Liver Regeneration and Cell Transplantation for End-Stage Liver Disease
Abstract
:1. Introduction
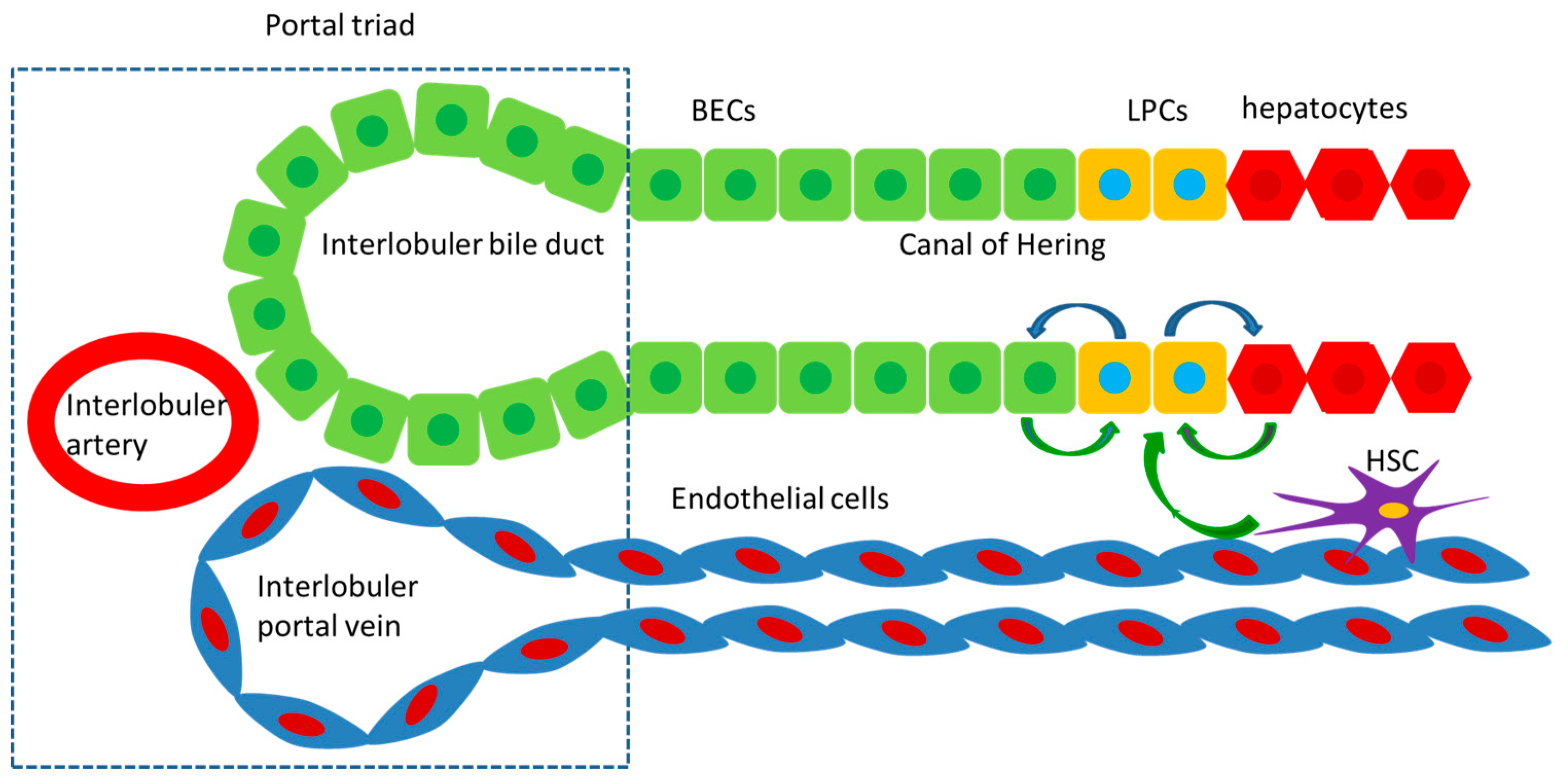
2. Histological Basis of Regeneration
3. The Typical Liver Regeneration
4. The Alternative Liver Regeneration
5. Regenerative Medicine and Cell Transplantation for ESLD
5.1. Hepatocytes Transplantation
5.2. Stem Cell Transplantation
5.2.1. LSCs
5.2.2. Nonhepatic Stem Cells
5.3. Challenges and Future Prospects of Regenerative Medicine
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fricker, Z.P.; Serper, M. Current Knowledge, Barriers to Implementation, and Future Directions in Palliative Care for End-Stage Liver Disease. Liver Transplant. 2019, 25, 787–796. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, E. Clinical application of stem cell in patients with end-stage liver disease: Progress and challenges. Ann. Transl. Med. 2020, 8, 564. [Google Scholar] [CrossRef] [PubMed]
- So, J.; Kim, A.; Lee, S.-H.; Shin, D. Liver progenitor cell-driven liver regeneration. Exp. Mol. Med. 2020, 52, 1230–1238. [Google Scholar] [CrossRef]
- Ali, M.; Payne, S.L. Biomaterial-based cell delivery strategies to promote liver regeneration. Biomater. Res. 2021, 25, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hua, J. Immune cells in liver regeneration. Oncotarget 2016, 8, 3628–3639. [Google Scholar] [CrossRef] [Green Version]
- De Rudder, M.; Dili, A.; Stärkel, P.; Leclercq, I. Critical Role of LSEC in Post-Hepatectomy Liver Regeneration and Failure. Int. J. Mol. Sci. 2021, 22, 8053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, J.; Ni, R.; Yang, Q.; Luo, L.; He, J. Contributions of biliary epithelial cells to hepatocyte homeostasis and regeneration in zebrafish. iScience 2021, 24, 102142. [Google Scholar] [CrossRef]
- Kiseleva, Y.V.; Antonyan, S.Z.; Zharikova, T.S.; Tupikin, K.A.; Kalinin, D.V.; Zharikov, Y.O. Molecular pathways of liver regeneration: A comprehensive review. World J. Hepatol. 2021, 13, 270–290. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K. Principles of Liver Regeneration and Growth Homeostasis. Compr. Physiol. 2013, 3, 485–513. [Google Scholar]
- Liu, M.; Chen, P. Proliferation inhibiting pathways in liver regeneration (Review). Mol. Med. Rep. 2017, 16, 23–35. [Google Scholar] [CrossRef]
- Huck, I.; Gunewardena, S.; Espanol-Suner, R.; Willenbring, H.; Apte, U. Hepatocyte Nuclear Factor 4 Alpha Activation Is Essential for Termination of Liver Regeneration in Mice. Hepatology 2019, 70, 666–681. [Google Scholar] [CrossRef]
- Nishiyama, K.; Nakashima, H.; Ikarashi, M.; Kinoshita, M.; Nakashima, M.; Aosasa, S.; Seki, S.; Yamamoto, J. Mouse CD11b+Kupffer Cells Recruited from Bone Marrow Accelerate Liver Regeneration after Partial Hepatectomy. PLoS ONE 2015, 10, e0136774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, Y.A.; Fu, Y.; Rodrigues, R.M.; He, Y.; Guan, Y.; Guillot, A.; Ren, R.; Feng, D.; Hidalgo, J.; Ju, C.; et al. Kupffer cell restoration after partial hepatectomy is mainly driven by local cell proliferation in IL-6-dependent autocrine and paracrine manners. Cell. Mol. Immunol. 2021, 18, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.-L.; Cai, X.; Yuan, X.; Liebe, R.; Dooley, S.; Li, H.; Wang, T.-L. Two sides of one coin: Massive hepatic necrosis and progenitor cell-mediated regeneration in acute liver failure. Front. Physiol. 2015, 6, 178. [Google Scholar] [CrossRef] [Green Version]
