Wearable Sensors for the Detection of Biomarkers for Wound Infection
Abstract
:1. Introduction
2. Wound Infection Biomarkers
3. Detection Methods for Wound Infection Biomarkers
4. Sensors for Wound Infection Biomarkers
4.1. Electrochemical Sensors
| Detection | Analyte | Method | Linear Range | LOD | Matrix | Wireless Data Transfer | Ref |
|---|---|---|---|---|---|---|---|
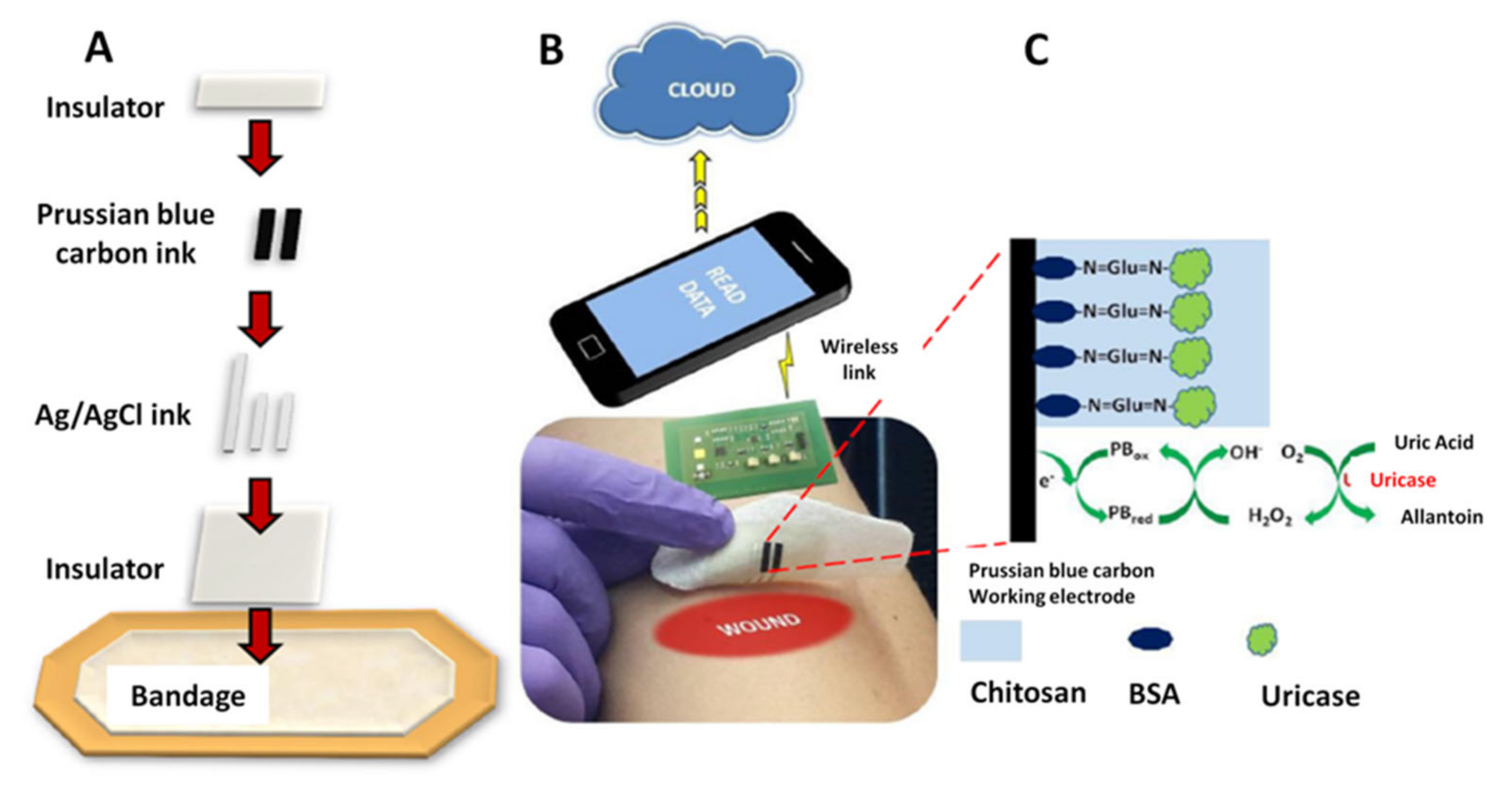
| AMP | UA | C-SPE/PB/uricase/Chi on wound dressing | 100–800 μM | NS | PBS | RFID, NFC | [9] |
| AMP | UA | Embroided ink coated/uricase thread (on gauze) | 0–800 μM | NS | Simulated wound fluid | - | [35] |
| POT | pH | C/PANI on Ecoflex substrate | 4–10 | - | Standard pH buffer solutions, emulated wounds | - | [71] |
| POT | pH | ITOE/PANI, can be attached to bandage + NFC probe | 4–10 | - | Emulated wound and emulate infected wound | NFC | [10] |
| POT | pH | C-SPE/PANI on PET film, attached to commercial transparent tape | 4–10 | - | Standard pH buffer solutions | Bluetooth | [8] |
| POT | pH | C electrode on commercial bandage | 2–13 | - | Acidic and alkaline solutions | Using 2.4 GHz ISM band | [72] |
| EIS | pH | Screen-printed CuO NR on IDE | 5–8.5 | - | Buffer solution, DMEM | - | [41] |
| SWV | pH | Riboflavin/LIG/polyimide | 2–8 | - | Buffer solution | - | [73] |
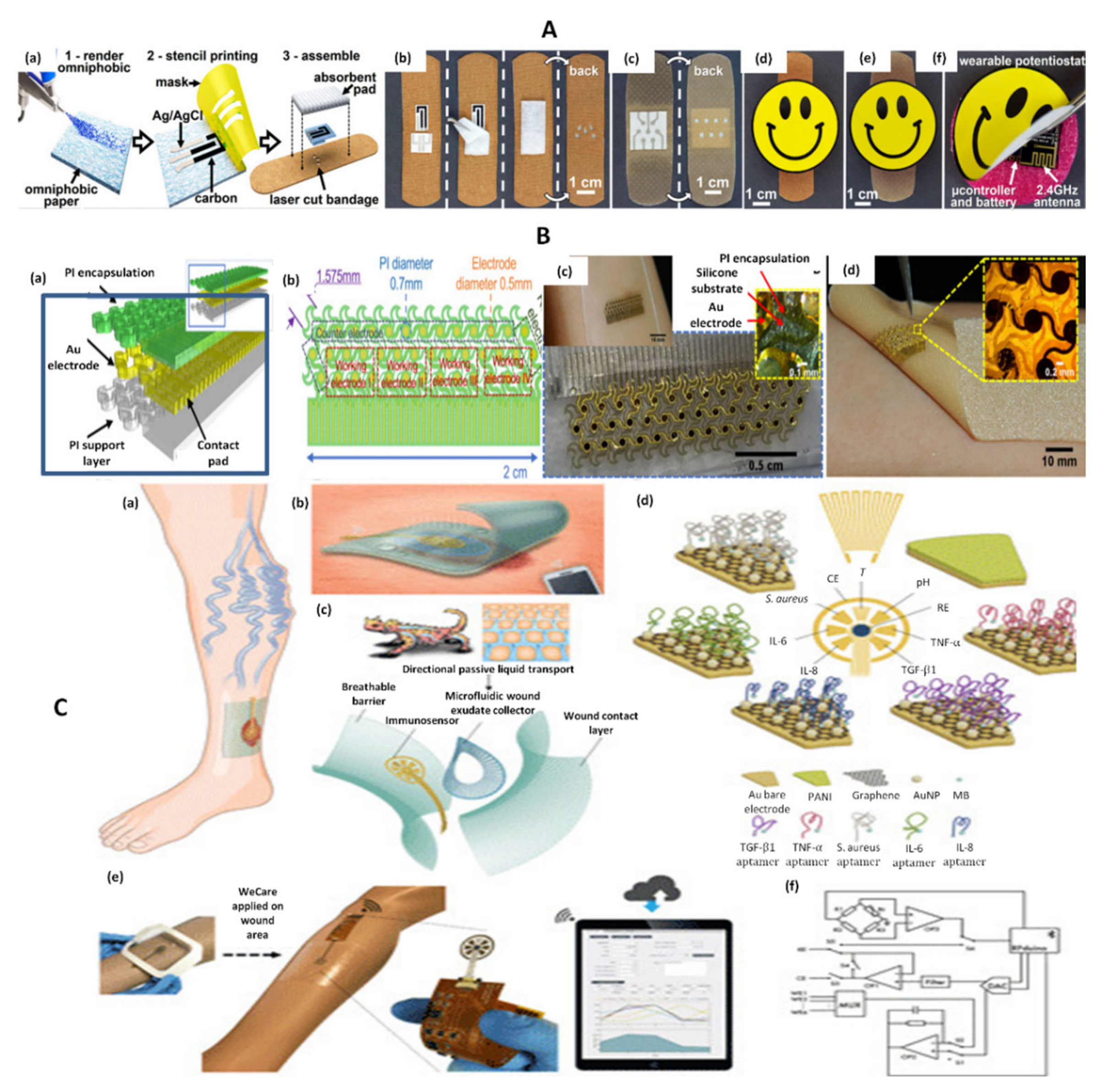
| AMP | UA | Screen-printed carbon/uricase on omniphobic paper | 0.22–0.75 mM | 0.2 mM | PBS | Using 2.4 GHz ISM band | [36] |
| EIS | pH | 5.5–8.5 | - | Standard pH buffer solutions | |||
| DPV | pH | LGG/MXene/PANI | 4–9 | - | Artificial wound exudate | - | [37] |
| AMP | UA | LGG/MXene/uricase | 50–1200 μM | 50 μM | Artificial wound exudate | - | |
| SWV | UA | CNT/PA | 100–1000 μM | NS | Simulated wound fluid | - | [29] |
| Pyo | 0.10–100 μM | 0.1 μM | Simulated wound fluid, bacteria culture media | ||||
| SWV | UA | CUA | 100–700 μM | 1 ± 0.4 μM | PBS, simulated wound fluid | - | [25] |
| Pyo | 1–250 μM | 1 ± 0.5 μM | PBS, Simulated wound fluid, bacteria culture | ||||
| Nitric oxide | 1–100 μM | 0.2 μM | PBS, simulated wound fluid, eukaryotic cell culture | ||||
| AMP | Oxygen | AuE/Nafion/PDMS on wound dressing | 58.5–178 [O2]% | NS | PBS | - | [38] |
| AMP | Lactate | AuE/PB/SWCNT/Chi/LO/SWCNT/Chi on wound dressing | 0.1–0.5 mM | PBS | - | ||
| SWV | TNF-α | AuE/AuNPs-GP/Apt-MB | 0–2 ng/mL | NS | Spiked serum, mice wounds, wound exudate | Bluetooth | [27] |
| IL-6 | 0–30 ng/mL | ||||||
| IL-8 | 0–30 ng/mL | ||||||
| TGF-β1 | 0–150 pg/mL | ||||||
| Staph. aureus | 0–1 × 109 CFU | ||||||
| pH | PANI/AuE | 4–9 |
| Detection | Analyte | Method | Linear Range | LOD | Matrix | Wireless Data Transfer | Ref |
|---|---|---|---|---|---|---|---|
