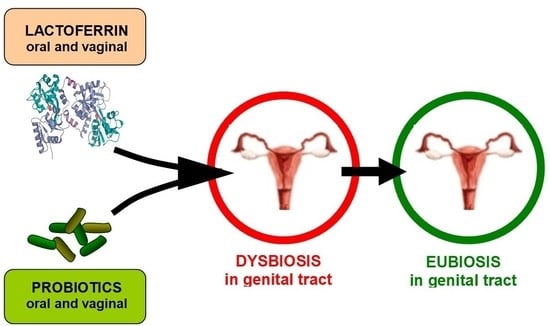
Antimicrobial and Prebiotic Activity of Lactoferrin in the Female Reproductive Tract: A Comprehensive Review
Abstract
1. Introduction
2. Immunity of the Female Genital Tract
3. Lactoferrin in the Female Genital Tract
4. Normal Microbiota of the Female Genital Tract and Factors That Can Affect It
- Antibiotic therapy (antibiotics killing Gram-positive bacteria, among others penicillin, cephalosporin, carbapenem, macrolides, polimyxin, bacitracin, vancomycin);
- Natural corticosteroids (secreted upon stress) and steroidal drugs;
- Nonsteroidal anti-inflammatory drugs (NSAIDs);
- Immunosuppressive drugs, radiotherapy;
- Chronic inflammatory illnesses including those of autoimmune etiology, and metabolic syndrome (diabetes, obesity, hypertension);
- Diseases with primary or secondary immunosuppression (for example, oncologic diseases);
- Infectious diseases (viral, bacterial, fungal, parasitic);
- Surgeries of the urinogenital tract;
- Mechanical contraception (rings, uterine spiral);
- Sexual life (in particular early-initiated, frequent, with various partners, oral and anal sex and digital vaginal penetration);
- Pregnancy;
- Hygiene habits (scant or excessive hygiene, application of tampons, disinfecting agents, vaginal irrigation);
- Lingerie (tight-fitting, cool, synthetic);
- Low socioeconomic status;
- Cigarettes, alcohol, narcotics;
- Diet (mainly monosaccharides);
- Microbiota in the oral cavity;
- Bathing in pools.
5. Abnormal Microbiota of the Female Genital Tract
- Bacterial infection without inflammatory state (bacterial vaginosis, BV);
- Bacterial infection with the domination of aerobic bacteria and accompanying inflammatory state (aerobic vaginitis, AV);
- Fungal inflammation of the vulva and vagina (vulvovaginal candidiasis, VVC);
- Trichomoniasis of the vagina (Trichomonas vaginitis, TV);
- Non-typical inflammation (C. trachomatis, Mycoplasma hominis, Mycoplasma genitalium, Ureaplasma urealyticum, and others).
6. Probiotics and Prebiotics, Activity and Safety
7. Probiotics and Prebiotics for Prophylaxis and Therapy of Female Tract Infections
8. Antimicrobial and Prebiotic Activity of Lactoferrin in the Genital Tract—In Vitro Tests
9. Antimicrobial and Prebiotic Activity of Lactoferrin in the Genital Tract—In Vivo Tests
10. Lactoferrin in the Diet and Dietary Supplements
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Döderlein, A. Das Scheidensekret und Seine Bedeutung für das Puerperalfi Eber; BOD: Leipzig, Germany, 1892. [Google Scholar]
- Artym, J.; Zimecki, M. Beneficial effect of lactoferrin on the microbiota from gastrointestinal tract. Adv. Microbiol. 2020, 59, 277–290. [Google Scholar] [CrossRef]
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Gabrielli, O. Prebiotics in human milk: A review. Dig. Liver Dis. 2006, 38 (Suppl. 2), S291–S294. [Google Scholar] [CrossRef]
- Vega-Bautista, A.; de la Garza, M.; Carrero, J.C.; Campos-Rodriguez, R.; Godinez-Victoria, M.; Drago-Serrano, M.E. The impact of lactoferrin on the growth of intestinal inhabitant bacteria. Int. J. Mol. Sci. 2019, 20, 4707. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D. Lactoferrin, a key molecule in immune and inflammatory processes. Biochem. Cell Biol. 2012, 90, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in Aseptic and Septic Inflammation. Molecules 2019, 24, 1323. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, S.; Spahis, S.; Pouliot, Y.; Levy, E. Lactoferrin, a Pleiotropic Protein in Health and Disease. Antioxid. Redox Signal. 2016, 24, 813–836. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Heyden, L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Jaskiewicz, A.; Tarasiuk, A.; Fichna, J. Lactoferrin: An overview of its main functions, immunomodulatory and antimicrobial role, and clinical significance. Crit. Rev. Food Sci. 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Superti, F.; De Seta, F. Warding Off Recurrent Yeast and Bacterial Vaginal Infections: Lactoferrin and Lactobacilli. Microorganisms 2020, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Agerberth, B.; Gudmundsson, G.H. Host antimicrobial defence peptides in human disease. Curr. Top. Microbiol. Immunol. 2006, 306, 67–90. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Cole, A.L. Antimicrobial polypeptides are key anti-HIV-1 effector molecules of cervicovaginal host defense. Am. J. Reprod. Immunol. 2008, 59, 27–34. [Google Scholar] [CrossRef]
- Kalia, M.; Singh, J.; Kaur, M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Madanchi, H.; Shoushtari, M.; Kashani, H.H.; Sardari, S. Antimicrobial peptides of the vaginal innate immunity and their role in the fight against sexually transmitted diseases. New Microbes New Infect. 2019, 34, 100627. [Google Scholar] [CrossRef]
- Alexander, D.B.; Iigo, M.; Yamauchi, K.; Suzui, M.; Tsuda, H. Lactoferrin: An alternative view of its role in human biological fluids. Biochem. Cell Biol. 2012, 90, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of lactobacilli and lactoferrin in the mucosal cervicovaginal defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef]
- Artym, J.; Zimecki, M. The role of lactoferrin in infections and inflammation. Forum Zakażeń 2013, 4, 329–345. [Google Scholar] [CrossRef]
- Jenssen, H.; Hancock, R.E. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.D. Antibiotic Properties and Applications of Lactoferrin. Curr. Pharm. Des. 2007, 13, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Artym, J. The role of lactoferrin in the iron metabolism. Part II. Antimicrobial and antiinflammatory effect of lactoferrin by chelation of iron. Post. Hig. Med. Dosw 2010, 64, 604–616. [Google Scholar]
- Superti, F. Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; Compos-Rodriguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin: Balancing ups and downs of inflammation due to microbial infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Campos-Rodriguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin and Peptide-derivatives: Antimicrobial Agents with Potential Use in Nonspecific Immunity Modulation. Curr. Pharm. Des. 2018, 24, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Gruden, S.; Ulrih, N.P. Diverse Mechanisms of Antimicrobial Activities of Lactoferrins, Lactoferricins, and Other Lactoferrin-Derived Peptides. Int. J. Mol. Sci. 2021, 22, 11264. [Google Scholar] [CrossRef]