- Van Haele, M.; Roskams, T. Hepatic Progenitor Cells: An Update. Gastroenterol. Clin. N. Am. 2017, 46, 409–420. [Google Scholar] [CrossRef]
- Katoonizadeh, A.; Nevens, F.; Verslype, C.; Pirenne, J.; Roskams, T. Liver regeneration in acute severe liver impairment: A clinicopathological correlation study. Liver Int. 2006, 26, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Wang, S.; Munker, S.; Jung, K.; Macías-Rodríguez, R.U.; Ruiz-Margáin, A.; Schierwagen, R.; Liu, H.; Shao, C.; Fan, C.; et al. Follistatin-controlled activin-HNF4α-coagulation factor axis in liver progenitor cells determines outcome of acute liver failure. Hepatology 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Itoh, T. Stem/progenitor cells in liver regeneration. Hepatology 2016, 64, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Raven, A.; Lu, W.-Y.; Man, T.Y.; Ferreira-Gonzalez, S.; O’Duibhir, E.; Dwyer, B.J.; Thomson, J.P.; Meehan, R.R.; Bogorad, R.; Koteliansky, V.; et al. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature 2017, 547, 350–354. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, X.; Li, W.; Feng, R.-X.; Li, L.; Yi, G.-R.; Zhang, X.-N.; Yin, C.; Yu, H.-Y.; Zhang, J.-P.; et al. Chronic Liver Injury Induces Conversion of Biliary Epithelial Cells into Hepatocytes. Cell Stem Cell 2018, 23, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Lotowska, J.M.; Sobaniec-Lotowska, M.E.; Sobaniec, P. Ultrastructural Profile Combined with Immunohistochemistry of a Hepatic Progenitor Cell Line in Pediatric Autoimmune Hepatitis: New Insights into the Morphological Pattern of the Disease. Cells 2021, 10, 1899. [Google Scholar] [CrossRef]
- Tarlow, B.D.; Pelz, C.; Naugler, W.E.; Wakefield, L.; Wilson, E.M.; Finegold, M.J.; Grompe, M. Bipotential Adult Liver Progenitors Are Derived from Chronically Injured Mature Hepatocytes. Cell Stem Cell 2014, 15, 605–618. [Google Scholar] [CrossRef] [Green Version]
- Kordes, C.; Sawitza, I.; Götze, S.; Herebian, D.; Häussinger, D. Hepatic stellate cells contribute to progenitor cells and liver regeneration. J. Clin. Investig. 2014, 124, 5503–5515. [Google Scholar] [CrossRef] [Green Version]
- Bellanti, F.; di Bello, G.; Iannelli, G.; Pannone, G.; Pedicillo, M.C.; Boulter, L.; Lu, W.-Y.; Tamborra, R.; Villani, R.; Vendemiale, G.; et al. Inhibition of nuclear factor (erythroid-derived 2)-like 2 promotes hepatic progenitor cell activation and differentiation. NPJ Regen. Med. 2021, 6, 28. [Google Scholar] [CrossRef]
