Diversity Variation of Silica-Scaled Chrysophytes Related to Differences in Physicochemical Variables in Estuaries of Rivers in an Arctic Watershed
Abstract
:1. Introduction
2. Site Description
2.1. Habitat Characteristics
2.2. Climate
3. Materials and Methods
3.1. Field and Laboratory Methods
3.2. Geographical Distribution
3.3. Statistical Analysis
3.4. Data Availability
4. Results
4.1. Diversity and Geographic Distribution of Chrysophytes
4.2. Addition of Autecology of Rare Species
4.3. Undetermined Species
4.4. Physicochemical Parameters and Species Composition and Richness
4.5. Specific Morphology of Some Chrysophytes Found in the Study Area
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wujek, D.E. Freshwater silica-scaled heterotrophic protista: Heliozoa, thaumatomonad flagellates, amoebae, and bicosoecids, from the Lake Itasca Region, Minnesota. Minn. Acad. Sci. J. 2015, 79, 1–13. [Google Scholar]
- Siver, P.A. The Biology of Mallomonas: Morphology, Taxonomy and Ecology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; p. 248. [Google Scholar]
- Siver, P.A. Synurophyte algae. In Freshwater Algae of North America: Ecology and Classification, 2nd ed.; Academic Press: Boston, MA, USA, 2015; pp. 607–651. [Google Scholar]
- Dürrschmidt, M.; Croome, R. Mallomonadaceae (Chrysophyceae) from Malaysia and Australia. Nord. J. Bot. 1985, 5, 285–298. [Google Scholar] [CrossRef]
- Neustupa, J.; Řezáčová, M. The genus Mallomonas (Mallomonadales, Synurophyceae) in several Southeast Asian urban water bodies—The biogeographical implications. Nova Hedwig. 2007, 84, 249–259. [Google Scholar] [CrossRef]
- Gusev, E.S.; Thant, N.T.H. Silica-scaled chrysophytes (Chrysophyceae and Synurophyceae) from Vietnam (Khanh Hoa and Quang Nam provinces). Nova Hedwig. 2011, 93, 191–199. [Google Scholar] [CrossRef]
- Gusev, E.S.; Kapustin, D.A.; Martynenko, N.A.; Guseva, E.E.; Kulikovskiy, M.S. Mallomonas gusakovii sp. nov. (Chrysophyceae, Synurales), a new species from Phu Quoc Island, Vietnam. Phytotaxa 2019, 406, 199–205. [Google Scholar] [CrossRef]
- Duff, K.E. Chrysophyte microfossils in arctic Siberian lakes. Chrysophytes: Progress and new horizons. Nova Hedwig. Beih. 1996, 114, 253–263. [Google Scholar]
- Kristiansen, J.; Düwel, L.; Wegeber, S. Silica-scaled chrysophytes from the Taymyr Peninsula, Northern Siberia. Nova Hedwig. 1997, 65, 337–351. [Google Scholar] [CrossRef]
- Gusev, E.S.; Guseva, E.E.; Gabyshev, V.A. Taxonomic composition of silica-scaled chrysophytes in rivers and lakes of Yakutia and Magadanskaya oblast (Russia). Nova Hedwig. Beih. 2018, 147, 105–117. [Google Scholar] [CrossRef]