- Teng, C.T. Lactoferrin gene expression and regulation: An overview. Biochem. Cell Biol. 2002, 80, 7–16. [Google Scholar] [CrossRef]
- Novak, R.M.; Donoval, B.A.; Graham, P.J.; Boksa, L.A.; Spear, G.; Hershow, R.C.; Chen, H.Y.; Landay, A. Cervicovaginal levels of lactoferrin, secretory leukocyte protease inhibitor, and RANTES and the effects of coexisting vaginoses in human immunodeficiency virus (HIV)-seronegative women with a high risk of heterosexual acquisition of HIV infection. Clin. Vaccine Immunol. 2007, 14, 1102–1107. [Google Scholar] [CrossRef]
- Cohen, M.S.; Britigan, B.E.; French, M.; Bean, K. Preliminary observations on lactoferrin secretion in human vaginal mucus: Variation during the menstrual cycle, evidence of hormonal regulation, and implications for infection with Neisseria gonorrhoeae. Am. J. Obstet. Gynecol. 1987, 157, 1122–1125. [Google Scholar] [CrossRef]
- Hein, M.; Valore, E.V.; Helmig, R.B.; Uldbjerg, N.; Ganz, T. Antimicrobial factors in the cervical mucus plug. Am. J. Obstet. Gynecol. 2002, 187, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Heller, K.A.; Greig, P.C.; Heine, R.P. Amniotic-fluid lactoferrin: A marker for subclinical intraamniotic infection prior to 32 weeks gestation. Infect. Dis. Obstet. Gynecol. 1995, 3, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M. Innate host defense of human vaginal and cervical mucosae. Curr. Top. Microbiol. Immunol. 2006, 306, 199–230. [Google Scholar] [CrossRef] [PubMed]
- Spear, G.T.; Kendrick, S.R.; Chen, H.Y.; Thomas, T.T.; Bahk, M.; Balderas, R.; Ghosh, S.; Weinberg, A.; Landay, A.L. Multiplex immunoassay of lower genital tract mucosal fluid from women attending an urban STD clinic shows broadly increased IL1β and lactoferrin. PLoS ONE 2011, 6, e19560. [Google Scholar] [CrossRef] [PubMed]
- Filardo, S.; Di Pietro, M.; Tranquilli, G.; Latino, M.A.; Recine, N.; Porpora, M.G.; Sessa, R. Selected Immunological Mediators and Cervical Microbial Signatures in Women with Chlamydia trachomatis Infection. mSystems 2019, 4, e00094-19. [Google Scholar] [CrossRef]
- Otsuki, K.; Yoda, A.; Toma, Y.; Shimizu, Y.; Saito, H.; Yanaihara, T. Lactoferrin and interleukin-6 interaction in amniotic infection. Adv. Exp. Med. Biol. 1998, 443, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, K.; Yoda, A.; Saito, H.; Mitsuhashi, Y.; Toma, Y.; Shimizu, Y.; Yanaihara, T. Amniotic Fluid Lactoferrin in Intrauterine Infection. Placenta 1999, 20, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, K.; Yoda, A.; Hirose, K.; Shimizu, Y.; Saito, H.; Yanaihara, T. Salivary Lactoferrin in Neonates with Chorioamnionitis. Showa Univ. J. Med. Sci. 1997, 9, 39–44. [Google Scholar] [CrossRef][Green Version]
- Adnane, M.; Meade, K.M.; O’Farrelly, C. Cervico-vaginal mucus (CVM)—An accessible source of immunologically informative biomolecules, Vet. Res. Commun. 2018, 42, 255–263. [Google Scholar] [CrossRef]
- Rein, M.F.; Shih, L.M.; Miller, J.R.; Guerrant, R.L. Use of Lactoferrin Assay in the Differential Diagnosis of Female Genital Tract Infections and Implications for the Pathophysiology of Bacterial Vaginosis. Sex. Trans. Dis. 1996, 23, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.R.; Sperandio, V. Inter-kingdom signaling: Chemical language between bacteria and host. Curr. Opin. Microbiol. 2009, 12, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Szachta, P.; Gałęcka, M.; Bartnicka, A. Biodiversity of vaginal microbiota. The role of gynecological probiotics in maitaning the balance of vaginal ecosystem. Forum Zak. 2015, 6, 139–143. [Google Scholar]
- van de Wijgert, J.H.; Borgdorff, H.; Verhelst, R.; Crucitti, T.; Francis, S.; Verstraelen, H.; Jespers, V. The vaginal microbiota: What have we learned after a decade of molecular characterization? PLoS ONE 2014, 9, e105998. [Google Scholar] [CrossRef] [PubMed]
- Pytka, M.; Kordowska-Wiater, M.; Jarocki, P. Microbiome of the womens genital tract. Adv. Microbiol. 2019, 58, 227–236. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Hida, K.; Shukair, S.; Wang, Y.Y.; Figueiredo, A.; Cone, R.; Hope, T.J.; Hanes, J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J. Virol. 2009, 83, 11196–11200. [Google Scholar] [CrossRef] [PubMed]
- Graver, M.A.; Wade, J.J. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 8. [Google Scholar] [CrossRef]
- Hummelen, R.; Macklaim, J.M.; Bisanz, J.E.; Hammond, J.-A.; McMillan, A.; Vongsa, R.; Vongsa, R.; Koenig, D.; Gloor, G.B.; Reid, G. Vaginal microbiome and epithelial gene array in post-menopausal women with moderate to severe dryness. PLoS ONE 2011, 6, e26602. [Google Scholar] [CrossRef]
- Hainer, B.L.; Gibson, M.V. Vaginitis: Diagnosis and Treatment. Am. Fam. Phys. 2011, 83, 807–815. [Google Scholar]
- Edwards, L. Dermatologic causes of vaginitis: A clinical review. Dermatol. Clin. 2010, 28, 727–735. [Google Scholar] [CrossRef]
- Mendling, W. Vaginal microbiota. Adv. Exp. Med. Biol. 2016, 902, 83–93. [Google Scholar] [CrossRef]
- Madej, A.; Mazanowska, N. Aerobic vaginitis. Menopause Rev. 2017, 16, 4–7. [Google Scholar]
- Srinivasan, S.; Fredricks, D.N. The human vaginal bacterial biota and bacterial vaginosis. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 750479. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.; Coleman, D.; O’Salilivan, M.; Stephens, N. Public health policies and management strategies for genital Chlamydia trachomatis infection. Risk Manag. Health Policy 2011, 4, 57–65. [Google Scholar] [CrossRef]
- FAO/WHO. Health and Nutrition Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; Food and Agriculture Organization of the United Nations and World Health Organization: Córdoba, Argentina, 2001; Available online: http://www.fao.org/3/a-a0512e.pdf (accessed on 20 September 2021).