| COL | pH | pH indicator dye embedded in hydrogel wound dressing + optoelectronic probe | 6.4–8.4 | - | Standard pH buffer solutions | RFID, NFC | [11] |
| COL | pH | pH indicator dye embedded in alginate microfibers | 6.2–8.2 | - | Agarose and standard pH solutions on pig skin | Smartphone photo | [42] |
| COL | pH | Curcumin embedded in PCL fibers | 6–9 | - | Standard pH buffer solutions | Smartphone photo | [43] |
| COL + FL | pH | O-QDs on commercial medical cotton cloth | 5–9 | - | PBS | - | [46] |
| COL | Bacteria | MB embedded in PVA/CMC | NS | NS | Bacteria suspension | - | [74] |
| FL | Bacteria | Florescent dye vesicles embedded in GelMA | NS | NS | Mice wounds | - | [44] |
| FL | H2O2 | Eu CP/PAN fiber mats | 20–200 μM | NS | Standard solutions, animal wounds | - | [26] |
| FL | O2 | Ru(dpp)3Cl2/paper | 5–26 ppm | NS | Oxygenated water, mice wounds | - | [45] |
4.1.1. Electrochemical Uric Acid Sensors
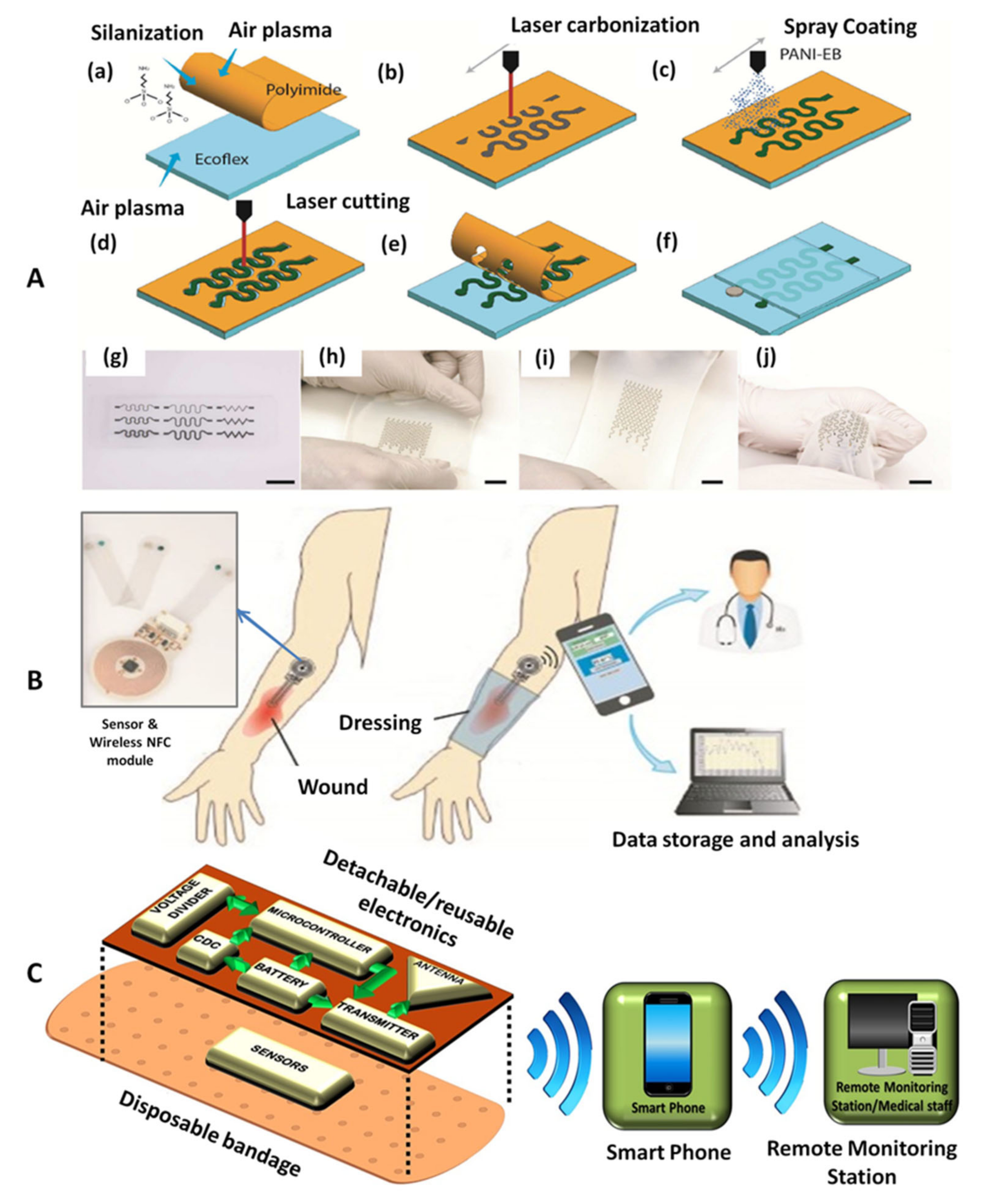
4.1.2. Electrochemical pH Sensors
4.1.3. Electrochemical Sensors for Multiple Analytes
4.2. Colorimetric Sensors
Colorimetric pH Sensors
4.3. Fluorimetric Sensors
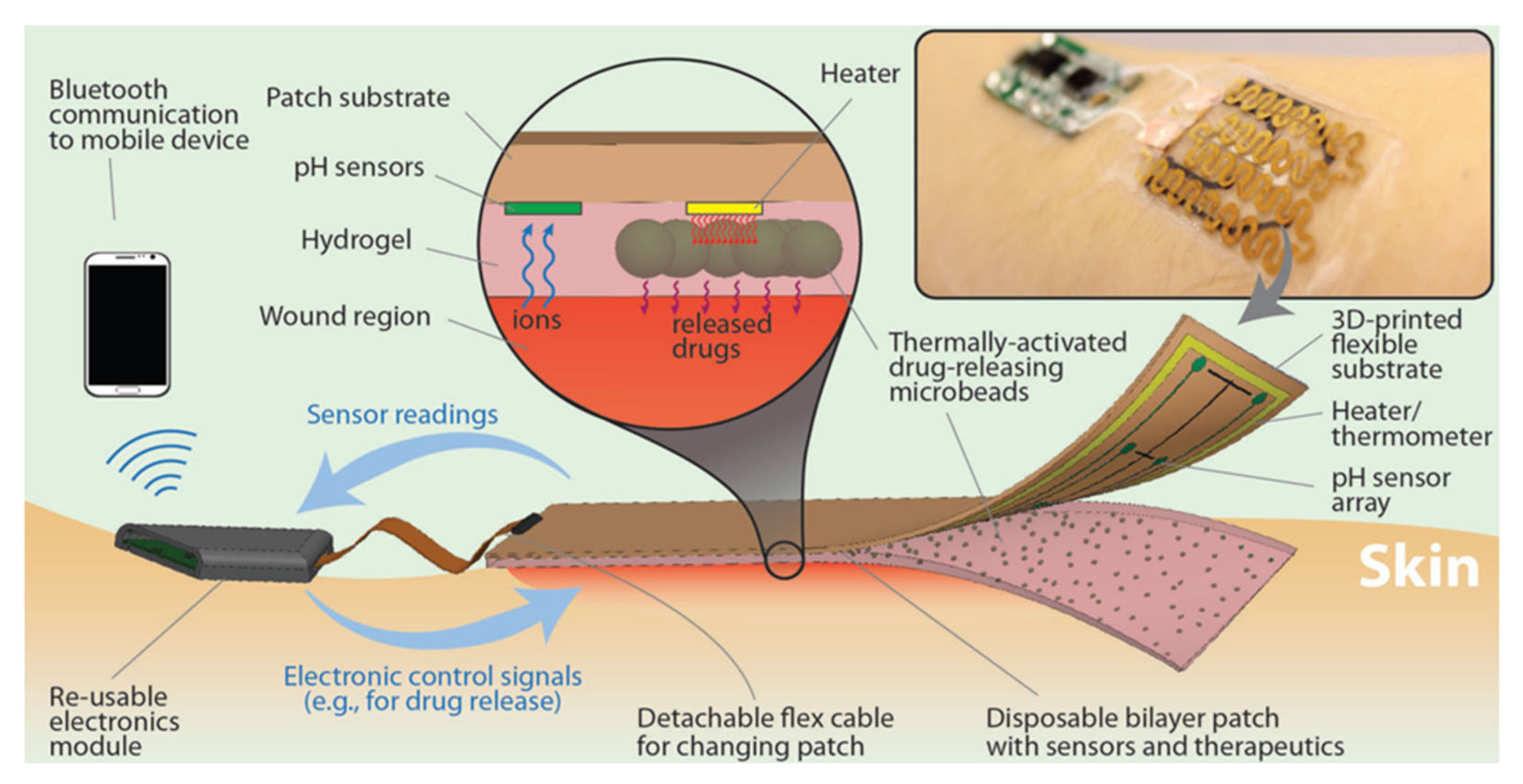
4.4. Theranostic Approaches
5. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, A.R.; Bernstein, J.M. Chronic Wound Infection: Facts and Controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutting, K.F. The Current Burden of Infected Wounds. Br. J. Health Care Manag. 2016, 22, 436–438. [Google Scholar] [CrossRef]
- Li, S.; Renick, P.; Senkowsky, J.; Nair, A.; Tang, L. Diagnostics for Wound Infections. Adv. Wound Care 2021, 10, 317–327. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Sazonov, E.; Daoud, W.A. Grand Challenges in Wearable Electronics. Front. Electron. 2021, 2, 1–4. [Google Scholar] [CrossRef]
- Nasiri, S.; Khosravani, M.R. Progress and Challenges in Fabrication of Wearable Sensors for Health Monitoring. Sens. Actuator A Phys. 2020, 312, 112105. [Google Scholar] [CrossRef]
- Mostafalu, P.; Tamayol, A.; Rahimi, R.; Ochoa, M.; Khalilpour, A.; Kiaee, G.; Yazdi, I.K.; Bagherifard, S.; Dokmeci, M.R.; Ziaie, B.; et al. Smart Bandage for Monitoring and Treatment of Chronic Wounds. Small 2018, 14, 1703509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassal, P.; Kim, J.; Kumar, R.; de Araujo, W.R.; Steinberg, I.M.; Steinberg, M.D.; Wang, J. Smart Bandage with Wireless Connectivity for Uric Acid Biosensing as an Indicator of Wound Status. Electrochem. Commun. 2015, 56, 6–10. [Google Scholar] [CrossRef]
- Rahimi, R.; Brener, U.; Chittiboyina, S.; Soleimani, T.; Detwiler, D.A.; Lelièvre, S.A.; Ziaie, B. Laser-Enabled Fabrication of Flexible and Transparent pH Sensor with near-Field Communication for in-Situ Monitoring of Wound Infection. Sens. Actuators B Chem. 2018, 267, 198–207. [Google Scholar] [CrossRef]
