treatment) and internal parts of Elysia cf. expansa showed different metabolite compositions. In particular, the ether extract of the external parts was characterized by a main UV positive spot at Rf 0.6 (light petroleum/diethyl ether, 1:1), along with a series of minor more polar (Rf 0.55-0.2) UV-visible compounds, whereas the extract of internal parts was constituted mainly by the typical lipids (fatty acid glycerides), as confirmed by 1H-NMR analysis. The external part extract was submitted to LH-20 Sephadex column chromatography to obtain two fractions containing the metabolites at Rf 0.6-0.2 These two fractions were separately purified by silica-gel column chromatography to give the main component, caulerpenyne (1), and enriched fractions containing minor related metabolites, which were further purified by reverse-phase HPLC, to afford two unprecedented molecules, named dihydrocaulerpenyne (4) and expansinol (5). The known pigment caulerpin (6) was also isolated from the extract. Caulerpenyne (1) [15] and caulerpin (6) [17,18] were identified by comparison of their 1H-NMR, mass spectra and [α] values with literature data. The new compounds 4 and 5, both structurally related to the main metabolite caulerpenyne (1), were characterized as described below.
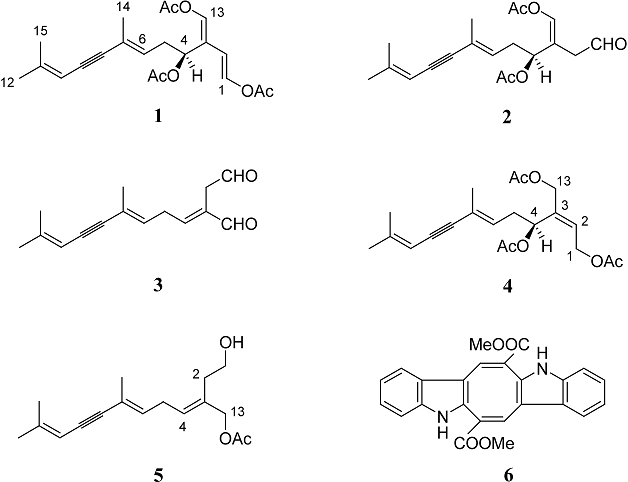
The molecular formula C21H28O6 of dihydrocaulerpenyne (4) was derived from the sodiated molecular peak at m/z 399.1781 in the HR-ESIMS spectrum. Preliminary NMR analysis of compound 4 displayed strong spectral similarities with caulerpenyne (1), suggesting a linear sesquiterpene skeleton with the same functionalization. In fact, the 1H-NMR spectrum of sesquiterpene 4 at 400MHz showed high fields signal attributable to three vinyl methyls at δ 1.82 (3H, bs, H3-15), δ 1.81 (3H, bs, H3-14) and δ 1.46 (3H, bs, H3-12), three acetoxy groups at δ 1.64 (3H, s, OAc-1), δ 1.57 (3H, s, OAc-4) and δ 1.63 (3H, s, OAc-13), and an allylic methylene at δ 2.49 (1H, m, H-5a) and 2.30 (1H, m, H-5b), analogous with caulerpenyne (1). On the other hand, the low field proton pattern of 4 was different from that of 1, as it displayed the same olefinic multiplets at δ 5.88 (1H, t, J=7.0 Hz, H-6) and δ 5.42 (1H, bs, H-10), along with a double doublet at δ 5.63 (1H, dd, J=6.6, 7.7 Hz, H-4), which was up-field shifted with respect to the corresponding proton in caulerpenyne (1), but it lacked the signals due to the 1,4-diacetoxy-1,4-butadiene moiety. In their place, two methylene multiplets at δ 4.77 (2H, m, H2-1) and at δ 4.55 (2H, ABq, J= 13.4 Hz, H2-13), and an additional vinyl signal at δ 5.72 (1H, t, J= 6.4 Hz, H-2) were observed in the spectrum of compound 4, suggesting the presence of a 1,4-diacetoxy-2-butene moiety. Analysis of the 13C-NMR spectrum confirmed this hypothesis, and 4 was thus identified as the 1,4-dihydroderivative of caulerpenyne (1). The stereochemistry at C-4 was assumed to be the same as caulerpenyne (1), whereas the Z-stereochemistry of the C-2/C-3 double bond was established by a series of n.O.e. difference experiments, whereby diagnostic n.O.e. effects were observed between H2-13 (δ 4.55) and H-2 (δ 5.72) as well as between H2-1 (δ 4.77) and H-4 (δ 5.63). All 1H- and 13C-NMR resonances (Table 1) were fully assigned by mono- and bi-dimensional NMR experiments (COSY, HSQC, HMBC) and were in complete agreement with the proposed structure.