- Hällfors, G.; Hällfors, S. Records of chrysophytes with siliceous scales (Mallomonadaceae and Paraphysomonadaceae) from Finnish inland waters. Flagellates in freshwater ecosystems. Hydrobiologia 1988, 161, 1–29. [Google Scholar] [CrossRef]
- Němcová, Y.; Kreidlová, J.; Kosová, A.; Neustupa, J. Lakes and pools of Aquitaine region (France)—A biodiversity hotspot of Synurales in Europe. Nova Hedwig. 2012, 95, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bessudova, A.Y.; Sorokovikova, L.M.; Tomberg, I.V.; Likhoshway, Y.V. Silica-scaled chrysophytes in large tributaries of Lake Baikal. Cryptogam. Algol. 2018, 39, 145–165. [Google Scholar] [CrossRef]
- Siver, P.A. The synurophyceae. In Freshwater Algae of North America; Wehr, J., Sheath, B., Eds.; Academic Press: Thornwood, NY, USA, 2003; pp. 523–558. [Google Scholar]
- Asmund, B.; Hilliard, D.K. Studies on Chrysophyceae from some ponds and lakes in Alaska. Hydrobiolology 1961, 7, 237–258. [Google Scholar] [CrossRef]
- McKenzie, C.; Kling, H. Scale-bearing Chrysophyceae (Mallomonadaceae and Paraphysomonadaceae) from Mackenzie Delta area lakes, Northwest Territories, Canada. Nord. J. Bot. 1989, 9, 103–112. [Google Scholar] [CrossRef]
- Kristiansen, J. Silica-scaled chrysophytes from west Greenland: Disko island and the Søndre Strømfjord region. Nord. J. Bot. 1992, 12, 525–536. [Google Scholar] [CrossRef]
- Forsström, L.; Sorvari, S.; Korhola, A.; Rautio, M. Seasonality of phytoplankton in subarctic Lake Saanajärvi in NW Finnish Lapland. Pol. Biol. 2005, 28, 846–861. [Google Scholar] [CrossRef]
- Němcová, Y.; Nováková, S.; Řezáčová-Škaloudová, M. Synura obesa sp. nov. (Synurophyceae) and other silica scaled chrysophytes from Abisko (Swedish Lapland). Nova Hedwig. 2008, 86, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Pichrtová, M.; Janatková, K.; Němcová, Y. Silica-scaled chrysophytes from Abisko (Swedish Lapland). Nord. J. Bot. 2011, 29, 112–118. [Google Scholar] [CrossRef]
- Kristiansen, J. Flagellates from Finnish Lappland. Bot. Tidsskr. 1964, 59, 315–333. [Google Scholar]
- Siver, P.A.; Voloshko, L.N.; Gavrilova, O.V.; Getsen, M.V. The scaled chrysophyte flora of the Bolshezemelskaya tundra (Russia). Nova Hedwig. Beih. 2005, 128, 125–150. [Google Scholar]
- Voloshko, L.N. A new species of the genus Mallomonas (Chrysophyceae, Synurophyceae) from lakes of the Vorkuta tundra. Bot. Zhurn. 2012, 97, 1226–1234. (In Russian) [Google Scholar]
- Voloshko, L.N. New taxa of the genus Mallomonas (Chrysophyceae, Synurophyceae) from lakes of the Polar Urals. Bot. Zhurn. 2009, 94, 1068–1076. (In Russian) [Google Scholar]
