Relationship Between Physicochemical Properties and Herbicidal Activity of 1,2,5-Oxadiazole N-Oxide Derivatives
Abstract
:Introduction

Results and Discussion
Lipophilicity determinations using an octadecylsilane (ODS) RP-HPLC column.
| Compounda | Rwb | Awb | Lflb | Erc | log k’wd | log Po/we | Vf | μf | qoN→O, f |
|---|---|---|---|---|---|---|---|---|---|
| 1 | -19 | 18 | -4 | -1.20 | - | 4.95 | 534 | 2.06 | 0.238 |
| 2 | -48 | 16 | -22 | -1.19 | 3.34 | 3.76 | 742 | 4.52 | 0.304 |
| 3 | -57 | 8 | -30 | -1.03 | 3.24 | 3.69 | 723 | 3.94 | 0.304 |
| 4 | -15 | -7 | -4 | -0.99 | 4.24 | 4.25 | 573 | 4.7 | 0.292 |
| 5 | 0 | 0 | 0 | -1.22 | - | 4.03 | 943 | 4.07 | 0.298 |
| 6 | -11 | -5 | -5 | -1.62 | 0.22 | 1.88 | 375 | 2.17 | 0.302 |
| 7 | 97 | -50 | -50 | -2.08 | 1.47 | 2.33 | 575 | 5.67 | 0.312 |
| 8 | -75 | -85 | -50 | -1.59 | 4.39 | 4.01 | 859 | 7.91 | 0.316 |
| 9 | -86 | -95 | -- | -- | 1.18 | 2.25 | 568 | 1.38 | 0.309 |
| 10 | -69 | -58 | -58 | -2.00 | 2.18 | 3.42 | 451 | 4.76 | 0.274 |
| 11 | -4 | -5 | -30 | -0.93 | 2.40 | 4.27 | 454 | 1.39 | 0.274 |
| 12 | -23 | -9 | -16 | -- | - | 2.66 | 475 | 2.02 | 0.280 |
| 13 | -17 | 13 | -6 | -1.17 | 2.02 | 3.08 | 660 | 5.20 | 0.276 |
| 14 | -- | -- | -- | -1.10 | 2.31 | 2.60 | 809 | 8.8 | 0.278 |
| 15 | -11 | -2 | -23 | -1.54 | 1.46 | 2.20 | 685 | 3.59 | 0.281 |
| 16 | -57 | -52 | -55 | -2.06 | 1.27 | 4.89 | 675 | 2.59 | 0.278 |
| 17 | -51 | -66 | -71 | -1.54 | 2.74 | 5.75 | 706 | 4.93 | 0.269 |
| 18 | -91 | -73 | -50 | -1.43 | - | 6.74 | 751 | 3.65 | 0.265 |
| Compound | log k’w | -a | b | r2,a |
|---|---|---|---|---|
| 1c | - | - | - | - |
| 2 | 3.34 | 25.1 | 0.45 | 0.9992 |
| 3 | 3.24 | 25.2 | 0.45 | 0.9996 |
| 4 | 4.24 | 29.1 | 0.53 | 0.9932 |
| 5c | - | - | - | - |
| 6 | 0.22 | 8.5 | 0.14 | 0.9722 |
| 7 | 1.47 | 13.5 | 0.24 | 0.9956 |
| 8 | 4.39 | 31.8 | 0.57 | 0.9914 |
| 9 | 1.18 | 11.9 | 0.21 | 0.9954 |
| 10 | 2.18 | 13.5 | 0.25 | 0.9920 |
| 11 | 2.40 | 19.1 | 0.34 | 0.9565 |
| 12b | - | - | - | - |
| 13 | 2.02 | 16.7 | 0.29 | 0.9980 |
| 14 | 2.31 | 21.1 | 0.37 | 0.9978 |
| 15 | 1.46 | 15.6 | 0.27 | 0.9972 |
