A Direct Route to 6,6’-Disubstituted-2,2’-Bipyridines by Double Diels-Alder/retro Diels-Alder Reaction of 5,5’-bi-1,2,4-Triazines
Abstract
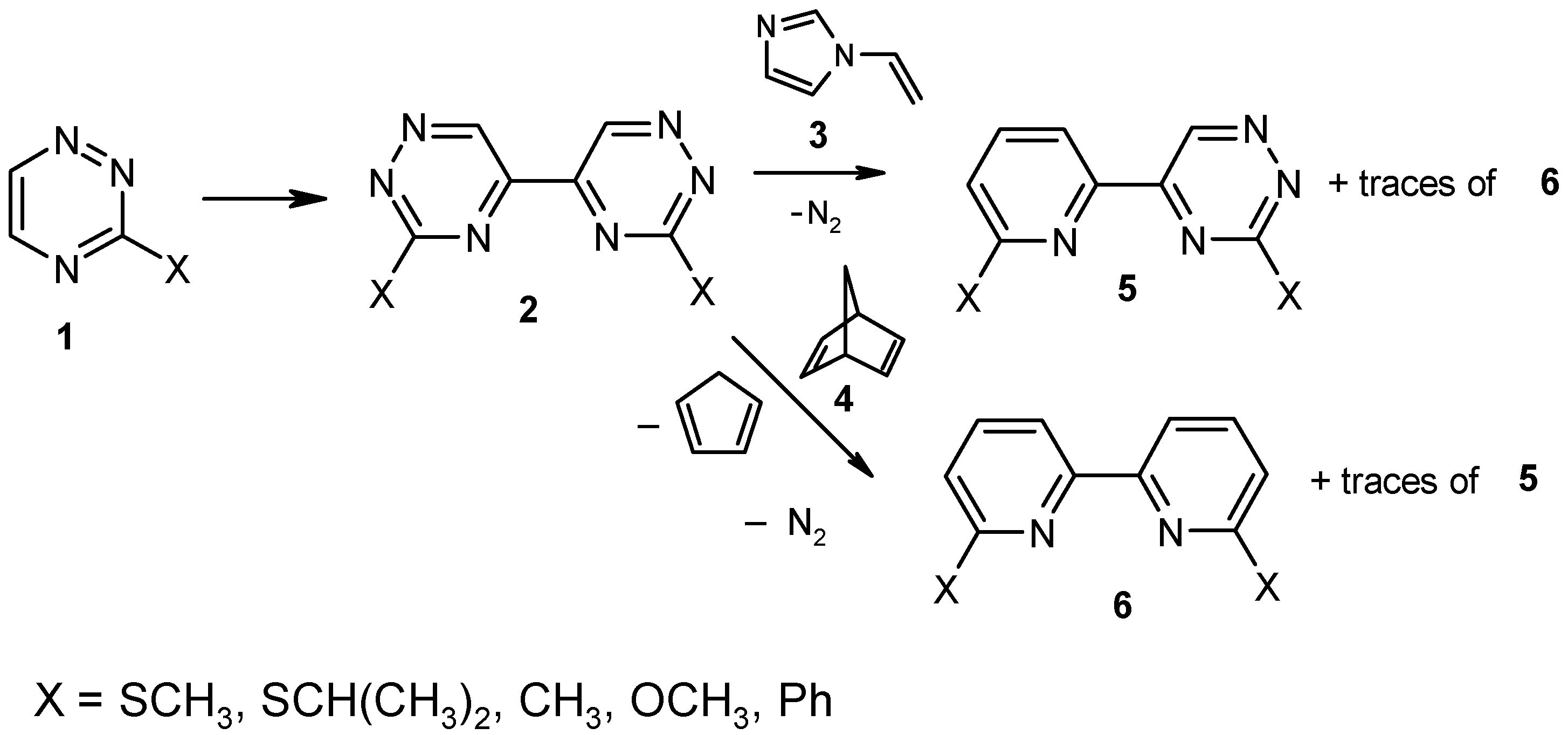
:Introduction
Results and Discussion
| Comp. | X | Yield (%) | M.p. (°C) | Lit. M.p. (°C) |
|---|---|---|---|---|
| 2a | -SCH3 | 94 | 166-167 | 168.5-170 [12] |
| Comp. | X | Reaction time (hrs) | Yield (%) | M.p. (°C) | Lit. M.p. (°C) |
|---|---|---|---|---|---|
| 6a | -SCH3 | 30 | 80 | 130-131 | 130-131 [17] |
Conclusions
Experimental
General
Typical procedure: Preparation of 3,3’-Bis(phenyl)-5,5’-bi-1,2,4-triazine (2e)
The synthesis of 6,6’-disubstituted-2,2’-bipyridines 6a-e. General procedure.