- Kassal, P.; Zubak, M.; Scheipl, G.; Mohr, G.J.; Steinberg, M.D.; Murković Steinberg, I. Smart Bandage with Wireless Connectivity for Optical Monitoring of pH. Sens. Actuators B Chem. 2017, 246, 455–460. [Google Scholar] [CrossRef]
- Atkinson, A.J.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Schooley, R.T.; Spilker, B.A.; et al. Biomarkers and Surrogate Endpoints: Preferred Definitions and Conceptual Framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Mota, F.A.R.; Pereira, S.A.P.; Araújo, A.R.T.S.; Passos, M.L.C.; Saraiva, M.L.M.F.S. Biomarkers in the Diagnosis of Wounds Infection: An Analytical Perspective. TrAC-Trends Anal. Chem. 2021, 143, 116405. [Google Scholar] [CrossRef]
- O’Callaghan, S.; Galvin, P.; O’Mahony, C.; Moore, Z.; Derwin, R. ‘Smart’ Wound Dressings for Advanced Wound Care: A Review. J. Wound Care 2020, 29, 394–406. [Google Scholar] [CrossRef]
- Brown, M.S.; Ashley, B.; Koh, A. Wearable Technology for Chronic Wound Monitoring: Current Dressings, Advancements, and Future Prospects. Front. Bioeng. Biotechnol. 2018, 6, 47. [Google Scholar] [CrossRef]
- Tegl, G.; Schiffer, D.; Sigl, E.; Heinzle, A.; Guebitz, G.M. Biomarkers for Infection: Enzymes, Microbes, and Metabolites. Appl. Microbiol. Biotechnol. 2015, 99, 4595–4614. [Google Scholar] [CrossRef]
- Ono, S.; Imai, R.; Ida, Y.; Shibata, D.; Komiya, T.; Matsumura, H. Increased Wound pH as an Indicator of Local Wound Infection in Second Degree Burns. Burns 2015, 41, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, D.G.; Haalboom, M.; Bowler, P.G.; Gamerith, C.; Sigl, E.; Heinzle, A.; Burnet, M.W.M. Elevated Wound Fluid pH Correlates with Increased Risk of Wound Infection. Wound Med. 2019, 26, 100166. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The Effects of pH on Wound Healing, Biofilms, and Antimicrobial Efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Upton, Z.; Edwards, H.; Finlayson, K.; Shooter, G.K. Elevated Uric Acid Correlates with Wound Severity. Int. Wound J. 2012, 9, 139–149. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Upton, Z.; Shooter, G.K. Uric Acid and Xanthine Oxidoreductase in Wound Healing. Curr. Rheumatol. Rep. 2014, 16, 396. [Google Scholar] [CrossRef]
- Castilla, D.M.; Liu, Z.-J.; Velazquez, O.C. Oxygen: Implications for Wound Healing. Adv. Wound Care 2012, 1, 225–230. [Google Scholar] [CrossRef]
- Löffler, M.; Zieker, D.; Weinreich, J.; Löb, S.; Königsrainer, I.; Symons, S.; Bühler, S.; Königsrainer, A.; Northoff, H.; Beckert, S. Wound Fluid Lactate Concentration: A Helpful Marker for Diagnosing Soft-Tissue Infection in Diabetic Foot Ulcers? Preliminary Findings. Diabet. Med. 2011, 28, 175–178. [Google Scholar] [CrossRef]