| 16 | 1.27 | 10.3 | 0.18 | 0.9851 |
| 17 | 2.74 | 18.47 | 0.34 | 0.9958 |
| 18c | - | - | - | - |
| 19 | 2.21 | 19.2 | 0.34 | 0.9864 |

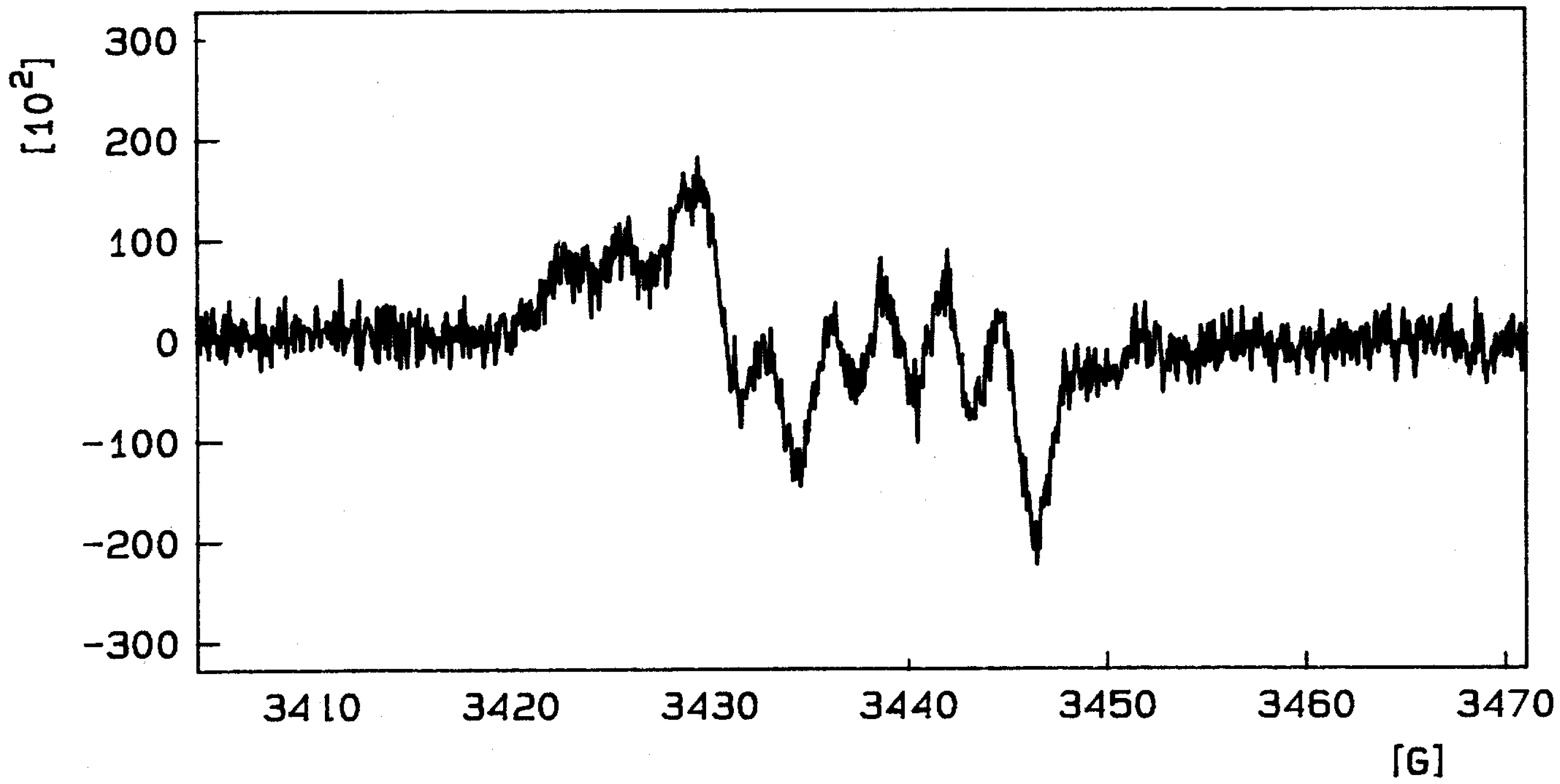
Cyclic Voltammetry and ESR

Calculation of quantum-chemical descriptors
Contributing factors to the biological response
| Er | logk´w | logPo/w | V | μ | qo | |
|---|---|---|---|---|---|---|
| Er | 1 | 0.25 | 0.03 | 0.02 | 0.05 | 0.01 |
| Logk´w | – | 1 | 0.92 (4 outliers) | 0.78 | 0.37 | 0.02 |
| logPo/w | – | – | 1 | 0.13 | 0.01 | 0.23 |
| V | – | – | – | 1 | 0.29 | 0.13 |
| μ | – | – | – | – | 1 | 0.17 |
| qo | – | – | – | – | – | 1 |
| N° | Multi-parameter regression | R-squared | Adj-R-squared | Prob> F | n b |
|---|---|---|---|---|---|
| I | aw = (167 ± 26) + (95±12) Er – (18±3) log k’w | 0.8866 | 0.8542 | 0.005 | 10 |
| II | aw = (132 ± 24) + (66±10)Er – (15±3) log P o/w | 0.7490 | 0.7074 | 0.0005 | 13 |
| III | lfl = (78 ± 20) + (56 ± 9) Er – (9±3) log k’w | 0.8270 | 0.7780 | 0.002 | 10 |
| IV | lfl = (74 ± 17) + (48±9) Er – (8±2) log P o/w | 0.8445 | 0.8000 | 0.0015 | 10 |
| rw | aw | lfl | |
|---|---|---|---|
| rw | 1 | 0.19 | 0.13 |
| aw | – | 1 | 0.67 |
| lfl | – | – | 1 |
Conclusions
Experimental
General
Acknowledgments
References
- Wermuth, C.G. (Ed.) “The Practice of Medicinal Chemistry”; Academic Press: New York, 1996.
- Basak, S.C.; Niemi, G.J.; Veith, G.D. Optimal characterization of structure for prediction of properties. J. Math. Chem. 1990, 4, 185–202. [Google Scholar] [CrossRef]
- Basak, S.B.; Monsrud, L.J.; Rosen, M.E.; Frane, C.M.; Magnuson, V.R. A comparative study lipophlicity and topological indices in biological correlation. Acta Pharm. Jugosl. 1986, 36, 81–95. [Google Scholar]
- Santo, M; Giacomelli, L.; Reta, M.; Cattana, R.; Silber, J.; Chana, A.; Rodriguez, M.; Ochoa, C. Role of weak molecular interaction in the mechanism of action a serie of antihelmintcs. Molecules. 2000, 5, 317–378. [Google Scholar] [CrossRef]