- Voloshko, L.N. The chrysophycean algae from glacial lakes of Polar Ural (Russia). Nova Hedwig. Beih. 2010, 136, 191–211. [Google Scholar] [CrossRef]
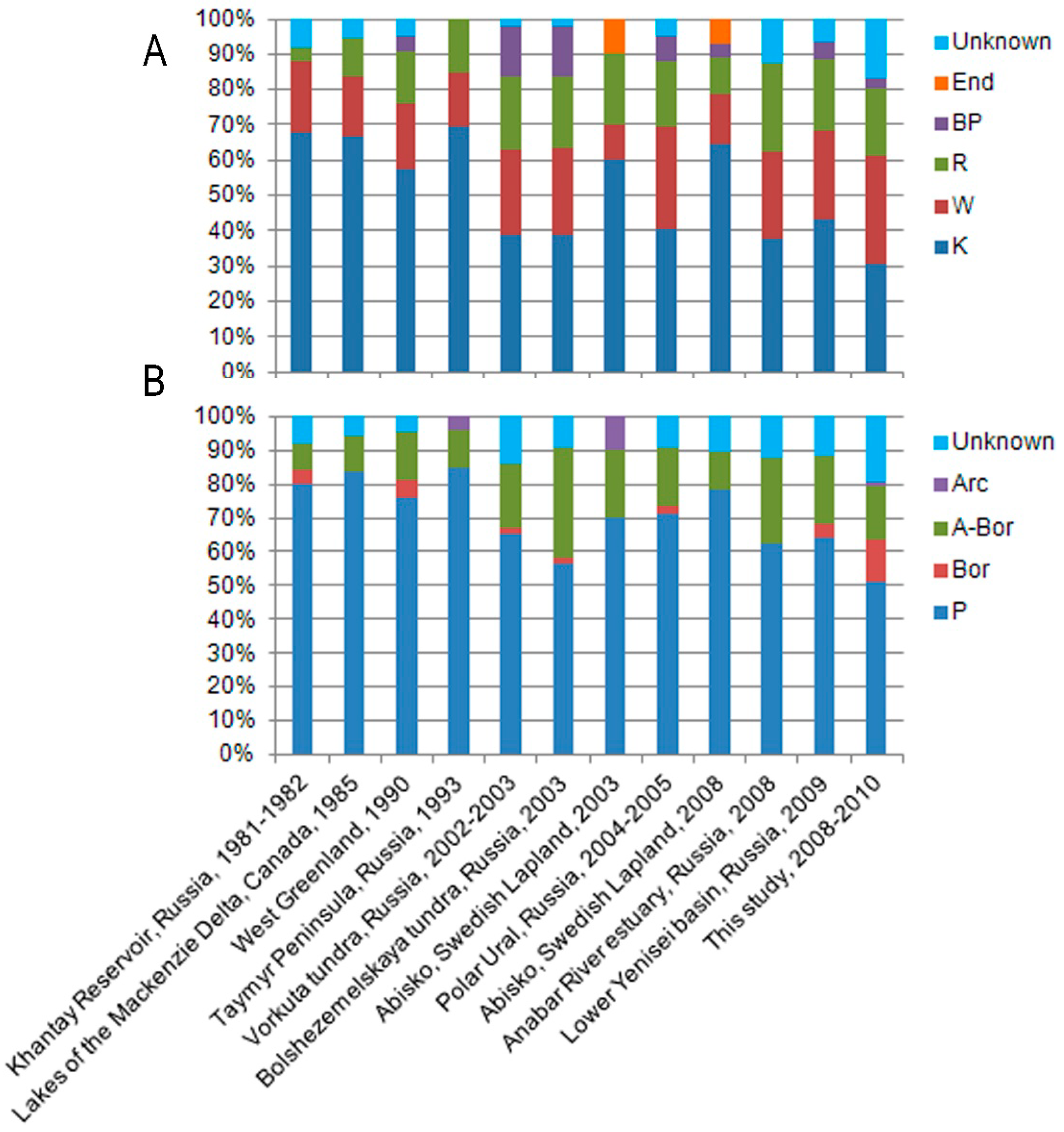
- Firsova, A.D.; Bessudova, A.Y.; Sorokovikova, L.M.; Tomberg, I.V.; Likhoshway, Y.V. The diversity of chrysophycean algae in an arctic zone of river and sea water mixing, Russia. Am. J. Plant Sci. 2015, 6, 2439–2452. [Google Scholar] [CrossRef] [Green Version]
- Bessudova, A.Y.; Bukin, Y.S.; Sorokovikova, L.M.; Firsova, A.D.; Tomberg, I.V. Silica-scaled Chrysophytes in small lakes of the Lower Yenisei basin, the Arctic. Nova Hedwig. 2018, 107, 315–336. [Google Scholar] [CrossRef]
- Balonov, I.M.; Kuzmina, A.E. Golden algae. In Proceedings of Limnological Institute, RAS of the Academy of Sciences of USSRP; Votintsev, K.K., Ed.; Hydrochemical and Hydrobiological Studies of the Khantay Reservoir, Nauka, Siberian Branch: Novosibirsk, Russia, 1986; pp. 59–70. (In Russian) [Google Scholar]
- Wolfe, A.P.; Siver, P.A. A hypothesis linking chrysophyte microfossils to lake carbon dynamics on ecological and evolutionary time scales. Glob. Planetary Chang. 2013, 111, 189–198. [Google Scholar] [CrossRef]
- Mushet, G.R.; Laird, K.R.; Das, B.; Hesjedal, B.; Leavitt, P.R.; Scott, K.A.; Simpson, G.L.; Wissel, B.; Wolfe, J.D.; Cumming, B.F. Regional climate changes drive increased scaled-chrysophyte abundance in lakesdownwind of Athabasca Oil Sands nitrogen emissions. J. Paleolimnol. 2017, 58, 419–435. [Google Scholar] [CrossRef] [Green Version]
- Schindler, D.W. The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millennium. Can. J. Fish. Aquat. Sci. 2001, 58, 18–29. [Google Scholar] [CrossRef]
- Paterson, A.M.; Winter, J.G.; Nicholls, K.H.; Clarks, B.J.; Ramcharan, C.W.; Yan, N.D.; Somers, K.M. Long-term changes in phytoplankton composition in seven Canadian Sheet lakes in response to multiple anthropogenic stressors. Can. J. Fish. Aquat. Sci. 2008, 65, 846–861. [Google Scholar] [CrossRef]
- Rühland, K.M.; Paterson, A.M.; Smol, J.P. Hemispheric-scale patterns of climate-related shifts in planktonic diatoms from North American and European lakes. Glob. Chang. Biol. 2008, 14, 2740–2754. [Google Scholar] [CrossRef]
- Field, C.B.; Barros, V.R.; Dokken, D.J.; Mach, K.J.; Mastrandrea, M.D.; Bilir, T.E.; Chatterjee, M.; Ebi, K.L.; Estrada, Y.O.; Genova, R.C.; et al. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2014: Impacts, Adaptation, and Vulnerability; Cambridge University Press: New York, NY, USA, 2014; pp. 1–32. [Google Scholar]