- Boykin, J.V. Wound Nitric Oxide Bioactivity. J. Wound Ostomy Cont. Nurs. 2010, 37, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Simoska, O.; Duay, J.; Stevenson, K.J. Electrochemical Detection of Multianalyte Biomarkers in Wound Healing Efficacy. ACS Sens. 2020, 5, 3547–3557. [Google Scholar] [CrossRef]
- Wu, K.; Wu, X.; Chen, M.; Wu, H.; Jiao, Y.; Zhou, C. H2O2-Responsive Smart Dressing for Visible H2O2 Monitoring and Accelerating Wound Healing. Chem. Eng. J. 2020, 387, 124127. [Google Scholar] [CrossRef]
- Gao, Y.; Nguyen, D.T.; Yeo, T.; Lim, S.B.; Tan, W.X.; Madden, L.E.; Jin, L.; Long, J.Y.K.; Aloweni, F.A.B.; Liew, A.Y.J.; et al. A Flexible Multiplexed Immunosensor for Point-of-Care in Situ Wound Monitoring. Sci. Adv. 2021, 7, eabg9614. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Ligi, D.; Canale, M.; Raffetto, J.D. Omics Profiles in Chronic Venous Ulcer Wound Fluid: Innovative Applications for Translational Medicine. Expert Rev. Mol. Diagn. 2014, 14, 737–762. [Google Scholar] [CrossRef] [PubMed]
- Jarošová, R.; McClure, S.E.; Gajda, M.; Jović, M.; Girault, H.H.; Lesch, A.; Maiden, M.; Waters, C.; Swain, G.M. Inkjet-Printed Carbon Nanotube Electrodes for Measuring Pyocyanin and Uric Acid in a Wound Fluid Simulant and Culture Media. Anal. Chem. 2019, 91, 8835–8844. [Google Scholar] [CrossRef]
- Alatraktchi, F.A.; Svendsen, W.E.; Molin, S. Electrochemical Detection of Pyocyanin as a Biomarker for Pseudomonas Aeruginosa: A Focused Review. Sensors 2020, 20, 5218. [Google Scholar] [CrossRef]
- Khan, S.; Lorenzelli, L.; Dahiya, R.S. Technologies for Printing Sensors and Electronics over Large Flexible Substrates: A Review. IEEE Sens. J. 2015, 15, 3164–3185. [Google Scholar] [CrossRef]
- Sharma, A.; Badea, M.; Tiwari, S.; Marty, J.L. Wearable Biosensors: An Alternative and Practical Approach in Healthcare and Disease Monitoring. Molecules 2021, 26, 748. [Google Scholar] [CrossRef]
- Harris, D. Electroanalytical Techniques. In Quantitative Chemical Analysis, 8th ed.; Fiorillo, J., Ed.; W. H. Freeman and Company: New York, NY, USA, 2010; pp. 361–392. [Google Scholar]
- Amine, A.; Mohammadi, H. Amperometry. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M.B.T.-E., Eds.; Academic Press: Oxford, UK, 2019; pp. 85–98. [Google Scholar] [CrossRef]
- Liu, X.; Lillehoj, P.B. Embroidered Electrochemical Sensors on Gauze for Rapid Quantification of Wound Biomarkers. Biosens. Bioelectron. 2017, 98, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Goswami, D.; Cuellar, H.E.; Castro, B.; Kuang, S.; Martinez, R.V. Early Detection and Monitoring of Chronic Wounds Using Low-Cost, Omniphobic Paper-Based Smart Bandages. Biosens. Bioelectron. 2018, 117, 696–705. [Google Scholar] [CrossRef]
