The Synthesis and Herbicidal Activity of 1-Alkyl-3-(α-hydroxysubstituted benzylidene)pyrrolidine-2,4-diones
Abstract
:Introduction
Results and Discussion
Synthesis and characterization of target compounds
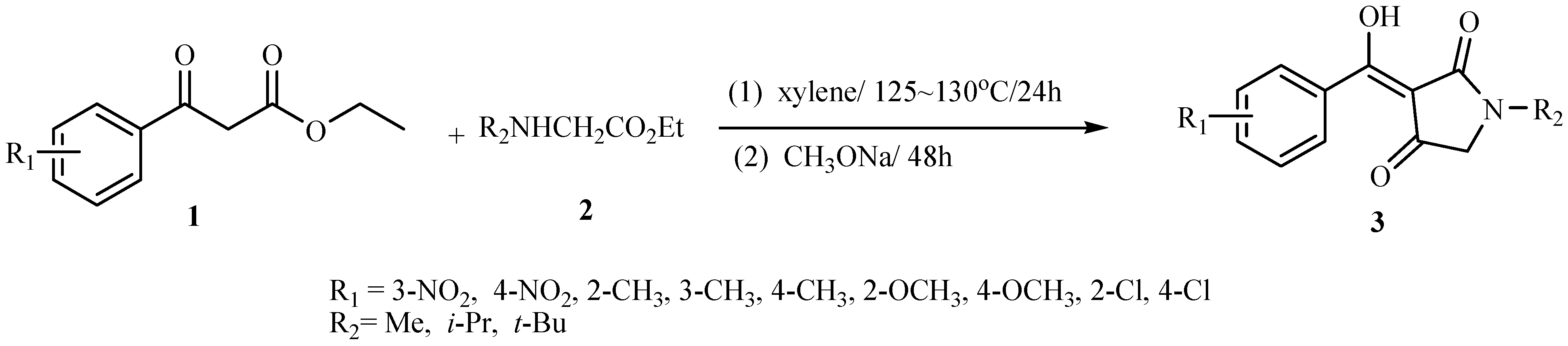
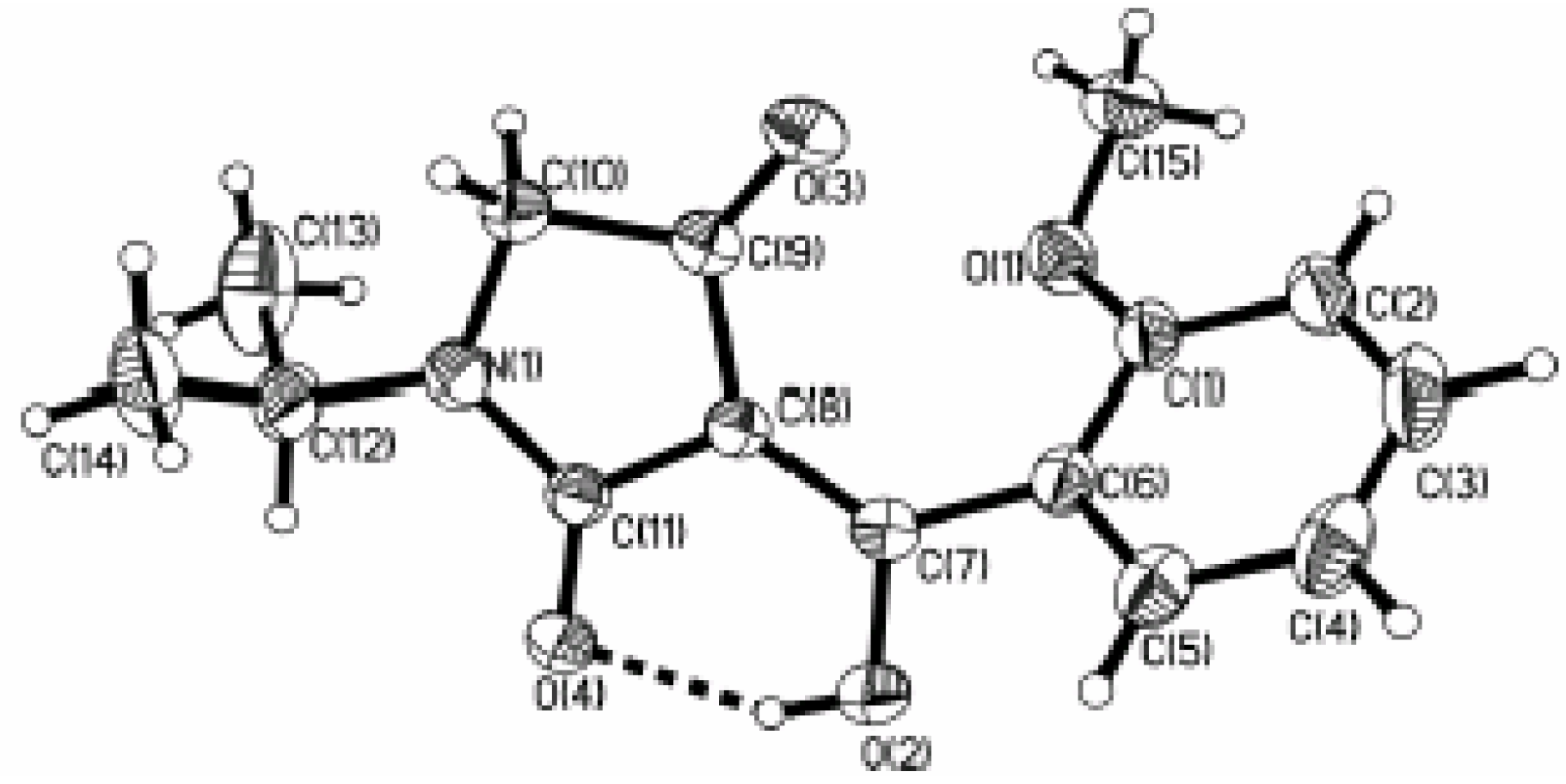
Biological evaluation
| Cpd No | R1 ; R2 | Brassica napus(ug/mL, inhibition %) | Echinochloa crusgalli (L.) Beauv(ug/mL, inhibition %) | |||
|---|---|---|---|---|---|---|
| 10 | 100 | 10 | 100 | Bleaching result at 100 ug/mL * | ||
| 3a | 3-NO2 ; Me | 0 | 22.4 | 5.2 | 42.8 | + |
| 3b | 4-NO2 ; Me | 5.1 | 40.9 | 9.0 | 51.9 | + |
| 3c | 4-Cl ; Me | 15.8 | 90.5 | 9.4 | 50.1 | + |
| 3d | 2-CH3 ; Me | 0 | 44.4 | 31.2 | 78.3 | ++ |
| 3e | 3-CH3 ; Me | 14.0 | 64.0 | 25.3 | 68.3 | ++ |
| 3f | 4-OCH3 ; Me | 7.9 | 50.7 | 16.2 | 78.8 | ++ |
| 3g | 3-NO2 ; i-Pr | 10.2 | 70.3 | 0 | 42 | + |
| 3h | 4-NO2 ; i-Pr | 4.5 | 34.2 | 0 | 1.6 | - |
| 3i | 2-Cl ; i-Pr | 38.9 | 66.8 | 51 | 77.9 | ++ |
| 3j | 4-Cl ; i-Pr | 16 | 77.2 | 25.1 | 68 | ++ |
| 3k | 2-OCH3 ; i-Pr | 3.0 | 58.7 | 77.3 | 78.1 | +++ |
| 3l | 4-OCH3 ; i-Pr | 11.9 | 71 | 79.6 | 80 | +++ |
| 3m | 2-CH3 ; i-Pr | 15.2 | 62.4 | 76.9 | 77.3 | +++ |
| 3n | 3-CH3 ; i-Pr | 3.3 | 82.1 | 49.2 | 75.5 | +++ |
| 3o | 4-CH3 ; i-Pr | 8.1 | 86.4 | 76.8 | 78.4 | +++ |
| 3p | 3-NO2; t-Bu | 22.1 | 80.1 | 0 | 0.5 | - |
| 3q | 4-NO2; t-Bu | 1.8 | 48.1 | 0 | 9.8 | - |
| 3r | 4-Cl; t-Bu | 13.8 | 98 | 0.4 | 20.8 | - |
| 3s | 2-OCH3; t-Bu | 41.2 | 69.5 | 74.9 | 82.9 | ++ |
| 3t | 4-OCH3; t-Bu | 5.4 | 77.7 | 78.7 | 80.3 | ++ |
| 3u | 2-CH3; t-Bu | 21.1 | 74.9 | 63.6 | 74.4 | ++ |
| 3v | 3-CH3; t-Bu | 24.9 | 85.0 | 4.9 | 48.5 | + |
| 3w | 4-CH3; t-Bu | 13.5 | 88.1 | 36.3 | 48 | + |
| Sulcotrione** | 3.5 | 38.1 | 29.4 | 45.1 | +++ | |
Conclusions
Experimental
Materials and instruments