Table 1.
NMR Spectroscopic Dataa (400 MHz, C6D6) for dihydro-caulerpenyne (4) and expansinol (5).
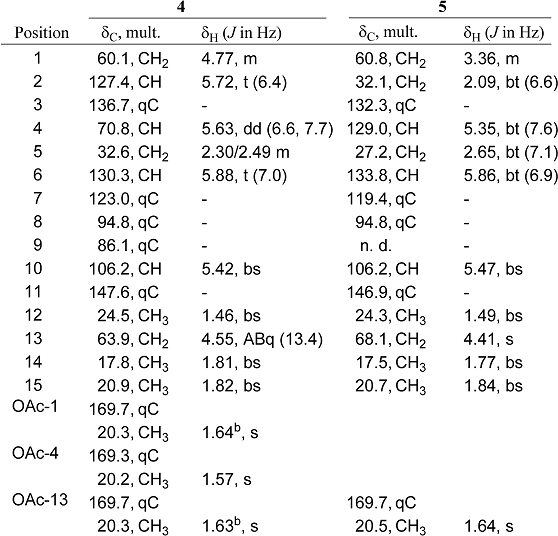 |
aAssignments aided by COSY, HSQC, HMBCbValues maybe interchanged
The more polar compound, expansinol (5), was quite unstable and displayed a sodiated molecular ion at m/z 299.1620 (C17H24O3). Spectral data analysis indicated a close relationship between 5 with compounds of caulerpenyne family, showing the structural differences with known caulerpenyne derivatives in the oxidized terminal part of the molecule. The 1H-NMR spectrum contained signals due to one acetoxy group (δ 1.64, 3H, s, OAc-13) and three vinyl methyls at δ 1.84 (3H, bs, H3-15), δ 1.77 (3H, bs, H3-14) and δ 1.49 (3H, bs, H3-12). Three olefinic multiplets at δ 5.86 (1H, bt, 6.9 Hz, H-6); δ 5.47 (1H, bs, H-10) and δ 5.35 (1H, bt, 7.6 Hz, H-4) supported the presence of three trisubstituted double bonds in accordance with caulerpenyne skeleton. In addition, the 1H-NMR spectrum showed signals attributed to two oxygen-bearing methylenes, one of which (δ 4.41, 2H, s, H2-13) was isolated and another one, resonating at δ 3.36 (2H, m, H2-1), which was further coupled to an allylic methylene at δ 2.09 (2H, bt, 6.6 Hz, H2-2). A multiplet at δ 2.65 (2H, bt, 7.1 Hz, H2-5) assigned to a bis-allylic methylene group completed the 1H-NMR spectrum. These data suggested that 5 was the monoacetyl ester of a diol derivative of caulerpenyne, i.e. 7 (the 3E-isomer) or 8 (the 3Z-isomer), previously obtained from caulerpenyne (1) by sodium borohydride reduction [15]. Unfortunately, the degradation of expansinol (5) prevented the recording of n.O.e. difference experiments, which had been planned to determine the stereochemistry of C-3/C-4 double bond.