- Sharifuzzaman, M.; Chhetry, A.; Zahed, M.A.; Yoon, S.H.; Park, C.I.; Zhang, S.; Chandra Barman, S.; Sharma, S.; Yoon, H.; Park, J.Y. Smart Bandage with Integrated Multifunctional Sensors Based on MXene-Functionalized Porous Graphene Scaffold for Chronic Wound Care Management. Biosens. Bioelectron. 2020, 169, 112637. [Google Scholar] [CrossRef]
- Ashley, B.K.; Brown, M.S.; Park, Y.; Kuan, S.; Koh, A. Skin-Inspired, Open Mesh Electrochemical Sensors for Lactate and Oxygen Monitoring. Biosens. Bioelectron. 2019, 132, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Simões, F.R.; Xavier, M.G. Electrochemical Sensors. In Nanoscience and Its Applications, 1st ed.; Da Roz, A.L., Ferreira, M., Leite, F.d.L., Oliveira, O.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 155–177. [Google Scholar] [CrossRef]
- Farghaly, O.A.; Abdel Hameed, R.S.; Abu-Nawwas, A.A.H. Analytical Application Using Modern Electrochemical Techniques. Int. J. Electrochem. Sci. 2014, 9, 3287–3318. [Google Scholar]
- Manjakkal, L.; Sakthivel, B.; Gopalakrishnan, N.; Dahiya, R. Printed Flexible Electrochemical pH Sensors Based on CuO Nanorods. Sens. Actuators B Chem. 2018, 263, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Tamayol, A.; Akbari, M.; Zilberman, Y.; Comotto, M.; Lesha, E.; Serex, L.; Bagherifard, S.; Chen, Y.; Fu, G.; Ameri, S.K.; et al. Flexible pH-Sensing Hydrogel Fibers for Epidermal Applications. Adv. Healthc. Mater. 2016, 5, 711–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, N.; Qin, J.; Feng, P.; Li, Z.; Song, B. Color-Changing Smart Fibrous Materials for Naked Eye Real-Time Monitoring of Wound pH. J. Mater. Chem. B 2019, 7, 2626–2633. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yao, D.; Qian, Z.; Hou, S.; Li, L.; Jenkins, A.T.A.; Fan, Y. Bacteria-Responsive Intelligent Wound Dressing: Simultaneous In Situ Detection and Inhibition of Bacterial Infection for Accelerated Wound Healing. Biomaterials 2018, 161, 11–23. [Google Scholar] [CrossRef]
- Ochoa, M.; Rahimi, R.; Zhou, J.; Jiang, H.; Yoon, C.K.; Maddipatla, D.; Narakathu, B.B.; Jain, V.; Oscai, M.M.; Morken, T.J.; et al. Integrated Sensing and Delivery of Oxygen for Next-Generation Smart Wound Dressings. Microsyst. Nanoeng. 2020, 6, 46. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Zhang, T.; Zhang, W.; Chen, W.; Cao, Y.; Chen, M.; Zhou, X. Orange-Emissive Carbon Quantum Dots: Toward Application in Wound pH Monitoring Based on Colorimetric and Fluorescent Changing. Small 2019, 15, 1902823. [Google Scholar] [CrossRef]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical Sensors Definitions and Classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Romanholo, P.V.V.; Razzino, C.A.; Raymundo-Pereira, P.A.; Prado, T.M.; Machado, S.A.S.; Sgobbi, L.F. Biomimetic Electrochemical Sensors: New Horizons and Challenges in Biosensing Applications. Biosens. Bioelectron. 