- Siddiqui, H.; Rawal, P.; Bihari, C.; Arora, N.; Kaur, S. Vascular Endothelial Growth Factor Promotes Proliferation of Epithelial Cell Adhesion Molecule–Positive Cells in Nonalcoholic Steatohepatitis. J. Clin. Exp. Hepatol. 2020, 10, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, M.; Ko, S.; Liu, Y.; Wang, H.; Sun, Y.; Solnica-Krezel, L.; Shin, D. Stat3 Regulates Liver Progenitor Cell-Driven Liver Regeneration in Zebrafish. Gene Expr. 2018, 18, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.O.; Lu, W.; Okabe, H.; Abrams, M.; Oertel, M.; Poddar, M.; Singh, S.; Forbes, S.J.; Monga, S.P. Hepatocyte-Specific β-Catenin Deletion During Severe Liver Injury Provokes Cholangiocytes to Differentiate Into Hepatocytes. Hepatology 2019, 69, 742–759. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.; Russell, J.O.; Tian, J.; Gao, C.; Kobayashi, M.; Feng, R.; Yuan, X.; Shao, C.; Ding, H.; Poddar, M.; et al. Hdac1 Regulates Differentiation of Bipotent Liver Progenitor Cells During Regeneration via Sox9b and Cdk8. Gastroenterology 2019, 156, 187–202.e14. [Google Scholar] [CrossRef]
- So, J.; Kim, M.; Lee, S.H.; Ko, S.; Lee, D.A.; Park, H.; Shin, D. Attenuating the Epidermal Growth Factor Receptor-Extracellular Signal-Regulated Kinase-Sex-Determining Region Y-Box 9 Axis Promotes Liver Progenitor Cell-Mediated Liver Regeneration in Zebrafish. Hepatology 2021, 73, 1494–1508. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Yamaguchi, J.; Kokuryo, T.; Ebata, T.; Yokoyama, Y.; Nagino, M. Loss of trefoil factor 1 inhibits biliary regeneration but accelerates the hepatic differentiation of progenitor cells in mice. Biochem. Biophys. Res. Commun. 2018, 506, 12–19. [Google Scholar] [CrossRef]
- Zeng, J.; Jing, Y.; Wu, Q.; Zeng, J.; Wei, L.; Liu, J. Autophagy Is Required for Hepatic Differentiation of Hepatic Progenitor Cells via Wnt Signaling Pathway. BioMed Res. Int. 2021, 2021, 6627506. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, H.; He, Y.; Lu, C.; Zhu, P.; Li, M.; Duan, J.; Fang, Z. Troglitazone inhibits hepatic oval cell proliferation by inducing cell cycle arrest through Hippo/YAP pathway regulation. Dig. Liver Dis. 2021. [Google Scholar] [CrossRef]
- Jung, K.; Kim, M.; So, J.; Lee, S.H.; Ko, S.; Shin, D. Farnesoid X Receptor Activation Impairs Liver Progenitor Cell-Mediated Liver Regeneration via the PTEN-PI3K-AKT-mTOR Axis in Zebrafish. Hepatology 2021, 74, 397–410. [Google Scholar] [CrossRef]
- Bizzaro, D.; Russo, F.P.; Burra, P. New Perspectives in Liver Transplantation: From Regeneration to Bioengineering. Bioengineering 2019, 6, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva Morais, A.; Vieira, S.; Zhao, X.; Mao, Z.; Gao, C.; Oliveira, J.M.; Reis, R.L. Advanced Biomaterials and Processing Methods for Liver Regeneration: State-of- the-Art and Future Trends. Adv. Healthc. Mater. 2020, 9, e1901435. [Google Scholar] [CrossRef] [PubMed]
- Matas, A.J.; Sutherland, D.E.; Steffes, M.W.; Mauer, S.M.; Sowe, A.; Simmons, R.L.; Najarian, J.S. Hepatocellular transplantation for metabolic deficiencies: Decrease of plasms bilirubin in Gunn rats. Science 1976, 192, 892–894. [Google Scholar] [CrossRef]