- Magnuson, J.J.; Robertson, D.M.; Benson, B.J.; Wynne, R.H.; Livingstone, D.M.; Arai, R.A.; Barry, R.G.; Card, V.; Kuusisto, E.; Granin, N.G.; et al. Historical trends in lake and river ice cover in the Northern Hemisphere. Science 2000, 289, 1743–1746. [Google Scholar] [CrossRef] [Green Version]
- Vuglinsky, V.; Valatin, D. Changes in ice cover duration and maximum ice thickness for rivers and lakes in the Asian part of Russia. Nat. Res. 2018, 9, 73–87. [Google Scholar] [CrossRef] [Green Version]
- Korneva, L.G. Recent invasion of planktonic diatom algae in the Volga River basin and their causes. Inland Wat. Boil. 2007, 1, 30–39. (In Russian) [Google Scholar]
- Korneva, L.G. Invasions of alien species of planktonic microalgae into the fresh waters of Holarctic (Review). Russ. J. Biol. Invasions 2014, 1, 9–37. [Google Scholar] [CrossRef]
- Korneva, L.G. Phytoplankton of Reservoirs of the Volga River Basin; Kostroma: Dom Pechati, Russia, 2015; p. 284. (In Russian) [Google Scholar]
- Bessudova, A.; Bukin, Y.; Likhoshway, Y. Dispersal of silica-scaled Chrysophytes in Northern Water bodies. Diversity 2021, 13, 284. [Google Scholar] [CrossRef]
- Kirillov, F.N.; Labutina, T.M. Biology of Vilyuy Reservoir; Nauka: Novosibirks, Russia, 1979; p. 272. (In Russian) [Google Scholar]
- Vasilyeva, I.I.; Remigaylo, P.A. Algae of Vilyuy Reservoir; Yakutia Scientific Center USSR Academy of Sciences Publishing House: Yakutsk, Russia, 1982; p. 115. (In Russian) [Google Scholar]
- Remigaylo, P.A. Phytoplankton of the Vilyuy River and Vilyuy Reservoir. Ph.D. Thesis, Novosibirsk University, Novosibirsk, Russia, 1995; p. 16. (In Russian). [Google Scholar]
- Vasilyeva-Kralina, I.I.; Remigaylo, P.A.; Gabushev, V.A.; Pshennikova, E.V.; Ivanova, A.P.; Kopyrina, L.I. Algae. In Diversity of Plants of Yakutia; Publishing House SB RAS: Novpsibirsk, Russia, 2005; pp. 150–272. (In Russian) [Google Scholar]
- Gabyshev, V.A.; Gabysheva, O.I. Phytoplankton of Big Rivers of Yakutia and Adjacent Territories of East Siberia; Korneva, L.G., Ed.; SibAK Press: Novosibirks, Russia, 2018; p. 414. (In Russian) [Google Scholar]
- Gabyshev, V.A.; Tsarenko, P.M.; Ivanova, A.P. Diversity and features of the spatial structure of algal communities of water bodies and watercourses in the Lena River estuary. Inland Water Biol. 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Gabyshev, V.A.; Tsarenko, P.M.; Ivanova, A.P. Algae of mouth area of Lena River. In The Biological Resources of the Ust-Lensky Nature Reserve: Fungi, Algae, Vegetation, Fishes, Birds, Muskoxen; Science: Novosibirsk, Russia, 2019; pp. 14–35. [Google Scholar]