- Linares, D.M.; Gómez, C.; Renes, E.; Fresno, J.M.; Tornadijo, M.E.; Ross, R.P.; Stanton, C. Lactic acid bacteria and bifidobacteria with potential to design natural biofunctional health-promoting dairy foods. Front. Microbiol. 2017, 8, 846. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Slizewska, K. Effect of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Bruck, W.M.; Redgrave, M.; Tuohy, K.M.; Lonnerdal, B.; Graverholt, G.; Hernell, O.; Gibson, G.R. Effects of bovine α-lactalbumin and casein glycomacropeptide-enriched infant formulae on faecal microbiota in healthy term infants. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Hilton, E.; Isenberg, H.D.; Alperstein, P.; France, K.; Borenstein, M.T. Ingestion of yoghurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann. Intern. Med. 1992, 116, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Harasim-Dylak, A.; Roguska, M.; Maździarz, A. Effectiveness of Trivagin in restoring and maintaining normal vaginal ecosystem in women treated for recurrent bacterial vaginosis. Curr. Gynecol. Oncol. 2011, 9, 245–252. [Google Scholar]
- Larsson, P.G.; Stray-Pedersen, B.; Ryttig, K.R.; Larsen, S. Human lactobacilli as supplementation of clindamycin to patients with bacterial vaginosis reduce the recurrence rate; a 6-month, double-blind, randomized, placebo-controlled study. BMC Womens Health 2008, 8, 3. [Google Scholar] [CrossRef]
- Mastromarino, P.; Macchia, S.; Meggiorini, L.; Trinchieri, V.; Mosca, L.; Perluigi, M.; Midulla, C. Effectiveness of Lactobacillus containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin. Microbiol. Infect. 2009, 15, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Barbonetti, A.; Cinque, B.; Vassallo, M.R.; Mineo, S.; Francavilla, S.; Cifone, M.G.; Francavilla, F. Effect of vaginal probiotic lactobacilli on in vitro-induced sperm lipid peroxidation and its impact on sperm motility and viability. Fertil. Steril. 2011, 95, 2485–2488. [Google Scholar] [CrossRef] [PubMed]
- Mastromarino, P.; Hemalatha, R.; Barbonetti, A.; Cinque, B.; Cifone, M.G.; Tammaro, F.; Francavilla, F. Biological control of vaginosis to improve reproductve health. Indian J. Med. Res. 2014, 2014, 80–86. [Google Scholar]
- Mastromarino, P.; Vitali, B.; Mosca, L. Bacterial vaginosis: A review on clinical trials with probiotics. New Microbiol. 2013, 36, 229–238. [Google Scholar]
- Senok, A.C.; Verstraelen, H.; Temmerman, M.; Botta, G.A. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst. Rev. 2009, 4, CD006289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Y.; Zheng, Y. Probiotics for the Treatment of Bacterial Vaginosis: A Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 3859. [Google Scholar] [CrossRef] [PubMed]
- Dugoua, J.J.; Machado, M.; Zhu, X.; Chen, X.; Koren, G.; Einarson, T.R. Probiotic safety in pregnancy: A systematic review and meta-analysis of randomized controlled trials of Lactobacillus, Bifidobacterium, and Saccharomyces spp. J. Obstet. Gynaecol. Can. 2009, 31, 542–552. [Google Scholar] [CrossRef]
- Elias, J.; Bozzo, P.; Einarson, A. Are probiotics safe for use during pregnancy and lactation? Can. Fam. Physician 2011, 57, 299–301. [Google Scholar]
- Vitali, B.; Cruciani, F.; Baldassarre, M.E.; Capursi, T.; Spisni, E.; Valerii, M.C.; Candela, M.; Turroni, S.; Brigidi, P. Dietary supplementation with probiotics during late pregnancy: Outcome on vaginal microbiota and cytokine secretion. BMC Microbiol. 2012, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.Y.; Feng, D.; Wei, D.M.; Mei, L.; Chen, H.; Wang, X.; Fang, F. Probiotics for vulvovaginal candidiasis in non-pregnant women. Cochrane Database Syst. Rev. 2017, 11, CD010496. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.; Pires, M.C.; Leao, T.L.; Hernandez, Z.P.; Rodriguez, M.L.; Martins, A.K.; Miranda, L.S.; Martins, F.S.; Nicoli, J.R. Selection of Lactobacillus strains as potential probiotics for vaginitis treatment. Microbiology 2016, 162, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Antonio, M.A.; Meyn, L.A.; Murray, P.J.; Busse, B.; Hillier, S.L. Vaginal colonization by probiotic Lactobacillus crispatus CTV-05 is decreased by sexual activity and endogenous Lactobacilli. J. Infect. Dis. 2009, 199, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, D. Gynaecologic probiotics—Practical guidebook. Food Forum 2018, 4, 16–19. [Google Scholar]
- McFall, M. Adaptive immunity. Care for the community. Nature 2007, 445, 153. [Google Scholar] [CrossRef]
- Nader-Macías, M.E.; De Gregorio, P.R.; Silva, J.A. Probiotic lactobacilli in formulas and hygiene products for the health of the urogenital tract. Pharmacol. Res. Perspect. 2021, 9, e00787. [Google Scholar] [CrossRef]
- Strus, M.; Chmielarczyk, A.; Kochan, P.; Adamski, P.; Chełmicki, Z.; Chełmicki, A.; Pałucha, A.; Heczko, P.B. Studies on the effects of probiotic Lactobacillus mixture given orally on vaginal and rectal colonization and on parameters of vaginal health in women with intermediate vaginal flora. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 163, 210–215. [Google Scholar] [CrossRef]
- Chen, P.W.; Ku, Y.W.; Chu, F.Y. Influence of bovine lactoferrin on the growth of selected probiotic bacteria under aerobic conditions. Biometals 2014, 27, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Maddox, I.S.; Ferguson, L.R.; Shu, Q. Influence of bovine lactoferrin on selected probiotic bacteria and intestinal pathogens. Biometals 2010, 23, 593–596. [Google Scholar] [CrossRef]
- Weinberg, E.D. The Lactobacillus anomaly: Total iron abstinence. Perspect. Biol. Med. 1997, 40, 578–583. [Google Scholar] [CrossRef]