- Mito, M.; Kusano, M.; Kawaura, Y. Hepatocyte transplantation in man. Transplant. Proc. 1992, 24, 3052–3053. [Google Scholar] [CrossRef]
- Dwyer, B.J.; Macmillan, M.T.; Brennan, P.N.; Forbes, S.J. Cell therapy for advanced liver diseases: Repair or rebuild. J. Hepatol. 2021, 74, 185–199. [Google Scholar] [CrossRef]
- Iansante, V.; Mitry, R.R.; Filippi, C.; Fitzpatrick, E.; Dhawan, A. Human hepatocyte transplantation for liver disease: Current status and future perspectives. Pediatr. Res. 2018, 83, 232–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cernigliaro, V.; Peluso, R.; Zedda, B.; Silengo, L.; Tolosano, E.; Pellicano, R.; Altruda, F.; Fagoonee, S. Evolving Cell-Based and Cell-Free Clinical Strategies for Treating Severe Human Liver Diseases. Cells 2020, 9, 386. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.; Russell, J.O.; Molina, L.M.; Monga, S.P. Liver Progenitors and Adult Cell Plasticity in Hepatic Injury and Repair: Knowns and Unknowns. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 23–50. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.-Y.; Bird, T.; Boulter, L.; Tsuchiya, A.; Cole, A.M.; Hay, T.; Guest, R.V.; Wojtacha, D.; Man, T.Y.; Mackinnon, A.; et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat. Cell Biol. 2015, 17, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Subba Rao, M.; Sasikala, M.; Nageshwar Reddy, D. Thinking outside the liver: Induced pluripotent stem cells for hepatic applications. World J. Gastroenterol. 2013, 19, 3385–3396. [Google Scholar] [PubMed]
- Pettinato, G.; Lehoux, S.; Ramanathan, R.; Salem, M.M.; He, L.-X.; Muse, O.; Flaumenhaft, R.; Thompson, M.T.; Rouse, E.A.; Cummings, R.D.; et al. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells. Sci. Rep. 2019, 9, 8920. [Google Scholar] [CrossRef] [Green Version]
- De Miguel, M.P.; Prieto, I.; Moratilla, A.; Arias, J.; Aller, M.A. Mesenchymal Stem Cells for Liver Regeneration in Liver Failure: From Experimental Models to Clinical Trials. Stem Cells Int. 2019, 2019, 3945672. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tsuchiya, A.; Terai, S. The development of mesenchymal stem cell therapy in the present, and the perspective of cell-free therapy in the future. Clin. Mol. Hepatol. 2021, 27, 70–80. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, J.H.; Jun, J.H.; Park, S.; Jung, J.; Bae, S.H.; Kim, G.J. Enhanced PRL-1 expression in placenta-derived mesenchymal stem cells accelerates hepatic function via mitochondrial dynamics in a cirrhotic rat model. Stem Cell Res. Ther. 2020, 11, 512. [Google Scholar] [CrossRef]
- Chen, H.; Tang, S.; Liao, J.; Liu, M.; Lin, Y. VEGF 165 gene-modified human umbilical cord blood mesenchymal stem cells protect against acute liver failure in rats. J. Gene Med. 2021, 23, e3369. [Google Scholar] [CrossRef] [PubMed]
- Nigro, A.L.; Gallo, A.; Bulati, M.; Vitale, G.; Paini, D.S.; Pampalone, M.; Galvagno, D.; Conaldi, P.G.; Miceli, V. Amnion-Derived Mesenchymal Stromal/Stem Cell Paracrine Signals Potentiate Human Liver Organoid Differentiation: Translational Implications for Liver Regeneration. Front. Med. 2021, 8, 746298. [Google Scholar] [CrossRef]