- Bessudova, A.Y.; Tomberg, I.V.; Firsova, A.D.; Kopyrina, L.I.; Likhoshway, Y.V. Silica-scaled chrysophytes in lakes Labynkyr and Vorota of the Sakha (Yakutia) Republic, Russia. Nova Hedwig. Beih. 2019, 148, 35–48. [Google Scholar] [CrossRef]
- Izyumenko, S.A. (Ed.) Climate of the Yakut ASSR. In Atlas; Gidrometeoizdat: Leningrad, Russia, 1968; p. 33. (In Russian) [Google Scholar]
- Chistyakov, G.E. River Water Resources of Yakutia; Nauka: Moskow, Russia, 1964; p. 255. (In Russian) [Google Scholar]
- Chistyakov, G.E. Hydropower Resources of Drainage Basins of the Yana River; Chistyakov, G.E., Nogovitsyn, D.D., Yakushev, M.V., Eds.; Nauka: Moskow, Russia, 1970; p. 214. (In Russian) [Google Scholar]
- Resources of Inland Waters of the USSR. Lena-Indigirka Region; Gidrometeoizdat: Leningrad, Russia, 1972; Volume 17, p. 651. (In Russian)
- Resources of Inland Waters of the USSR. North-East; Gidrometeoizdat: Leningrad, Russia, 1966; Volume 19, p. 602. (In Russian)
- Guidelines for Collection and Processing of Samples at Hydrobiological Studies of Fresh Waters; Nauka: Leningrad, Russia, 1981; p. 32. (In Russian)
- Wiebe, P.H.; Benfield, M.C. From the Hensen net toward four-dimensional biological oceanography. Prog. Oceanogr. 2003, 56, 7–136. [Google Scholar] [CrossRef]
- Alyokin, O.A.; Semyonov, A.D.; Skopintsev, B.A. Guidelines for Chemical Analysis of Inland Waters; Gidrometeoizdat: Leningrad, Russia, 1973; p. 269. (In Russian) [Google Scholar]
- Semyonov, A.D. Guidelines for Chemical Analysis of Inland Water; Gidrometeizdat: Leningrad, Russia, 1977; p. 540. (In Russian) [Google Scholar]
- Sokolova, S.A.; Anisova, S.N. (Eds.) List of Commercial Fishing Standards: Maximum Allowable Concentrations (MACs) and Safe Reference Levels of Contaminants in Commercial Fishing Waters; VNIRO: Moskow, Russia, 1999; p. 304. (In Russian) [Google Scholar]
- Kristiansen, J. Dispersal and biogeography of silica-scaled chrysophytes. Biodiv. Conserv. 2008, 17, 419–426. [Google Scholar] [CrossRef]
- Voloshko, L.N. Golden Algae of Waters of North Russia; Komarov Botanical Institute of the Russian Academy of Sciences: Sankt-Petersburg, Russia, 2017; p. 378. (In Russian) [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package, 2014. R Package Version 2.2-0. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 13 August 2021).
- Oksanen, J. Vegan: An Introduction to Ordination. 2015. Available online: http://cran.r-project.org/web/packages/vegan/vignettes/introvegan.pdf (accessed on 13 August 2021).