We therefore decided to synthesize the model diol derivatives 7 and 8, starting from natural caulerpenyne, and compare their NMR data with that of expansinol (5). An aliquot of sample of 1 was submitted to NaBH4 reduction as reported in the literature [15] giving the expected mixture of 7 and 8 (ratio 5:1).

Figure 1.
Selected 1H- and 13C-NMR values for compounds 7 (3E-isomer) and 8 (3Z-isomer).
The 1H-NMR spectrum of this mixture showed distinct H2-2, H-4 and H2-13 signals for both diols, so NMR analysis (13C NMR, HSQC, and n.O.e. difference experiments) was directly conducted on the mixture, in order to identify the two isomers. The proton and carbon assignments of the terminal diol moieties of compounds 7 and 8 are reported in Figure 1. Comparison of these data with those of 5 clearly indicated the 3E-stereochemistry for the natural mono-acetyl derivative. Particularly diagnostic was the 13C-NMR shift assigned to C-2 (32.1 ppm).
Conclusions
Analogously with Elysia species from the Caribbean area, but in contrast with Oxynoidean sacoglossans, Elysia cf. expansa has revealed a Caulerpa-derived metabolism, dominated by the presence of caulerpenyne (1) (14% of ether extract of the external part). In addition, two caulerpenyne-related molecules, compounds 4 and 5, and the pigment caulerpin (6) have been detected in trace amounts in the extract (ca. 0.2%, 0.1% and 0.3% of ether extract of the external part, respectively). The ethereal extracts of several Caulerpa spp. samples, collected in small amounts from the same habitat as the sacoglossan were also analyzed by HPLC chromatography, showing the presence of caulerpenyne (1) and caulerpin (6) in all samples, whereas compounds 4 and 5 were not detectable. This finding raises the question of whether compounds 4 and 5 may be the result of transformation of 1 by the sacoglossan or whether the molluscs may simply accumulate very minor algal compounds. A series of caulerpenyne-derived products related to compounds 4 and 5, have in fact been reported from several tropical Caulerpa algae [19,20,21,22]. However, in contrast to the majority of Caulerpa-feeder sacoglossans, the defensive toxins, oxytoxin-1 (2) and oxytoxin-2 (3), were not found in Elysia cf. expansa. It has been suggested that these compounds are obtained by oxynoidean and elysioidean sacoglossans by transforming dietary caulerpenyne (1) [6,12,13,16], but they have also been reported to be produced in Caulerpa algae by a wound-activated defensive mechanism [23,24]. An alternative hypothesis suggests that the two toxins are either minor metabolites selectively accumulated from algae or they are formed during the suctorial feeding of the alga by a wound-activated mechanism. Recently, it has been rigorously proven that the sacoglossan Oxynoe olivacea contains two different kinds of lipases that selectively can hydrolyze one of the two enol-acetates displayed by caulerpenyne [16]. These results are further supported by the data reported in this work. In fact, the presence in Elysia cf. expansa of large amounts of caulerpenyne proves that this sesquiterpenoid can be accumulated by the mollusc without a relevant wound-activated degradation. On the other side, it is noteworthy to observe the close chemical analogy between Indo-Pacific Elysia cf. expansa and the co-generic Caribbean E. nisbeti [6]. Both sacoglossans seem to be unable to biotransform dietary caulerpenyne, with an important accumulation of this metabolite. In summary, the structural analogy of compounds 4 and 5 with those described in other Caulerpa algae seems to support a dietary accumulation in the mollusc of minor algal metabolites whereas the absence of toxins 2 and 3 suggests that Oxynoidea and Elysioidea sacoglossans possess different hydrolytic enzymatic systems.