- Takeuchi, S.; Tsuchiya, A.; Iwasawa, T.; Nojiri, S.; Watanabe, T.; Ogawa, M.; Yoshida, T.; Fujiki, K.; Koui, Y.; Kido, T.; et al. Small extracellular vesicles derived from interferon-γ pre-conditioned mesenchymal stromal cells effectively treat liver fibrosis. NPJ Regen. Med. 2021, 6, 19. [Google Scholar] [CrossRef]
- Schmelzle, M.; Duhme, C.; Junger, W.; Salhanick, S.D.; Chen, Y.; Wu, Y.; Toxavidis, V.; Csizmadia, E.; Han, L.; Bian, S.; et al. CD39 Modulates Hematopoietic Stem Cell Recruitment and Promotes Liver Regeneration in Mice and Humans After Partial Hepatectomy. Ann. Surg. 2013, 257, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Schwerfeld-Bohr, J.; Chi, H.; Worm, K.; Dahmen, U. Influence of Hematopoietic Stem Cell-Derived Hepatocytes on Liver Regeneration after Sex-Mismatched Liver Transplantation in Humans. J. Investig. Surg. 2012, 25, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Myerson, D.; Parkin, R.K. Donor-derived hepatocytes in human hematopoietic cell transplant recipients: Evidence of fusion. Virchows Arch. 2018, 474, 365–374. [Google Scholar] [CrossRef]
- Wang, X.; Maretti-Mira, A.C.; Wang, L.; Deleve, L.D. Liver-Selective MMP-9 Inhibition in the Rat Eliminates Ischemia-Reperfusion Injury and Accelerates Liver Regeneration. Hepatology 2019, 69, 314–328. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Siegel, C.T.; Shuai, L.; Lai, J.; Zeng, L.; Zhang, Y.; Lai, X.; Bie, P.; Bai, L. Repair of liver mediated by adult mouse liver neuro-glia antigen 2-positive progenitor cell transplantation in a mouse model of cirrhosis. Sci. Rep. 2016, 6, 21783. [Google Scholar] [CrossRef]
- Zhang, Z.; Gong, Y.; Guo, Y.; Hai, Y.; Yang, H.; Yang, S.; Liu, Y.; Ma, M.; Liu, L.; Li, Z.; et al. Direct transdifferentiation of spermatogonial stem cells to morphological, phenotypic and functional hepatocyte-like cells via the ERK1/2 and Smad2/3 signaling pathways and the inactivation of cyclin A, cyclin B and cyclin E. Cell Commun. Signal. 2013, 11, 67. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Niu, M.; Sun, M.; Yuan, Q.; Yao, C.; Hou, J.; Wang, H.; Wen, L.; Fu, H.; Zhou, F.; et al. Transdifferentiation of human male germline stem cells to hepatocytes in vivo via the transplantation under renal capsules. Oncotarget 2017, 8, 14576–14592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, R.H.G.; Liew, J.X.K.; Wee, A.; Masilamani, J.; Chang, S.K.Y.; Phan, T.T. Safety Evaluation of Human Cord-Lining Epithelial Stem Cells Transplantation for Liver Regeneration in a Porcine Model. Cell Transplant. 2020, 29, 963689719896559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overi, D.; Carpino, G.; Cardinale, V.; Franchitto, A.; Safarikia, S.; Onori, P.; Alvaro, D.; Gaudio, E. Contribution of Resident Stem Cells to Liver and Biliary Tree Regeneration in Human Diseases. Int. J. Mol. Sci. 2018, 19, 2917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, L.; Liu, R.; Qin, L.