- Aguirre, G.; Cerecetto, H.; Di Maio, R.; Gonzalez, M.; Porcal, W.; Seoane, G.; Ortega, M.A.; Aldana, I.; Monge, A.; Denicola, A. Benzo[1,2-c]1,2,5-oxadiazole N-oxide derivatives as potential antitrypanosomal drugs. Structure-activity relationships. Part II. Arch. Pharm. Med. Chem. 2002, 335, 15–21. [Google Scholar] [CrossRef]
- Manhold, R.; Kubinyi, H.; Timmerman, H. “Lipophilicity in Drug Action and Toxicology”. In “Methods and Principles in Medicinal Chemistry”; Pilska, V., Testa, B, Van de Waterbeemd, H., Eds.; VCH: Weinheim, New York, 1996; Vol. 4. [Google Scholar]
- Hadjipavlou-Litina, D.; Hantzch, C. Quantitative Structure-Activity Relationships of the Benzodiazepines. A Review and Reevaluation. Chem. Rev. 1994, 91, 1483–1505. [Google Scholar] [CrossRef]
- Hsieh, M. M.; Dorsey, J.G. Accurate determination of log k'w in reversed-phase liquid chromatography. Implications for quantitative structure-retention relationships. J. Chromatogr. 1993, 631, 63–78. [Google Scholar] [CrossRef]
- Ghose, A.; Crippen, G. Atomic physicochemical parameters for three-dimensional-structure-directed quantitative structure-activity relationships. 2. Modeling dispersive and hydrophobic interactions. J. Chem. Inf. Comput. Sci. 1987, 27, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Michels, J.J.; Dorsey, J.G. Estimation of the reversed-phase liquid chromatographic lipophilicity parameter log k'w using ET-30 solvatochromism. J. Chromatogr. 1990, 499, 435–451. [Google Scholar] [CrossRef]
- Dorsey, J.; Khaledi, M. Hydrophobicity estimations by reversed-phase liquid chromatography. J. Chromatogr. A 1993, 656, 485–499. [Google Scholar] [CrossRef]
- Lambert, W.J. Modeling oil-water partitioning and membrane permeation using reversed-phase chromatography. J. Chromatogr. A 1993, 656, 469–484. [Google Scholar] [CrossRef]
- Gancia, E.; Montana, J.G.; Manallack, D.T. Theoretical hydrogen bonding parameters for drug design. J. Mol. Graph. Model. 2001, 19, 349–362. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Lobanov, V.S.; Karelson, M. QSPR: Correlation and Quantitative Prediction of Chemical and Physical Properties from Structure. Chem. Soc. Rev. 1995, 279–287. [Google Scholar] [CrossRef]