General procedure for the synthesis of compounds 3a~3w: Preparation of 1-methyl-3-(α-hydroxy-3’-nitrobenzylidene)pyrrolidine-2,4-dione (3a)
Acknowledgements
References
- Wu, Y. C.; Hu, F. Z.; Yang, H. Z. Research and develoment on the inhibitors of 4-hydroxyphenyl-pyruvate dioxygenase. Nongyaoxue Xuebao 2001, 3, 1–10. (in Chinese). [Google Scholar]
- Zhu, Y. Q.; Hu, F. Z.; Yang, H. Z. Reviews on 4-hydroxyphenylpyruvate dioxygenase enzyme and the structure-activity relationships of its inhibitors. Huaxue Tongbao 2004, 67, w018/1–W018/7. (in Chinese). [Google Scholar]
- Jones, R. C. F.; Begley, M. J.; Peterson, G. E.; Sumaria, S. Acylation of pyrrolidine-2,4-dione. J. Chem. Soc., Perkin Trans. 1990, 1, 1959–67. [Google Scholar]
- Rinehart, K. L.; MacKellar, F. A.; Grostic, M. F.; Olson, E. C.; Wnuk, R. J.; Branfman, A. R.; Tirandamycin, I. Structure assignment. J. Am. Chem. Soc. 1971, 93, 4943–4945. [Google Scholar]
- Matsuo, K.; Kimura, M.; Kinuta, T.; Tarkai, N.; Tanaka, K. Syntheses and antimicrobial activities of 3-acyltetramic acid derivatives. Chem. Pharm. Bull. 1984, 32, 4197–4204. [Google Scholar]
- Zhu, Y. Q.; Hu, F. Z.; Zou, X. M.; Yao, C. S.; Li, Y. H.; Yang, H. Z. Synthesis of 1-benzyl-3-(α-hydroxy-(un)substituted enzylidene)pyrrolidine-2,4-diones and biological activity. Chin. J. Org. Chem. (in press).
- Kakidani, H.; Hirai, K. Three-dimentional modeling of plant 4-hydroxyphenylpyruvate dioxygenase, a molecular target of triketone-type herbicides. J. Pestic. Sci. 2003, 28, 409–415. [Google Scholar]
- Fritze, I. M.; Linden, L.; Freigang, J.; Auerbach, G.; Huber, R.; Steinbacher, S. The Crystal structures of Zea mays and Arabidopsis 4-hydroxyphenylpyruvate dioxygenase. Plant Physiol. 2004, 134, 1388–1400. [Google Scholar]