Acknowledgments
References and Notes
- Branowska, D. Molecules 2005, 10, 265–273.
- (a)Lehn, J.-M. Supramolecular Chemistry: Concepts and Perspectives; VCH: Weinheim.Steel, P. J. Adv. Heterocycl. Chem. 1997, 67, 1.
- Kaes, C.; Katz, A.; Hosseini, M. Chem. Rev. 2000, 100, 3553.
- Fanta, P. E. Synthesis 1974, 9.
- Newkome, G. R.; Patri, A. K.; Holder, E.; Schubert, S. Eur. J. Org. Chem. 2004, 235.
- Newkome, G. R.; Hager, D. C. J. Am. Chem. Soc. 1978, 100, 5567.
- Kawai, T.; Furukawa, N. Tetrahedron Lett. 1984, 25, 2549.
- Oae, S.; Takeda, T.; Wakabayashi, S. Tetrahedron Lett. 1988, 29, 4445.
- Pabst, G. R.; Sauer, J. Tetrahedron Lett. 1998, 39, 6687.
- Rykowski, A.; Branowska, D.; Kielak, J. Tetrahedron Lett. 2000, 41, 3657.
- Branowska, D. Synthesis 2003, 2096.
- Krass, D. K.; Chen, T.-K.; Paudler, W. W. J. Heterocyclic Chem. 1973, 10, 343.
- Neunhoeffer, H.; Henning, H.; Frühauf, H.-W.; Mutterer, M. Tetrahedron Lett. 1969, 3147.
- Paudler, W. W.; Chen, T.-K. J. Heterocycl. Chem. 1970, 7, 767.
- Branowska, D.; Kielak, J. Polish J. Chem. 2003, 77, 1149.
- Branowska, D.; Rykowski, A. Synlett 2002, 1892.
- Kawai, T.; Furokawa, N.; Oae, S. Tetrahedron Lett. 1984, 25, 2549.
- Tiecco, M.; Tingoli, M.; Testaferri, L.; Bartoli, D.; Chianeli, D. Tetrahedron 1989, 45, 2857.
- Newkome, G. R.; Puckett, W. E.; Kiefer, G. E.; Gupta, V. K.; Xia, Y.; Coreil, M.; Hackney, M. A. J. Org. Chem. 1982, 47, 4116.
- Manandhar, S.; Singh, R. P.; Eggers, G. V.; Shreeve, J. M. J. Org. Chem. 2002, 67, 6415.
- Constable, E. C.; Elder, S. M.; Healy, J.; Tocher, D. A. J. Chem. Soc., Dalton Trans. 1990, 1405.
- Perrin, D.D.; Armarego, W. L. F. Purification of Laboratory Chemicals; Pergamon: Oxford, U.K., 1992. [Google Scholar]
- Sample Availability: Available from the author.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Branowska, D. A Direct Route to 6,6’-Disubstituted-2,2’-Bipyridines by Double Diels-Alder/retro Diels-Alder Reaction of 5,5’-bi-1,2,4-Triazines. Molecules 2005, 10, 274-278. https://doi.org/10.3390/10010274
Branowska D. A Direct Route to 6,6’-Disubstituted-2,2’-Bipyridines by Double Diels-Alder/retro Diels-Alder Reaction of 5,5’-bi-1,2,4-Triazines. Molecules. 2005; 10(1):274-278. https://doi.org/10.3390/10010274
Chicago/Turabian StyleBranowska, D. 2005. "A Direct Route to 6,6’-Disubstituted-2,2’-Bipyridines by Double Diels-Alder/retro Diels-Alder Reaction of 5,5’-bi-1,2,4-Triazines" Molecules 10, no. 1: 274-278. https://doi.org/10.3390/10010274
APA StyleBranowska, D. (2005). A Direct Route to 6,6’-Disubstituted-2,2’-Bipyridines by Double Diels-Alder/retro Diels-Alder Reaction of 5,5’-bi-1,2,4-Triazines. Molecules, 10(1), 274-278. https://doi.org/10.3390/10010274