- Monge, A.; Palop, J.A.; Lopez de Cerain, A.; Senador, V.; J.Martinez-Crespo, F.; Sainz, Y.; Narro, S.; Garcia, E.; Gonzalez, M.; Hamilton, E.; Barker, A. J.; Clarke, E. D.; Greenhow, D. T. Hypoxia-Selective Agents Derived from Quinoxaline 1,4-Di-N-oxides. J. Med. Chem. 1995, 38, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Cerecetto, H.; Gonzalez, M. N-Oxides as hypoxia selective cytotoxins. Mini Rev. Med. Chem. 2001, 1, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Cerecetto, H.; Dias, E.; Di Maio, R.; González, M.; Pacce, S.; Saenz, P.; Seoane, G.; Suescun, L.; Mombrú, A.; Fernández, G.; Lema, M.; Villalba, J. Synthesis and Herbicidal Activity of N-Oxide Derivatives. J. Agric. Food Chem. 2000, 48, 2995–3002. [Google Scholar] [CrossRef] [PubMed]
- Prezhdo, V.V.; Vasshchenko, E.V.; Prezhdo, O.V.; Pushko, A. Structure and properties of hydrogen bonded complexes of pyridine-N-oxide and its derivatives. J. Mol. Struc. 1999, 510, 69–83. [Google Scholar] [CrossRef]
- Caron, G.; Carrupt, P.A.; Testa, B.; Ermondi, G.; Gasco, A. Insight into the lipophilicity of the aromatic N-oxide moiety. Pharm. Res. 1996, 13, 1186–1190. [Google Scholar]
- Valkó, K.; Snyder, L.R.; Glajch, J.L. Retention in reversed-phase liquid chromatography as a function of mobile-phase composition. J. Chromatogr. 1993, 656, 501–520. [Google Scholar] [CrossRef]
- Kaliszan, R. “Structure and Retention in Chromatography. A Chemometric Approach”; Harwood Academic Publishers: London, 1997; pp. 155–203. [Google Scholar]
- Hsieh, M.M.; Dorsey, J.G. Bioavailability estimation by reversed-phase liquid chromatography: high bonding density C-18 phases for modeling biopartitioning processes. Anal. Chem. 1995, 67, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.R.; Hartwick, R.A. (Eds.) “High Performance Liquid Chromatography”; J. Wiley & Sons: New York, 1989.
- Myers, R. H. (Ed.) “Classical and Modern Regression with Applications”; P.W.S. Publishers: Boston, 1986.
- Riddick, J.A.; Bunger, W.B. (Eds.) “Techniques of Chemistry”, 3rd Ed.; Wiley-Interscience: New York, 1970.