- Chen, P.W.; Jheng, T.T.; Shyu, C.L.; Mao, F.C. Antimicrobial potential for the combination of bovine lactoferrin or its hydrolysate with lactoferrin-resistant probiotics against foodborne pathogens. J. Dairy Sci. 2013, 96, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Somkuti, G.A. Hydrolytic breakdown of lactoferricin by lactic acid bacteria. J. Ind. Microbiol. Biotechnol. 2010, 37, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.E.; Jeong, J.J.; Choi, S.Y. Lactobacillus rhamnosus HN001 and Lactobacillus acidophilus La-14 attenuate Gardnerella vaginalis-infected bacterial vaginosis in mice. Nutrients 2017, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- Bertuccini, L.; Russo, R.; Iosi, F.; Superti, F. Lactobacilli and lactoferrin: Biotherapeutic effects for vaginal health. J. Funct. Foods 2018, 45, 86–94. [Google Scholar] [CrossRef]
- Sessa, R.; Di Pietro, M.; Filardo, S.; Bressan, A.; Mastromarino, P.; Biasucci, A.V.; Rosa, L.; Cutone, A.; Berlutti, F.; Paesano, R.; et al. Lactobacilli-lactoferrin interplay in Chlamydia trachomatis infection. Pathog. Dis. 2017, 75, 5. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Liu, S.; Wang, H.; Su, H.; Liu, Z. Enhanced antifungal activity of bovine lactoferrin-producing probiotic Lactobacillus casei in the murine model of vulvovaginal candidiasis. BMC Microbiol. 2019, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Oda, H.; Wakabayashi, H.; Yamauchi, K.; Abe, F. Lactoferrin and bifidobacteria. Biometals 2014, 27, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Liepke, C.; Adermann, K.; Raida, M.; Mägert, H.J.; Forssmann, W.G.; Zucht, H.D. Human milk provides peptides highly stimulating the growth of bifidobacteria. Eur. J. Biochem. 2002, 269, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Kim, W.S.; Kumura, H.; Shimazaki, K. Screening of Bifidobacterium spp. based on in vitro growth responses to bovine lactoferrin. Int. J. Food Sci. Technol. 2010, 45, 453–458. [Google Scholar] [CrossRef]
- Kim, W.S.; Ohashi, M.; Tanaka, T.; Kumura, H.; Kim, G.-Y.; Kwon, I.-K.; Goh, J.-S.; Shimazaki, K. Growth-promoting effects of lactoferrin on L. acidophilus and Bifidobacterium spp. Biometals 2004, 17, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Miyakawa, H.; Ishibashi, N.; Tamura, Y.; Hayasawa, H.; Shimamura, S. Effect of iron-free and metal-bound forms of lactoferrin on the growth of bifidobacteria, E. coli and S. aureus. Biosci. Microflora 1996, 15, 1–7. [Google Scholar] [CrossRef][Green Version]
- Andrés, M.T.; Viejo-Díaz, M.; Fierro, J.F. Human lactoferrin induces apoptosis-like cell death in Candida albicans: Critical role of K+-channel-mediated K+ efflux. Antimicrob. Agents Chemother. 2008, 52, 4081–4088. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Carter, D.A. The Antifungal Activity of Lactoferrin and Its Derived Peptides: Mechanisms of Action and Synergy with Drugs against Fungal Pathogens. Front. Microbiol. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Lupetti, A.; Paulusma-Annema, A.; Welling, M.M.; Dogterom-Ballering, H.; Brouwer, C.P.; Senesi, S.; Van Dissel, J.T.; Nibbering, P.H. Synergistic activity of the N-terminal peptide of human lactoferrin and fluconazole against Candida species. Antimicrob. Agents Chemother. 2003, 47, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, H.; Samaranayake, L.P.; Tenovuo, J.; Pang, K.M.; Hamada, T. The fungicidal effect of human lactoferrin on Candida albicans and Candida krusei. Arch. Oral. Biol. 1993, 38, 1057–1063. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Abe, S.; Okutomi, T.; Tansho, S.; Kawase, K.; Yamaguchi, H. Cooperative anti-Candida effects of lactoferrin or its peptides in combination with azole antifungal agents. Microbiol. Immunol. 1996, 40, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Leitch, E.C.; Willcox, M.D. Lactoferrin increases the susceptibility of S. epidermidis biofilms to lysozyme and vancomycin. Curr. Eye Res. 1999, 19, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ajello, M.; Greco, R.; Giansanti, F.; Massucci, M.T.; Antonini, G.; Valenti, P. Anti-invasive activity of bovine lactoferrin towards group A Streptococci. Biochem. Cell Biol. 2002, 80, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kieckens, E.; Rybarczyk, J.; Cox, E.; Vanrompay, D. Antibacterial and immunomodulatory activities of bovine lactoferrin against Escherichia coli O157:H7 infections in cattle. Biometals 2018, 31, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Masci, J.R. Complete response of severe, refractory oral candidiasis to mouthwash containing lactoferrin and lysozyme. AIDS 2000, 14, 2403–2404. [Google Scholar] [CrossRef] [PubMed]
- Trümpler, U.; Straub, P.W.; Rosenmund, A. Antibacterial prophylaxis with lactoferrin in neutropenic patients. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 310–313. [Google Scholar] [CrossRef]
- Weber-Dąbrowska, B.; Zimecki, M.; Kruzel, M.L.; Kochanowska, I.; Lusiak-Szelachowska, M. Alternative therapies in antibiotic-resistant infection. Adv. Med. Sci. 2006, 51, 242–244. [Google Scholar]