Experimental
General
Silica-gel chromatography was performed using pre-coated Merck F254 plates and Merck Kieselgel 60 powder. Sephadex LH-20 for molecular exclusion chromatography was purchased from Pharmacia (Uppsala, Sweden). HPLC purification was carried out on a Shimadzu LC-10AD liquid chromatograph equipped with an UV SPD-10A wavelength detector. Optical rotations were measured on a Jasco DIP 370 digital polarimeter. IR spectra were recorded on a BioRad FTS 155 FT-IR spectrophotometer. NMR experiments were recorded at ICB-NMR Service Centre. 1D- and 2D-NMR spectra were acquired in C6D6 (δ values are reported referred to the C6H6 signal at 7.15 ppm) on a Bruker Avance-400 operating at 400 MHz, using an inverse probe fitted with a gradient along the Z-axis. 13C-NMR were recorded on a Bruker DPX-300 operating at 300 MHz (δ values are reported referenced to C6D6, 128.0 ppm) using a dual probe. High resolution ESIMS were performed on a Micromass Q-TOF MicroTM coupled with a Waters Alliance 2695 HPLC. The instrument was calibrated by using a PEG mixture from 200 to 1000 amu (resolution specification 5000 FWHM, deviation <5 ppm RMS in the presence of a known lock mass).
Biological material
70 specimens of Elysia cf. expansa (average size 2.5 cm) were collected off Mandapam, Tamil Nadu (India), in January 2001, at a depth of 3-7 meters. The molluscs were immediately frozen and stored at –20°C till the extraction. The animals, showing a distinctive black line along parapodial edges, were tentatively identified as Elysia cf. expansa. A voucher specimen is deposited at ICB (code I 12). Samples of different co-occurring Caulerpa spp. were also deposited at ICB (codes I 33, I 35, I 45, I 66, I 67, I 69).
Extraction and isolation procedure
E. expansa (70 individuals) was first extracted with portions of acetone under ultrasound irradiation (each 100 mL x 3) to obtain metabolites present in the external part of the mollusc. The organic fraction was evaporated under vacuum and the resulting aqueous suspension was partitioned between diethyl ether and water. The organic phase was concentrated affording 770 mg of crude external part extract. The whole animal residue was homogenized with a pestle and extracted with acetone (3 x 50 mL). After removing the organic solvent the aqueous suspension was extracted with diethyl ether. The organic portion was evaporated affording 70 mg of crude internal part extract. Both extracts were analysed by TLC chromatography and 1H-NMR spectroscopy. The external ether soluble fraction (770 mg), was chromatographed on a Sephadex LH-20 column (eluent: 1:1 CHCl3/CH3OH) to give five fractions: I (150 mg), II (220 mg), III (120 mg), IV (250 mg) and V (40 mg). Fractions III and IV were submitted to further purification on a silica-gel column (light petroleum ether with increasing amounts of diethyl ether). Fraction III yielded several fractions, some of which were subjected to HPLC purification (Chromasil C18, CH3OH/H2O gradient, flow 1 mL/min) to give caulerpenyne (1, 50 mg) and compound 4 (1.2 mg). Fraction IV was submitted first to silica-gel column chromatography and then to HPLC (Chromasil C18, CH3OH/H2O gradient, flow 1 mL/min), again affording caulerpenyne (1, 60.0 mg), along with expansinol (5, 0.4 mg). Fraction V was subjected to silica-gel column chromatography (petroleum ether/diethyl ether) to give caulerpin 6 (2.0 mg). Samples of Caulerpa spp. were extracted with acetone (3x50 mL) and, after removing the organic solvent, the residual aqueous fractions were extracted with diethyl ether. Thin layer chromatography of Caulerpa ethereal extracts revealed only the presence of caulerpenyne (1) and caulerpin (6) in comparison with those of the animals.
Characterization data
Dihydrocaulerpenyne (4): Rf = 0.6 (1:1 light petroleum ether-diethyl ether); [α]D25= - 49.5° (c= 1 mg, CHCl3); IR νmax (liquid film): 2983, 2922, 2848, 1743, 1431, 1371, 1226, 1025 cm-1; UV (CH3OH) λmax (log ε) 283 (4.33), 270 (4.46), 203 (4.40) nm; HRESIMS: m/z 399.1781 (calculated for C21H28O6Na: 399.1784); 1H- and 13C-NMR data are given in Table 1.