- Olea-Azar, C.; Rigol, C.; Mendizabal, F.; Briones, R.; Cerecetto, H.; Di Maio, R.; Gonzalez, M.; Porcal, W.; Risso, M. Electrochemical and microsomal production of free radicals from 1,2,5-oxadiazole N-oxide as potential antiprotozoal drugs. Spectrochim. Acta 2003, 59A, 69–75. [Google Scholar] [CrossRef]
- Cerecetto, H.; Di Maio, R.; Gonzalez, M.; Risso, M.; Saenz, P.; Seoane, G.; Denicola, A.; Peluffo, G.; Quijano, C.; Olea-Azar, C. 1,2,5-Oxadiazole N-Oxide Derivatives and Related Compounds as Potential Antitrypanosomal Drugs: Structure-Activity Relationships. J. Med. Chem. 1999, 42, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Monge, A.; Lopez de Cerain, A.; Ezpeleta, O.; Cerecetto, H.; Dias, E.; Di Maio, R.; Gonzalez, M.; Onetto, S.; Risso, M.; Seoane, G.; Zinola, F; Olea-Azar, C. 1,2,5-Oxadiazole N-Oxide Derivatives as Hypoxia-Selective Cytotoxins. Structure-Activity Relationships. Die Pharmazie 1998, 53, 698–702. [Google Scholar]
- Olea-Azar, C.; Atria, A. M.; Di Maio, R.; Seaone, G.; Cerecetto, H. Electron Spin Resonance and Cyclic Voltammetry Studies of Nitrofurane and Nitrothiophene Analogues of Nifurtimox. Spectrosc. Lett. 1998, 31, 849–857. [Google Scholar] [CrossRef]
- Olea-Azar, C.; Atria, A. M.; Mendizabal, F.; Di Maio, R.; Seoane, G.; Cerecetto, H. Cyclic Voltammetry and Electron Paramagnetic Resonance Studies of Some Analogues of Nifurtimox. Spectrosc. Lett. 1998, 31, 99–109. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Zoebisch, E.G.; Healy, A.F.; Stewart, J.J.P. AM1: A New General Purpose Quantum Mechanical Molecular Model. J. Amer. Chem. Soc. 1985, 107, 3902–3909. [Google Scholar] [CrossRef]
- Sample availability: Not Applicable
© 2005 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes
Share and Cite
Fernandez, L.; Santo, M.; Reta, M.; Giacomelli, L.; Cattana, R.; Silber, J.; Risso, M.; Cerecetto, H.; Gonzalez, M.; Olea-Azar, C. Relationship Between Physicochemical Properties and Herbicidal Activity of 1,2,5-Oxadiazole N-Oxide Derivatives. Molecules 2005, 10, 1197-1208. https://doi.org/10.3390/10091197
Fernandez L, Santo M, Reta M, Giacomelli L, Cattana R, Silber J, Risso M, Cerecetto H, Gonzalez M, Olea-Azar C. Relationship Between Physicochemical Properties and Herbicidal Activity of 1,2,5-Oxadiazole N-Oxide Derivatives. Molecules. 2005; 10(9):1197-1208. https://doi.org/10.3390/10091197
Chicago/Turabian StyleFernandez, L., M. Santo, M. Reta, L. Giacomelli, R. Cattana, J. Silber, M. Risso, H. Cerecetto, M. Gonzalez, and C. Olea-Azar. 2005. "Relationship Between Physicochemical Properties and Herbicidal Activity of 1,2,5-Oxadiazole N-Oxide Derivatives" Molecules 10, no. 9: 1197-1208. https://doi.org/10.3390/10091197
APA StyleFernandez, L., Santo, M., Reta, M., Giacomelli, L., Cattana, R., Silber, J., Risso, M., Cerecetto, H., Gonzalez, M., & Olea-Azar, C. (2005). Relationship Between Physicochemical Properties and Herbicidal Activity of 1,2,5-Oxadiazole N-Oxide Derivatives. Molecules, 10(9), 1197-1208. https://doi.org/10.3390/10091197