- Zavaleta, N.; Figueroa, D.; Rivera, J.; Sanchez, J.; Alfaro, S.; Lonnerdal, B. Efficacy of rice-based oral rehydration solution containing recombinant human lactoferrin and lysozyme in Peruvian children with acute diarrhea. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Lai, Y.W.; Chen, C.S.; Chu, T.W.; Lin, W.; Yen, C.C.; Lin, M.F.; Tu, M.Y.; Chen, C.M. Probiotic Lactobacillus casei expressing human lactoferrin elevates antibacterial activity in the gastrointestinal tract. Biometals 2010, 23, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.P.; Bennett, S.H.; Hwang, F.F.; Yu, C. Neonatal small bowel epithelia: Enhancing anti-bacterial defense with lactoferrin and Lactobacillus GG. Biometals 2004, 17, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Otsuki, K.; Sasaki, Y.; Sawada, M.; Mitsukawa, K.; Chiba, H.; Nagatsuka, M.; Okai, T.; Kato, A. Preventive effect of recombinant human lactoferrin in rabbit preterm delivery model. Am. J. Obstet. Gynecol. 2005, 192, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, Y.; Otsuki, K.; Yoda, A.; Shimizu, Y.; Saito, H.; Yanaihara, T. Effect of lactoferrin on lipopolysaccharide (LPS) induced preterm delivery in mice. Acta Obstet. Gynecol. Scand. 2000, 79, 355–358. [Google Scholar]
- Otsuki, K.; Yakuwa, K.; Sawada, M.; Hasegawa, A.; Sasaki, Y.; Mitsukawa, K.; Chiba, H.; Nagatsuka, M.; Saito, H.; Okai, T. Recombinant human lactoferrin has preventive effects on lipopolysaccharide-induced preterm delivery and production of inflammatory cytokines in mice. J. Perinat. Med. 2005, 33, 320–323. [Google Scholar] [CrossRef]
- Sasaki, Y.; Otsuki, K.; Hasegawa, A.; Sawada, M.; Chiba, H.; Negishi, M.; Nagatsuka, M.; Okai, T. Preventive effect of recombinant human lactoferrin on lipopolysaccharide-induced preterm delivery in mice. Acta Obstet. Gynecol. Scand. 2004, 83, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Yakuwa, K.; Otsuki, K.; Nakayama, K.; Hasegawa, A.; Sawada, M.; Mitsukawa, K.; Chiba, H.; Nagatsuka, M.; Okai, T. Recombinant human lactoferrin has a potential to suppresses uterine cervical ripening in preterm delivery in animal model. Arch. Gynecol. Obstet. 2007, 275, 331–334. [Google Scholar] [CrossRef]
- Li, W.; Fu, K.; Lv, X.; Wang, Y.; Wang, J.; Li, H.; Tian, W.; Cao, R. Lactoferrin suppresses lipopolysaccharide-induced endometritis in mice via down-regulation of the NF-κB pathway. Int. Immunopharmacol. 2015, 28, 695–699. [Google Scholar] [CrossRef]
- De Alberti, D.; Russo, R.; Terruzzi, F.; Nobile, V.; Ouwehand, A.C. Lactobacilli vaginal colonisation after oral consumption of Respecta® complex: A randomised controlled pilot study. Arch. Gynecol. Obstet. 2015, 292, 861–867. [Google Scholar] [CrossRef]
- Russo, R.; Edu, A.; De Seta, F. Study on the effects of an oral lactobacilli and lactoferrin complex in women with intermediate vaginal microbiota. Arch. Gynecol. Obstet. 2018, 298, 139–145. [Google Scholar] [CrossRef]
- Russo, R.; Karadja, E.; De Seta, F. Evidence-based mixture containing Lactobacillus strains and lactoferrin to prevent recurrent bacterial vaginosis: A double blind, placebo controlled, randomised clinical trial. Benef. Microbes 2019, 10, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, K.; Imai, N. Effects of lactoferrin in 6 patients with refractory bacterial vaginosis. Biochem. Cell Biol. 2017, 95, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Pino, A.; Giunta, G.; Randazzo, C.L.; Caruso, S.; Caggia, C.; Cianci, A. Bacterial biota of women with bacterial vaginosis treated with lactoferrin: An open prospective randomized trial. Microb. Ecol. Health Dis. 2017, 28, 135747. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Saccone, G.; Ammendola, A.; Salzano, E.; Iannicelli, M.; De Rosa, R.; Nazzaro, G.; Locci, M. Vaginal lactoferrin in prevention of preterm birth in women with bacterial vaginosis. J. Matern. Fetal Neonatal Med. 2021, 34, 3704–3708. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, K.; Tokunaka, M.; Oba, T.; Nakamura, M.; Shirato, M.; Okai, T. Administration of oral and vaginal prebiotic lactoferrin for a women with a refractory vaginitis recurring preterm delivery: Appearance of lactobacillus in vaginal flora followed by term delivery. J. Obset. Gynaecol. Res. 2014, 40, 583–585. [Google Scholar] [CrossRef]
- Russo, R.; Superti, F.; Karadja, E.; De Seta, F. Randomised clinical trial in women with recurrent vulvovaginal candidiasis: Efficacy of probiotics and lactoferrin as maintenance treatment. Mycoses 2019, 62, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Costantino, D.; Guaraldi, C. Preliminary evaluation of a vaginal cream containing lactoferrin in the treatment of vulvovaginal candidosis. Minerva Ginecol. 2008, 60, 121–125. [Google Scholar] [PubMed]
- Ali, A.E.; Fathi, M.A.; Mohammed, A.I. Efficacy of Lactoferrin in Prevention of Premature Rupture of Membrane. Egypt. J. Hosp. Med. 2021, 85, 2823–2827. [Google Scholar] [CrossRef]
- Giunta, G.; Giuffrida, L.; Mangano, K.; Fagone, P.; Cianci, A. Influence of lactoferrin in preventing preterm delivery: A pilot study. Mol. Med. Rep. 2012, 5, 162–166. [Google Scholar] [CrossRef]
- Vesce, F.; Giugliano, E.; Bignardi, S.; Cagnazzo, E.; Colamussi, C.; Marci, R.; Valente, N.; Seraceni, S.; Maritati, M.; Contini, C. Vaginal lactoferrin administration before genetic amniocentesis decreases amniotic interleukin-6 levels. Gynecol. Obstet. Invest. 2014, 77, 245–249. [Google Scholar] [CrossRef]
- Maritati, M.; Comar, M.; Zanotta, N.; Seraceni, S.; Trentini, A.; Corazza, F.; Vesce, F.; Contini, C. Influence of vaginal lactoferrin administration on amniotic fluid cytokines and its role against inflammatory complications of pregnancy. J. Inflamm. 2017, 14, 5. [Google Scholar] [CrossRef]
- Trentini, A.; Maritati, M.; Rosta, V.; Cervellati, C.; Manfrinato, M.C.; Hanau, S.; Greco, P.; Bonaccorsi, G.; Bellini, T.; Contini, C. Vaginal lactoferrin administration decreases oxidative stress in the amniotic fluid of pregnant women: An open-label randomized pilot study. Front. Med. 2020, 7, 555. [Google Scholar] [CrossRef] [PubMed]