Expansinol (5): Rf = 0.35 (1:1light petroleum ether-diethyl ether); [α]D25= - 14.0° (c= 0.4 mg, CHCl3); IR νmax (liquid film): 2929, 2856, 1740, 1443, 1381, 1234, 1034 cm-1; UV (CH3OH) λmax (log ε) 283 (4.27) 269 (4.33) 203 (4.31) nm; HRESIMS: m/z 299.1620 (calculated for C17H24O3Na: 299.1623); 1H- and 13C-NMR data are given in Table 1.
Acknowledgements
We thank ICB Mass Service and ICB NMR Service Centre (Mrs. D. Melck is kindly acknowledged), Mr. C. Iodice for spectrophotometric measurements and Mr. R. Turco for graphical work. This work was partially supported by a bilateral CNR-CSIR project.
References
- Jensen, K.R. A review of sacoglossan diets, with comparative notes on radular and buccal anatomy. Malacol. Rev. 1980, 13, 55–77. [Google Scholar]
- Clark, K.B.; Busacca, M. Feeding specificity and chloroplast retention in four tropical ascoglossa, with a discussion of the extent of chloroplast symbiosis and the evolution of the order. J. Moll. Stud. 1978, 44, 272–282. [Google Scholar]
- Marin, A.; Ros, J. Ultrastructural and ecological aspects of the development of chloroplast retention in the sacoglossan gastropod Elysia timida. J. Moll. Stud. 1993, 59, 95–104. [Google Scholar] [CrossRef]
- Jensen, K.R. Evolution of sacoglossans (Mollusca, Opisthobranchia) and the ecological association with their food plants. Evol. Ecol. 1997, 11, 305–335. [Google Scholar] [CrossRef]
- Cimino, G.; Fontana, A.; Gavagnin, M. Marine opisthobranch molluscs: chemistry and ecology in sacoglossans and dorids. Curr. Org. Chem. 1999, 3, 327–372. [Google Scholar]
- Gavagnin, M.; Mollo, E.; Monatanaro, D.; Ortea, J.; Cimino, G. Chemical studies of Caribbean sacoglossans: dietary relationships with green algae and ecological implications. J. Chem. Ecol. 2000, 26, 1563–1578. [Google Scholar]
- Horgen, F.D.; delos Santos, D.B.; Goetz, G.; Sakamoto, B.; Kan, Y.; Nagai, H.; Scheuer, P.J. A new depsipeptide from the sacoglossan mollusk Elysia ornata and the green alga Bryopsis species. J. Nat. Prod. 2000, 63, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Becerro, M.A.; Goetz, G.; Paul, V.J.; Scheuer, P.J. Chemical defenses of the sacoglossan mollusk Elysia rufescens and its host alga Bryopsis sp. J. Chem. Ecol. 2001, 27, 2287–2299. [Google Scholar]
- Paul, V.J.; Van Alstyne, K.L. Use of ingested algal diterpenoids by Elysia imedae Macnae (Opisthobranchia: Ascoglossa) as antipredator defenses. J. Exp. Mar. Biol. Ecol. 1988, 119, 15–29. [Google Scholar]
- Gavagnin, M.; Marin, A.; Mollo, E.; Crispino, A.; Villani, G.; Cimino, G. Secondary metabolites from Mediterranean Elysioidea: origin and biological role. Comp. Biochem. Physiol. 1994, 108B, 107–115. [Google Scholar]
- Gavagnin, M.; Spinella, A.; Marin, A.; Castelluccio, F.; Cimino, G. Polypropionates from the Mediterranean mollusk Elysia timida. J. Nat. Prod. 1994, 57, 298–304. [Google Scholar] [CrossRef]