2021, 185, 113242. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, R.; Keskin, M. Biosensors: Components, Mechanisms, and Applications. In Analytical Techniques in Bioscience; Egbuna, C., Patrick-Iwuanyanwu, K., Shah, M.A., Ifemeje, J., Rasul, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 179–190. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Rocha-Santos, T.A.; Duarte, A.C. Review of Analytical Figures of Merit of Sensors and Biosensors in Clinical Applications. TrAC-Trends Anal. Chem. 2010, 29, 1172–1183. [Google Scholar] [CrossRef]
- Baig, N.; Sajid, M.; Saleh, A. Recent Trends in Nanomaterial-Modified Electrodes for Electroanalytical Applications. TrAC-Trends Anal. Chem. 2019, 111, 47–61. [Google Scholar] [CrossRef]
- Kumar, N.; Shetti, N.P.; Jagannath, S.; Aminabhavi, T.M. Electrochemical Sensors for the Detection of SARS-CoV-2 Virus. Chem. Eng. J. 2022, 430, 132966. [Google Scholar] [CrossRef] [PubMed]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical Sensor and Biosensor Platforms Based on Advanced Nanomaterials for Biological and Biomedical Applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Ozoemena, K.I.; Carrara, S. Biomedical Electrochemical Sensors for Resource-Limited Countries. Curr. Opin. Electrochem. 2017, 3, 51–56. [Google Scholar] [CrossRef]
- Hart, J.P.; Crew, A.; Crouch, E.; Honeychurch, K.C.; Pemberton, R.M. Screen-Printed Electrochemical (Bio)Sensors in Biomedical, Environmental and Industrial Applications. In Comprehensive Analytical Chemistry, 1st ed.; Cheng, Y.P., Ma, T.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 49, pp. 497–557. [Google Scholar] [CrossRef]
- Williams, D.E. Electrochemical Sensors for Environmental Gas Analysis. Curr. Opin. Electrochem. 2020, 22, 145–153. [Google Scholar] [CrossRef]
- Gan, X.; Zhao, H. Understanding Signal Amplification Strategies of Nanostructured Electrochemical Sensors for Environmental Pollutants. Curr. Opin. Electrochem. 2019, 17, 56–64. [Google Scholar] [CrossRef]
- Du, H.; Xie, Y.; Wang, J. Nanomaterial-Sensors for Herbicides Detection Using Electrochemical Techniques and Prospect Applications. TrAC-Trends Anal. Chem. 2021, 135, 116178. [Google Scholar] [CrossRef]
- Ananda Murthy, H.C.; Gebremedhn Kelele, K.; Ravikumar, C.R.; Nagaswarupa, H.P.; Tadesse, A.; Desalegn, T. Graphene-Supported Nanomaterials as Electrochemical Sensors: A Mini Review. Results Chem. 2021, 3, 100131. [Google Scholar] [CrossRef]