- Trentini, A.; Maritati, M.; Cervellati, C.; Manfrinato, M.C.; Gonelli, A.; Volta, C.A.; Vesce, F.; Greco, P.; Dallocchio, F.; Bellini, T.; et al. Vaginal lactoferrin modulates PGE2, MMP-9, MMP-2, and TIMP-1 amniotic fluid concentrations. Mediat. Inflamm. 2016, 2016, 3648719. [Google Scholar] [CrossRef]
- Zimecki, M.; Właszczyk, A.; Cheneau, P.; Brunel, A.S.; Mazurier, J.; Spik, G.; Kubler, A. Immunoregulatory effects of a nutritional preparation containing bovine lactoferrin taken orally by healthy individuals. Arch. Immunol. Ther. Exp. 1998, 46, 231–240. [Google Scholar]
- Zimecki, M.; Spiegel, K.; Właszczyk, A.; Kübler, A.; Kruzel, M.L. Lactoferrin increases the output of neutrophil precursors and attenuates the spontaneous production of TNF-alpha and IL-6 by peripheral blood cells. Arch. Immunol. Ther. Exp. 1999, 47, 113–118. [Google Scholar]
- Zimecki, M.; Właszczyk, A.; Wojciechowski, R.; Dawiskiba, J.; Kruzel, M. Lactoferrin regulates the immune responses in post-surgical patients. Arch. Immunol. Ther. Exp. 2001, 49, 325–333. [Google Scholar]
- Franco, I.; Perez, M.D.; Conesa, C.; Calvo, M.; Sanchez, L. Effect of technological treatments on bovine lactoferrin: An overview. Food Res. Int. 2018, 106, 173–182. [Google Scholar] [CrossRef]
- Paulsson, M.A.; Svensson, U.; Kishore, A.R.; Naidu, A.S. Thermal Behavior of Bovine Lactoferrin in Water and Its Relation to Bacterial Interaction and Antibacterial Activity. J. Dairy Sci. 1993, 76, 3711–3720. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Yamauchi, K.; Abe, F. Quality control of commercial bovine lactoferrin. Biometals 2018, 31, 313–319. [Google Scholar] [CrossRef]
- Arciniega-Martínez, I.M.; Campos-Rodríguez, R.; Drago-Serrano, M.E.; Sánchez-Torres, L.E.; Cruz-Hernández, T.R.; Reséndiz-Albor, A.A. Modulatory Effects of Oral Bovine Lactoferrin on the IgA Response at Inductor and Effector Sites of Distal Small Intestine from BALB/c Mice. Arch. Immunol. Ther. Exp. 2016, 64, 57–63. [Google Scholar] [CrossRef]
- Debbabi, H.; Dubarry, M.; Rautureau, M.; Tomé, D. Bovine lactoferrin induces both mucosal and systemic immune response in mice. J. Dairy Res. 1998, 65, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Wakabayashi, H.; Yamauchi, K.; Teraguchi, S.; Hayasawa, H. Bovine lactoferrin and lactoferricin derived from milk: Production and applications. Biochem. Cell Biol. 2002, 80, 109–112. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Olszewska, P.; Pazdrak, B.; Krupinska, A.M.; Actor, J.K. New insights into the systemic effects of oral lactoferrin: Transcriptome profiling. Biochem. Cell Biol. 2021, 99, 47–53. [Google Scholar] [CrossRef]
- FDA 2011. Generally Recognized as Safe (GRAS) Notification 000423 for Cow’s Milk-Derived Lactoferrin as a Component of Cow’s Milk-Based Infant Formulas, Cow’s Milk Products, and Chewing Gum. Available online: https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory (accessed on 12 May 2021).
- EFSA Panel on Dietetic Products, Nutrition and Allergies: Scientific opinion on bovine lactoferrin. EFSA J. 2012, 10, 2701. [CrossRef]
- Amini, A.A.; Nair, L.S. Lactoferrin: A biologically active molecule for bone regeneration. Curr. Med. Chem. 2011, 18, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Artym, J. A remedy against obesity? The role of lactoferrin in the metabolism of glucose and lipids. Post. Hig. Med. Dosw. 2012, 66, 937–953. [Google Scholar] [CrossRef]
- Artym, J.; Zimecki, M.; Kruzel, M.L. Lactoferrin for Prevention and Treatment of Anemia and Inflammation in Pregnant Women: A Comprehensive Review. Biomedicines 2021, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Luigi Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef]
- Godinez-Chaparro, B.; Guzmán-Mejía, F.; Drago-Serrano, M.E. Lactoferrin and Its Potential Impact for the Relief of Pain: A Preclinical Approach. Pharmaceuticals 2021, 14, 868. [Google Scholar] [CrossRef] [PubMed]
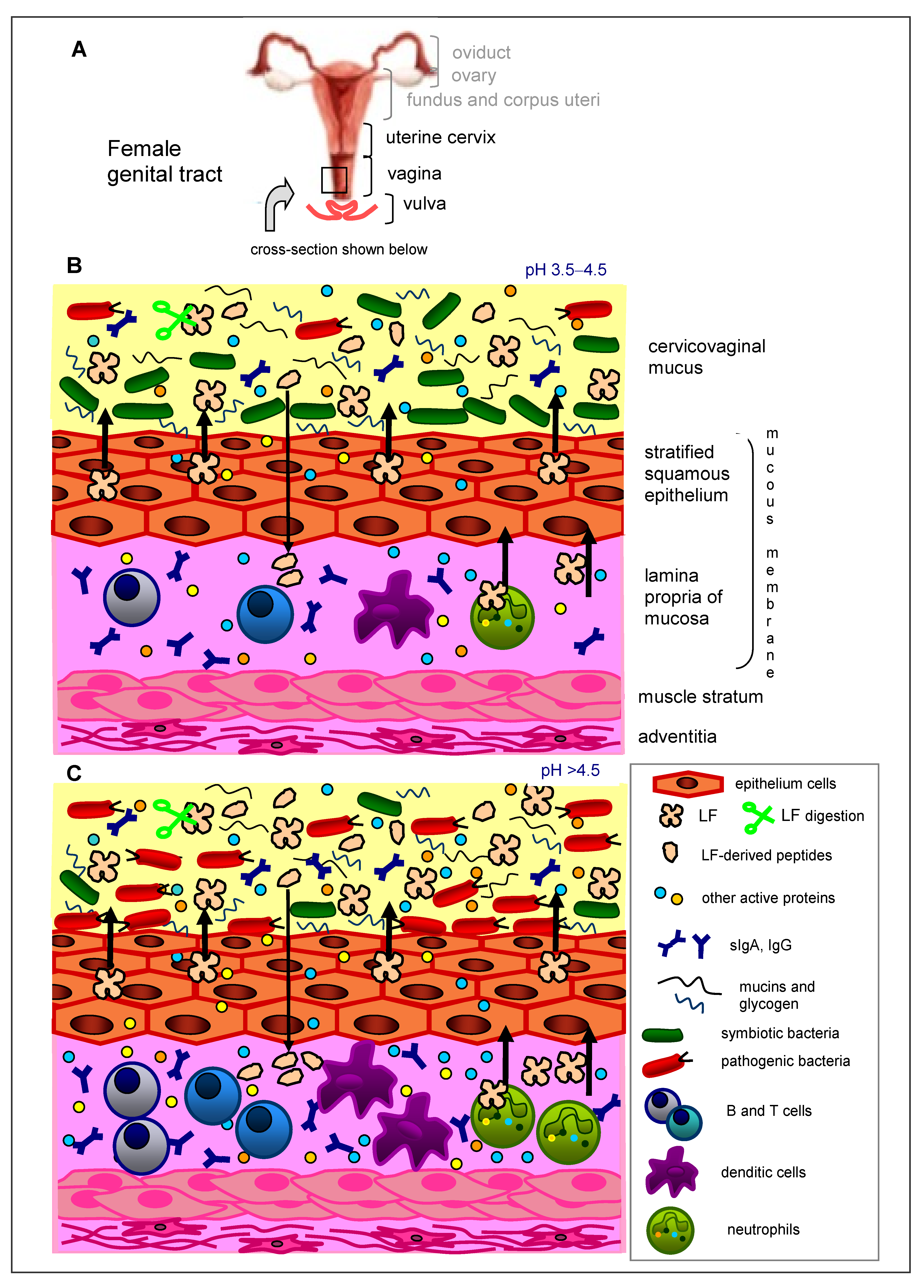
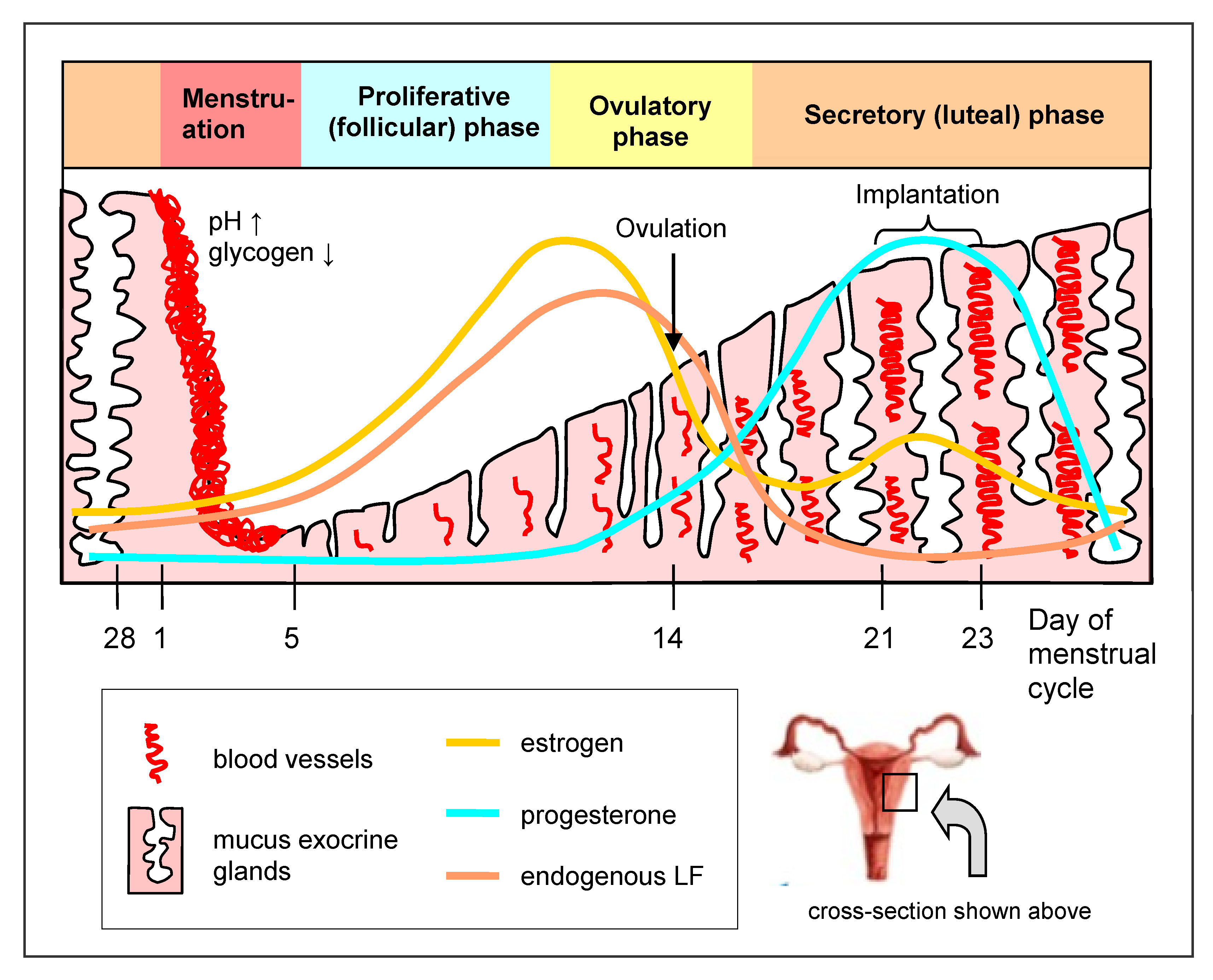
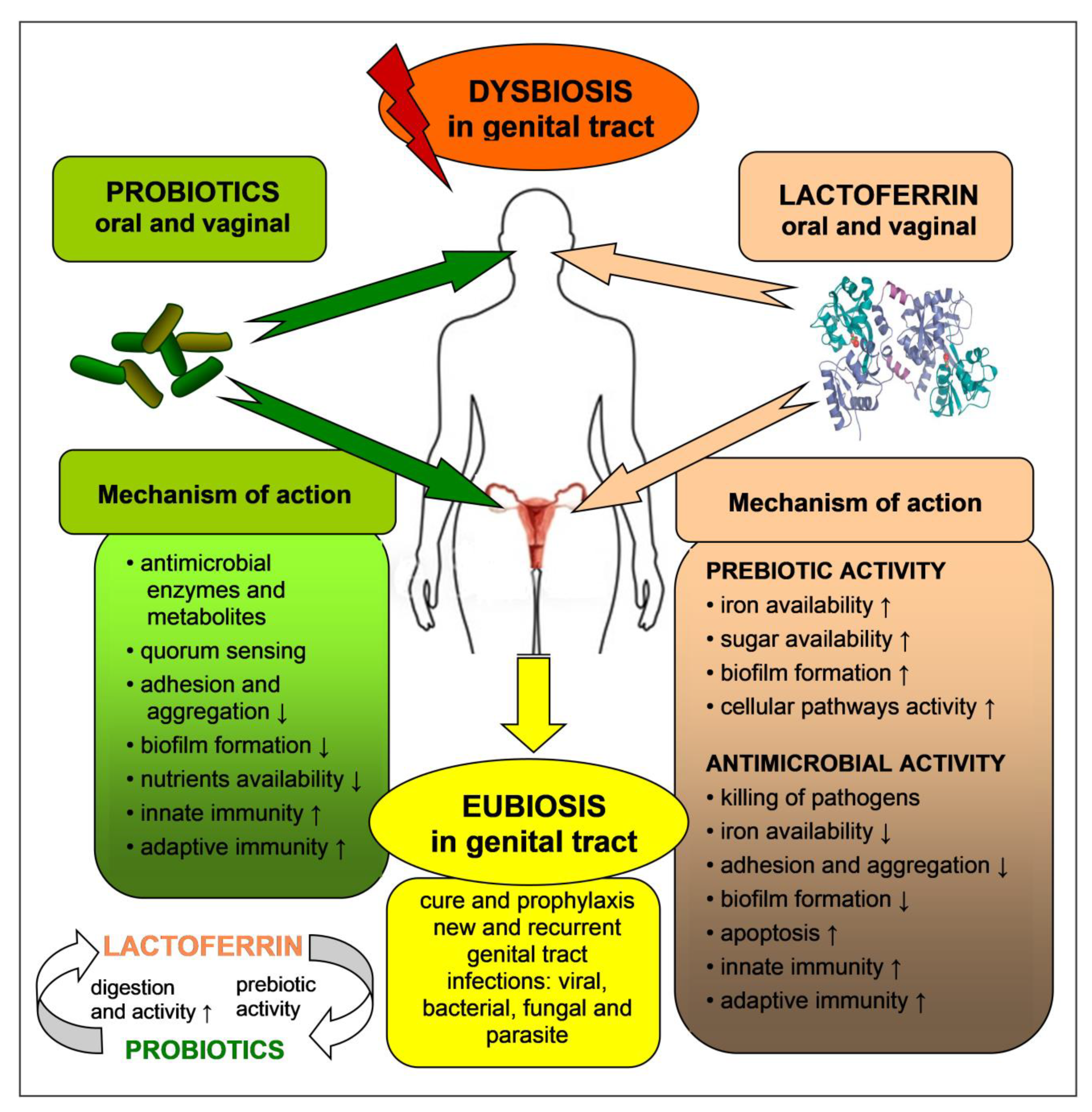
| Type of Study, Number of Participants, Country | Type of LF, Dose, Mode and Time of LF Application | Clinical/Laboratory Effects | References |
|---|---|---|---|
| Randomized, double-blind, placebo-controlled clinical study; n = 40 (women, healthy volunteers, of fertile age, 18–50 years); Italy | BLF RCXTM (50 mg) plus probiotics (5 × 109 CFU): L. acidophilus La-14 (ATCC SD5212) plus L. rhamnosus HN001 (AGAL NM07/09514) in Respecta® complex (Giellepi S.p.A. Health Science, Seregno, Italy) in p.o. capsule Placebo: identical capsule containing 100 mg maltodextrin; 2 capsules/day, after breakfast, for 14 consecutive days | Vaginal swabs, collected at weeks 0, 1, 2 and 3 and analyzed for the consumed microorganisms by qPCR; vaginal pH determined Vaginal L. acidophilus and L. rhamnosus levels on days 14 and 21 ↑ Transient vaginal colonization (at least double levels from baseline) L. acidophilus on days 14 and 21, in 12 and 16, respectively, out of 20 women Transient colonization of L. rhamnosus on days 7 and 21, in 16 and 17, respectively, out of 20 women Vaginal pH (4.06–4.24) → No significant change in placebo group No AEs neither correlated nor unrelated | [113] |