- Cimino, G.; Crispino, A.; Di Marzo, V.; Gavagnin, M.; Ros, J.D. Oxytoxins, bioactive molecules produced by the marine opisthobranch mollusc Oxynoe olivacea from a diet-derived precursor. Experientia 1990, 46, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Gavagnin, M.; Marin, A.; Castelluccio, F.; Villani, G.; Cimino, G. Defensive relationship between Caulerpa prolifera and its shelled sacoglossan predators. J. Exp. Mar. Biol. Ecol. 1994, 175, 197–210. [Google Scholar] [CrossRef]
- Fontana, A.; Ciavatta, M.L.; Mollo, E.; Naik, C.G.; Wahidulla, S.; D’Souza, L.; Cimino, G. Volvatellin, caulerpenyne-related product from the sacoglossan Volvatella sp. J. Nat. Prod. 1999, 62, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Amico, V.; Oriente, G.; Piattelli, M.; Tringali, C. Caulerpenyne, an unusual sesquiterpenoid from the green alga Caulerpa prolifera. Tetrahedron Lett. 1978, 38, 3593–3596. [Google Scholar] [CrossRef]
- Cutignano, A.; Notti, V.; D’Ippolito, G.; Domenech Coll, A.; Cimino, G.; Fontana, A. Lipase-mediated production of defensive toxins in the marine mollusc Oxynoe olivacea. Org. Biomol. Chem. 2004, 2, 3167–3171. [Google Scholar] [CrossRef] [PubMed]
- Maiti, B.C.; Thomas, R.H.; Mahendran, M. The structure of caulerpin, a pigment from Caulerpa algae. J. Chem. Res. Synop. 1978, 126–127. [Google Scholar]
- Schwede, J.G.; Cardellina II, J.H.; Grodi, S.H.; James, T.R.; Blackman, A. Distribution of the pigment caulerpin in species of the green alga Caulerpa. Phytochemistry 1987, 26, 155–158. [Google Scholar] [CrossRef]
- Guerriero, A.; Meinesz, A.; D’Ambrosio, M.; Pietra, F. Isolation of toxic and potentially toxic sesqui- and monoterpenes from the tropical green seaweed Caulerpa taxifolia which has invaded the region of Cap Martin and Monaco. Helv. Chim. Acta 1992, 75, 689–695. [Google Scholar] [CrossRef]
- Guerriero, A.; Marchetti, F.; D’Ambrosio, M.; Senesi, S.; Dini, F.; Pietra, F. New ecotoxicologically and biogenetically relevant terpenes of the tropical green seaweed Caulerpa taxifolia which is invading the Mediterranean. Helv. Chim. Acta 1993, 76, 855–864. [Google Scholar]
- Guerriero, A.; Depentori, D.; D’Ambrosio, M.; Durante, M.; Dini, F.; Pietra, F. Chlorophyll-photosensitised photodegradation of caulerpenyne; a potential harmful sesquiterpenoid from tropical green seaweeds in the genus Caulerpa. J. Chem. Soc. Chem. Commun. 1994, 2083–2084. [Google Scholar] [CrossRef]
- Guerriero, A.; D’Ambrosio, M. Epoxycaulerpenynes: reactivity and diastereoselective and highly regioselective synthesis by dimethyldioxirane oxidation of caulerpenyne. Eur. J. Org. Chem. 1999, 1985–1990. [Google Scholar] [CrossRef]
- Jung, V.; Pohnert, G. Rapid wound-activated transformation of the green algal defensive metabolite caulerpenyne. Tetrahedron 2001, 57, 7169–7172. [Google Scholar] [CrossRef]
- Jung, V.; Thibaut, T.; Meinesz, A.; Pohnert, G. Comparison of the wound-activated transformation of caulerpenyne by invasive and non-invasive Caulerpa species of the Mediterranean. J. Chem. Ecol. 2002, 28, 2091–2104. [Google Scholar]
- Sample Availability: Samples of the compounds 1, 4 and 6 are available from authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.