-Y.; Cheng, P.; Liu, B.-W.; Zhang, B.-Y.; Ding, S.-Z.; Li, X.-L. Transplantation of bone marrow-derived endothelial progenitor cells and hepatocyte stem cells from liver fibrosis rats ameliorates liver fibrosis. World J. Gastroenterol. 2018, 24, 237–247. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, K.; Lee, S.B.; Seo, D.; Yoon, S.; Kim, S.J.; Jang, K.; Jung, Y.K.; Lee, K.G.; Factor, V.M.; et al. Small molecule-mediated reprogramming of human hepatocytes into bipotent progenitor cells. J. Hepatol. 2019, 70, 97–107. [Google Scholar] [CrossRef]
- Park, S.; Hwang, S.I.; Kim, J.; Hwang, S.; Kang, S.; Yang, S.; Kim, J.; Kang, W.; Kim, K.-H.; Han, D.W.; et al. The therapeutic potential of induced hepatocyte-like cells generated by direct reprogramming on hepatic fibrosis. Stem Cell Res. Ther. 2019, 10, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, B.; Sun, D.; Du, Y.; Jia, J.; Sun, S.; Xu, J.; Liu, Y.; Xiang, C.; Chen, S.; Xie, H.; et al. A two-step lineage reprogramming strategy to generate functionally competent human hepatocytes from fibroblasts. Cell Res. 2019, 29, 696–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentile, S.D.; Kourouklis, A.P.; Ryoo, H.; Underhill, G.H. Integration of Hydrogel Microparticles With Three-Dimensional Liver Progenitor Cell Spheroids. Front. Bioeng. Biotechnol. 2020, 8, 792. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.E.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Kim, Y.W.; Lee, S.B.; Kang, K.; Yoon, S.; Choi, D.; Park, S.-H.; Jeong, J. Hepatic patch by stacking patient-specific liver progenitor cell sheets formed on multiscale electrospun fibers promotes regenerative therapy for liver injury. Biomaterials 2021, 274, 120899. [Google Scholar] [CrossRef] [PubMed]
- Kuse, Y.; Taniguchi, H. Present and Future Perspectives of Using Human-Induced Pluripotent Stem Cells and Organoid Against Liver Failure. Cell Transplant. 2019, 28 (Suppl. S1), 160S–165S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Cell Type | Location |
|---|---|
| LSCs | Liver |
| MSCs | Umbilical cord blood, adipose tissue, cartilage, bone marrow |
| Hematopoietic stem cells | Bone marrow, umbilical cord blood |
| EPCs | Peripheral vessels, bone marrow |
| SSCs | Testis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Lu, L.; Cai, X. Liver Regeneration and Cell Transplantation for End-Stage Liver Disease. Biomolecules 2021, 11, 1907. https://doi.org/10.3390/biom11121907
Li Y, Lu L, Cai X. Liver Regeneration and Cell Transplantation for End-Stage Liver Disease. Biomolecules. 2021; 11(12):1907. https://doi.org/10.3390/biom11121907
Chicago/Turabian StyleLi, Yan, Lungen Lu, and Xiaobo Cai. 2021. "Liver Regeneration and Cell Transplantation for End-Stage Liver Disease" Biomolecules 11, no. 12: 1907. https://doi.org/10.3390/biom11121907
APA StyleLi, Y., Lu, L., & Cai, X. (2021). Liver Regeneration and Cell Transplantation for End-Stage Liver Disease. Biomolecules, 11(12), 1907. https://doi.org/10.3390/biom11121907