| Randomized, double-blind, placebo-controlled clinical study; n = 40 (women of fertile age, 18–50 years, with symptomatic vaginal dysbiosis, signs or symptoms of vaginitis/vaginosis); Italy | BLF RCXTM (50 mg) plus probiotics (5 × 109 CFU): L. acidophilus GLA-14 (LMG S-29159) plus L. rhamnosus HN001 (AGAL NM07/09514) in Respecta® complex (Giellepi S.p.A. Health Science, Lissone, Italy) in p.o. capsule Placebo: identical capsule containing 100 mg maltodextrin; 1 capsule/day, for 15 consecutive days | Vaginal swabs, collected at days 0 and 15 and analyzed for the consumed microorganisms by qPCR; symptoms, pH of vaginal secretions and Nugent score assessed through the study Symptoms (in particular, itching, vaginal discharge and fishy odor) ↓ Nugent score ↓ (from intermediate 5.0 to normal 2.9) Vaginal pH ↓ (from 4.42 to 4.09) Vaginal colonization of both probiotic strains at the end of the study ↑ No significant change in placebo group No AEs, neither correlated nor unrelated | [114] |
| Randomized, double-blind, placebo-controlled clinical study; n = 48 (adult women with recurrent BV, treated with metronidazole 500 mg twice daily for 7 days); Italy | BLF RCXTM (50 mg) plus probiotics (5 × 109 CFU): L. acidophilus GLA-14 (LMG S-29159) plus L. rhamnosus HN001 (ATCC SD5675) in Respecta® complex (Giellepi S.p.A. Health Science, Lissone, Italy) in p.o. capsule Placebo: identical capsule containing 100 mg maltodextrin; 2 capsules/day, for 5 consecutive days, followed by a further 10 consecutive days at a dosage of 1 capsule/day (induction phase) During the maintenance phase (6 months following the induction phase), 1 capsule/day, for 10 consecutive days per month, starting the first day of menstrual cycle (prophylactic treatment) since the menstrual blood increases the vaginal pH and contributes to increasing the risk of BV recurrences | Normalization of Nugent score, remission of symptoms, recurrences during a 6-month follow-up period were assessed Nugent score ↓ Recurrence rate ↓ Symptoms of BV (vaginal discharge and itching) ↓ | [115] |
| n = 6 (5 pregnant women and 1 non-pregnant woman, with a history of multiple pregnancy losses or preterm delivery and refractory BV E. coli, Enterococcus, Gardnerella vaginalis, Staphylococcus); Japan | BLF (NRL Pharma, Kawasaki, Japan) in intravaginal suppositories, 150 mg/day and p.o. tablets, 700 mg/day, starting before pregnancy or from 11th–21st gestational week until delivery | Normalization of vaginal flora (appearance and gradual predominance of Lactobacillus) Patients achieved pregnancy and delivered at term No AEs in mothers and newborns | [116] |
| Open, prospective, randomized clinical study; n = 60 (sexually active women aged 18–45 years, with symptomatic acute BV); Italy | BLF (AG Pharma s.r.l. Rome, Italy), in vaginal tablets 100 mg or 200 mg, 1 tablet/day for 10 days | Outcomes were a clinical evaluation based on Amsel criteria and Nugent scores Vaginal pH and structure of the vaginal bacterial biota and its dynamics during the study, determined by culture-dependent and molecular-based techniques Vaginal pH ↓ (2 weeks after stopping treatment, 60% and 89% of patients had pH < 4.5 in 100 mg and 200 mg of BLF, respectively) Nugent score ↓ (2 weeks after stopping treatment, 43% and 75% of patients had Nugent score ≤ 3 in 100 mg and 200 mg of BLF, respectively) Occurrence of vaginal bacteria species associated with BV: Gardnerella, Prevotella and Lachnospira ↓ Occurrence of Streptococcus spp., Staphylococcus spp., Escherichia coli ↓ Occurrence of Candida spp. ↓ Occurrence of Lactobacillus species ↑ Bacterial biota balance was maintained up to 2 weeks after treatment, was stopped only in 200 mg BLF-treated patients | [117] |
| Single-center retrospective cohort clinical study; n = 125 (pregnant women in the first trimester with BV diagnosis, with a history of prior spontaneous preterm birth); Italy | LF * in vaginal tablets, 300 mg/day for 21 days | Incidence of preterm birth < 37 weeks of gestation was the primary outcome Rate of preterm birth ↓ Gestational age at delivery ↑ (37.7 weeks in LF group vs. 35.9 weeks in control group without LF) Rate of admission for threatened preterm labor ↓ (45% vs. 70.8%) No differences in other outcomes (chorioamnionitis, preterm PROM < 34 weeks and neonatal outcomes) No cases of late miscarriage were reported No AEs were reported | [118] |
| Case report; 38-year-old multiparous women with 3 preterm PROM, diagnosed as having refractory vaginitis (Streptococcus group B and Staphylococcus), not cured with estriol and antibiotics; Japan | BLF (NRL Pharma, Kawasaki, Japan) in intravaginal tablets, 150 mg/day, and p.o. tablets, 700 mg/day, for 41 weeks (13 weeks before pregnancy and 38 weeks after, until delivery) | Appearance of Lactobacillus in vaginal flora, the patient achieved pregnancy 3 months later and delivered a healthy infant After the delivery, LF application was discontinued and 1 and 3 months after, no Lactobacillus was detected in vaginal discharge cultures | [119] |
| Randomized, double-blind, placebo-controlled clinical study; n = 48 (women of fertile age, 18–50 years, with positive cultures for Candida spp., symptomatic acute episodes of VVC and with documented anamnestic history of recurrences confirmed by culture analysis, during the induction phase, being treated with standard antifungal therapy— clotrimazole 100 mg daily for 7 days); Italy | BLF RCXTM (50 mg) plus probiotics (5 × 109 CFU): L. acidophilus GLA-14 (LMG S-29159) plus L. rhamnosus HN001 (ATCC SD5675) in Respecta® complex (Giellepi S.p.A. Health Science, Lissone, Italy) in p.o. capsule Placebo: identical capsule containing 100 mg maltodextrin; 2 capsules/day, for 5 consecutive days, followed by a further 10 consecutive days at a dosage of 1 capsule/day (acute treatment) During the maintenance phase (6 months following the induction phase), 1 capsule/day for 10 consecutive days per month, in the premenstrual phase (prophylactic treatment) The timeline was related to the consideration that in