- Si, Y.; Lee, H.J. Carbon Nanomaterials and Metallic Nanoparticles-Incorporated Electrochemical Sensors for Small Metabolites: Detection Methodologies and Applications. Curr. Opin. Electrochem. 2020, 22, 234–243. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, Y.; Zhang, T. Materials, structures, and functions for flexible and stretchable biomimetic sensors. Acc. Chem. Res. 2019, 52, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Guesada-González, D.; Merkoçi, A. Nanomaterial-based devices for point-of-care diagnostic applications. Chem. Soc. Rev. 2018, 47, 4697–4709. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tilley, R.D.; Gooding, J.J. Challenges and solutions in developing ultrasensitive biosensors. J. Am. Chem. Soc. 2019, 141, 1162–1170. [Google Scholar] [CrossRef]
- Bahadir, E.B.; Sezgintürk, M.K. Applications of Graphene in Electrochemical Sensing and Biosensing. TrAC-Trends Anal. Chem. 2016, 76, 1–14. [Google Scholar] [CrossRef]
- Nag, A.; Mitra, A.; Mukhopadhyay, S.C. Graphene and Its Sensor-Based Applications: A Review. Sens. Actuators A 2017, 270, 177–194. [Google Scholar] [CrossRef]
- Brownson, D.A.C.; Banks, C.E. Graphene Electrochemistry: An Overview of Potential Applications. Analyst 2010, 135, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Murthy, C.N.; Prabha, C.R. Recent Advances in Carbon Nanotube Based Electrochemical Biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, R.; Ochoa, M.; Tamayol, A.; Khalili, S.; Khademhosseini, A.; Ziaie, B. Highly Stretchable Potentiometric pH Sensor Fabricated via Laser Carbonization and Machining of Carbon−Polyaniline Composite. ACS Appl. Mater. Interfaces 2017, 9, 9015–9023. [Google Scholar] [CrossRef]
- Farooqui, M.F.; Shamim, A. Low Cost Inkjet Printed Smart Bandage for Wireless Monitoring of Chronic Wounds. Sci. Rep. 2016, 6, 28949. [Google Scholar] [CrossRef] [Green Version]
- Barber, R.; Cameron, S.; Devine, A.; McCombe, A.; Pourshahidi, L.K.; Cundell, J.; Roy, S.; Mathur, A.; Casimero, C.; Papakonstantinou, P.; et al. Laser Induced Graphene Sensors for Assessing pH: Application to Wound Management. Electrochem. Commun. 2021, 123, 106914. [Google Scholar] [CrossRef]
- He, M.; Ou, F.; Wu, Y.; Sun, X.; Chen, X.; Li, H.; Sun, D.; Zhang, L. Smart Multi-Layer PVA Foam/ CMC Mesh Dressing with Integrated Multi-Functions for Wound Management and Infection Monitoring. Mater. Des. 2020, 194, 108913. [Google Scholar] [CrossRef]