- Wu, C. S.; Huang, J. L.; Sun, Y. S.; Yang, D. Y. Mode of action of 4-hydroxyphenylpyruvate dioxygenase inhibition by triketone-type inhibitors. J. Med. Chem. 2002, 45, 2222–2228. [Google Scholar]
- Zhu, Y. Q.; Song, H. B.; Yao, C.S.; Gao, Y.; Hu, F. Z.; Zou, X. M.; Yang, H. Z. 3-(a-Hydroxy-2-methoxylbenzylidene)-1-isopropylpyrrolidine-2,4-dione. Acta Cryst. 2004, E60, o599–o601. [Google Scholar]
- Yang, X.F. Study on bioassay methods for determining activities of sulfonyurea herbicides. Acta Scient. Nat.Univ. Nankaniensis. 1994, 88–93. (in Chinese). [Google Scholar]
- Speziale, A. J.; Jaworski, E.G. N-Substituted glycinate and alaninate eaters. J. Org. Chem. 1960, 25, 728–732. [Google Scholar] [CrossRef]
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Zhu, Y.-Q.; Yao, C.-S.; Zou, X.-M.; Hu, F.-Z.; Liu, B.; Li, Y.-H.; Yang, H.-Z. The Synthesis and Herbicidal Activity of 1-Alkyl-3-(α-hydroxysubstituted benzylidene)pyrrolidine-2,4-diones. Molecules 2005, 10, 427-434. https://doi.org/10.3390/10020427
Zhu Y-Q, Yao C-S, Zou X-M, Hu F-Z, Liu B, Li Y-H, Yang H-Z. The Synthesis and Herbicidal Activity of 1-Alkyl-3-(α-hydroxysubstituted benzylidene)pyrrolidine-2,4-diones. Molecules. 2005; 10(2):427-434. https://doi.org/10.3390/10020427
Chicago/Turabian StyleZhu, You-Quan, Chang-Sheng Yao, Xiao-Mao Zou, Fang-Zhong Hu, Bin Liu, Yong-Hong Li, and Hua-Zheng Yang. 2005. "The Synthesis and Herbicidal Activity of 1-Alkyl-3-(α-hydroxysubstituted benzylidene)pyrrolidine-2,4-diones" Molecules 10, no. 2: 427-434. https://doi.org/10.3390/10020427
APA StyleZhu, Y.-Q., Yao, C.-S., Zou, X.-M., Hu, F.-Z., Liu, B., Li, Y.-H., & Yang, H.-Z. (2005). The Synthesis and Herbicidal Activity of 1-Alkyl-3-(α-hydroxysubstituted benzylidene)pyrrolidine-2,4-diones. Molecules, 10(2), 427-434. https://doi.org/10.3390/10020427