the premenstrual (luteal) phase, the vagina becomes more vulnerable to the pathogens | Efficacy evaluation based on clinical overall cure rate: vaginal discharge or itching and negative cultures; recurrence rate during the 6-month follow-up period Itching ↓ (at 3 months, women without itching: 70.8% in the Respecta® group vs. 8.3% in the placebo group; at 6 months: 83.3% vs. 0%) Vaginal discharge ↓ (at 3 months women without discharge were 66.7% in Respecta® group vs. 8.3% in placebo group; at 6 months: 70.8% vs. 20.8%) Overall cure rate defined as the absence of any symptoms and yeasts indicated in culture from vaginal swabs ↓ (at 3 months: 66.7% vs. 8.3%; at 6 months: 70.8% vs. 0%) Recurrence rate was defined as the presence of any VVC symptoms and yeasts in culture from vaginal swabs ↓ (at 3 months: 33.3% vs. 91.7%; at 6 months: 29.2% vs. 100%) | [120] |
| n = 34 (women aged 25–45 years with signs and symptoms of acute VVC); Italy | BLF * 4% in vaginal cream, 5 g of cream in the vagina and 2 cm applied on the vulva twice a day for 7 days | Clinical and microscopic examination was performed Response to all the characteristic symptoms of VVC ↑ 27 patients completely recovered, 5 showed a good improvement, and 2 were still suffering from VVC at the end of treatment | [121] |
| Open-label cohort clinical study; n = 7 (pregnant women asymptomatically affected by Chlamydia trachomatis and showing a high concentration of IL-6 in cervical fluids); Italy | BLF, 20% iron-saturated (Morinaga Milk Ind., Tokyo, Japan), intravaginal, 100 mg every 8 h (daily doses 300 mg/person) for 30 days In vitro test on the anti-chlamydial effect of BLF: human epithelial HeLa-229 cell line was treated with 100 μg/mL BLF | In vivo test: C. trachomatis in cervicovaginal smears and IL-6 concentration in the cervical fluid were assessed 6 out of 7 cervical specimens were negative for C. trachomatis IL-6 in cervical fluids ↓ Patients achieved pregnancy and delivered at term No maternal and neonatal AEs In vitro test: inhibitory effect of BLF on C. trachomatis entry to cells, decrease in IL-6 and IL-8 levels induced by infection with C. trachomatis | [86] |
| One-center, placebo- controlled, cohort clinical study; n = 48 (pregnant women at risk of PROM); Egypt | rHLF * 100 mg, p.o. twice/day for 30 days before meals Placebo as control | Patient’s hospital stay → Duration of PROM to delivery → Mode of delivery (vaginal/cesarean section) → Risk of PROM ↓ | [122] |
| Open-label, pilot clinical study; n = 21 (26–32 weeks pregnant women, suffering from IDA and abnormal vaginal flora, at risk of preterm delivery); Italy | BLF (Lattoferrina®, AG Pharma, Rome, Italy) p.o., 100 mg/day b.i.d. (daily doses 200 mg), before meals, every day for 1 month FeSO4 520 mg in p.o. tablets, as control | Outcomes were clinical characteristics and structure of the vaginal bacterial biota, determined by a culture-based method Normal vaginal microbiota ↑ (vaginal infection disappearance) Cervicovaginal IL-6 (vaginal inflammatory response) ↓ In both groups, cervical length and funneling (in the ultrasound data) did not change at follow-up after 10 and 30 days; pregnancy continued regularly, and all women had term delivery after 37 weeks | [123] |
| Randomized, open-label study; n = 60 (pregnant women undergoing genetic amniocentesis at the 16th gestational week, at risk of inflammation); Italy | BLF (Difesan®, Progine Farmaceutici, Firenze, Italy), 300 mg in vaginal tablet, once 4 h or 12 h prior amniocentesis | Amniotic IL-6 ↓ | [124] |
| Decreased levels of amniotic pro-inflammatory mediators: IL-9, IL-15, IFN-γ, IP-10, TNF-α, IL-1α, MCP-3, IL-2RA, IL-12p40, IFN-α2, IL-2, IL-4, eotaxin, PDGF-BB, RANTES, IL-18, MIF ↓ Increased levels of amniotic anti-inflammatory mediators: IL-17, FGF-b, G-CSF, GM-CSF, MCP-1, IL-3, SDF-1α ↑ | [125] | ||
| BLF (Difesan®, Progine Farmaceutici, Firenze, Italy), 300 mg in a vaginal tablet, once every 4 h or 12 h prior to amniocentesis In vitro test on the antioxidant effect of LTF: human monocytic U937 cell line was treated with 50 μg/mL LTF # for 4 h or 12 h | Decreased oxidative stress in vivo: Amniotic TBARS concentration ↓ Amniotic TAS ↑ Decreased oxidative stress in vitro: TBARS concentration ↓ | [126] | |
| Prospective, randomized study; n = 111 (pregnant women undergoing genetic amniocentesis at the 16–18th gestational week, at risk of inflammation); Italy | BLF (Difesan®, Progine Farmaceutici, Firenze, Italy), 300 mg in vaginal tablet, once every 4 h prior to amniocentesis | Regulation of the inflammatory markers in the amniotic fluid: PGE2, MMP-9 and TIMP-1 (inhibitor of MMP-1) ↓ MMP-2 ↑ TIMP-2 (inhibitor of MMP-2) → | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artym, J.; Zimecki, M. Antimicrobial and Prebiotic Activity of Lactoferrin in the Female Reproductive Tract: A Comprehensive Review. Biomedicines 2021, 9, 1940. https://doi.org/10.3390/biomedicines9121940
Artym J, Zimecki M. Antimicrobial and Prebiotic Activity of Lactoferrin in the Female Reproductive Tract: A Comprehensive Review. Biomedicines. 2021; 9(12):1940. https://doi.org/10.3390/biomedicines9121940
Chicago/Turabian StyleArtym, Jolanta, and Michał Zimecki. 2021. "Antimicrobial and Prebiotic Activity of Lactoferrin in the Female Reproductive Tract: A Comprehensive Review" Biomedicines 9, no. 12: 1940. https://doi.org/10.3390/biomedicines9121940
APA StyleArtym, J., & Zimecki, M. (2021). Antimicrobial and Prebiotic Activity of Lactoferrin in the Female Reproductive Tract: A Comprehensive Review. Biomedicines, 9(12), 1940. https://doi.org/10.3390/biomedicines9121940