- Mazzara, F.; Patella, B.; Aiello, G.; O’Riordan, A.; Torino, C.; Vilasi, A.; Inguanta, R. Electrochemical Detection of Uric Acid and Ascorbic Acid Using R-GO/NPs Based Sensors. Electrochim. Acta 2021, 388, 138652. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO Nanostructures: Synthesis, Characterization, Growth Mechanisms, Fundamental Properties, and Applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Malekzad, H.; Sahandi Zangabad, P.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble Metal Nanoparticles in Biosensors: Recent Studies and Applications. Nanotechnol. Rev. 2017, 6, 301–329. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, K.; Xu, H.; Yan, W.; Jin, Q.; Cui, D. Detection Platforms for Point-of-Care Testing Based on Colorimetric, Luminescent and Magnetic Assays: A Review. Talanta 2019, 202, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Piriya, V.S.A.; Joseph, P.; Daniel, S.C.G.K.; Lakshmanan, S.; Kinoshita, T.; Muthusamy, S. Colorimetric Sensors for Rapid Detection of Various Analytes. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Zhang, M.; Bhandari, B.; Yang, C.H. Novel pH-Sensitive Films Containing Curcumin and Anthocyanins to Monitor Fish Freshness. Food Hydrocoll. 2020, 100, 105438. [Google Scholar] [CrossRef]
- Li, T.; Li, Z.; Huang, T.; Tian, L. Carbon Quantum Dot-Based Sensors for Food Safety. Sens. Actuators A Phys. 2021, 331, 113003. [Google Scholar] [CrossRef]
- Nazri, N.A.A.; Azeman, N.H.; Luo, Y.; A Bakar, A.A. Carbon Quantum Dots for Optical Sensor Applications: A Review. Opt. Laser Technol. 2021, 139, 106928. [Google Scholar] [CrossRef]
- Darwish, I.A.; Khalil, N.Y.; Bakheit, A.H.; Alzoman, N.Z. A Highly Sensitive Fluorimetric Method For Determination of Lenalidomide in Its Bulk Form and Capsules via Derivatization with Fluorescamine. Chem. Cent. J. 2012, 6, 118. [Google Scholar] [CrossRef] [Green Version]

| Wound Infection Biomarkers | |
|---|---|
Physiochemical parameters
| Signaling molecules
|
Enzymes
| Bacteria
|
Metabolites
| Bacteria metabolites
|
| Sensor Type | Advantages | Disadvantages |
|---|---|---|
| Electrochemical |
|
|
| Colorimetric |
|
|
| Fluorimetric |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pusta, A.; Tertiș, M.; Cristea, C.; Mirel, S. Wearable Sensors for the Detection of Biomarkers for Wound Infection. Biosensors 2022, 12, 1. https://doi.org/10.3390/bios12010001
Pusta A, Tertiș M, Cristea C, Mirel S. Wearable Sensors for the Detection of Biomarkers for Wound Infection. Biosensors. 2022; 12(1):1. https://doi.org/10.3390/bios12010001
Chicago/Turabian StylePusta, Alexandra, Mihaela Tertiș, Cecilia Cristea, and Simona Mirel. 2022. "Wearable Sensors for the Detection of Biomarkers for Wound Infection" Biosensors 12, no. 1: 1. https://doi.org/10.3390/bios12010001
APA StylePusta, A., Tertiș, M., Cristea, C., & Mirel, S. (2022). Wearable Sensors for the Detection of Biomarkers for Wound Infection. Biosensors, 12(1), 1. https://doi.org/10.3390/bios12010001