- Škaloud, P.; Škaloudová, M.; Jadrná, I.; Bestová, H.; Pusztai, M.; Kapustin, D.A.; Siver, P.A. Comparing morphological and molecular estimates of species diversity in the freshwater genus Synura (stramenopiles): A model for understanding diversity of eukaryotic microorganisms. J. Phycol. 2020, 56, 574–591. [Google Scholar] [CrossRef] [PubMed]
- Jo, B.Y.; Kim, J.I.; Škaloud, P.; Siver, P.A.; Shin, W. Multigene phylogeny of Synura (Synurophyceae) and descriptions of four new species based on morphological and DNA evidence. Europ. J. Phycol. 2016, 51, 413–430. [Google Scholar] [CrossRef] [Green Version]
- Škaloudová, M.; Škaloud, P. A new species of Chrysosphaerella (Chrysophyceae: Chromulinales), Chrysosphaerella rotundata, sp. nov., from Finland. Phytotaxa 2013, 130, 34–42. [Google Scholar] [CrossRef]
- Dürrschmidt, M. Studies on scale-bearing Chrysophyceae from the Giessen area, Federal Republic of Germany. Nord. J. Bot. 1984, 4, 123–143. [Google Scholar] [CrossRef]
- Thomsen, H.A.; Zimmermann, B.; Moestrup, O.; Kristiansen, J. Some new freshwater species of Paraphysomonas (Chrysophyceae). Nord. J. Bot. 1981, 1, 559–581. [Google Scholar] [CrossRef]
- Kristiansen, J. On the species of Paraphysomonas (Chrysophyceae) in some Greek lakes. Nova Hedwig. 1983, 38, 65–72. [Google Scholar]
- Kling, H.J.; Kristiansen, J. Scale-bearing Chrysophyceae (Mallomonadaceae) from Central and Northern Canada. Nord. J. Bot. 1983, 3, 269–290. [Google Scholar] [CrossRef]
- Nicholls, K.H. Five Paraphysomonas species (Chrysophyceae) new to North America, with notes on three other rarely reported species. Can. J. Bot. 1985, 63, 1208–1212. [Google Scholar] [CrossRef]
- Hickel, B.; Maass, I. Scaled chrysophytes, including heterotrophic nanoflagellates from the lake district in Holstein, northern Germany. Nova Hedwig. Beih. 1989, 95, 233–257. [Google Scholar]
- Wujek, D.E.; O’Kelly, C.J. Silica-scaled Chrysophyceae (Mallomonadaceae and Paraphysomonadaceae) from New Zealand freshwaters. N. Z. J. Bot. 1992, 30, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Ikävalko, J. Observations on silica-scaled flagellates (Chrysophyceae and Synurophyceae) in the brackish water of Pojo Bay, SW coast of Finland. Ann. Bot. Fen. 1994, 31, 1–27. [Google Scholar]
- Hällfors, G. Checklist of Baltic Sea Phytoplankton species (including some heterotrophic protistan groups). Balt. Sea Environ. Proc. 2004, 95, 1–208. [Google Scholar]
- Preisig, H.R.; Hibberd, D.J. Ultrastructure and taxonomy of Paraphysomonas (Chrysophyceae) and related genera 2. Nord. J. Bot. 1982, 2, 601–638. [Google Scholar] [CrossRef]
- Dürrschmidt, M. Studies on the Chrysophyceae from South Chilean inland waters by means of scanning and transmission electron microscopy, II. Archiv für Hydrobiologie 63. Algol. Stud. 1982, 63, 121–163. [Google Scholar]
- Nicholls, K.H. Eight chrysophyceae new to North America. Phycologia 1984, 23, 213–221. [Google Scholar] [CrossRef]
- Barreto, S. The silica-scaled chrysophyte flora of Hungary. Nova Hedwig. Beih. 2005, 128, 11–41. [Google Scholar]
- Pichrtová, M.; Němcová, Y.; Škaloud, P.; Rott, E. Silica-scaled chrysophytes from North Tyrol (Austria) including a description of Mallomonas tirolensis sp. nov. Nova Hedwig. Beih. 2013, 142, 69–85. [Google Scholar]
- Wei, Y.-X.; Yuan, X.-P. Studies on silica-scaled chrysophytes from the Daxinganling mountains and Wudalianchi Lake regions, China. Nova Hedwig. 2015, 101, 299–312. [Google Scholar] [CrossRef]
- Nielsen, Y. Spiniferomonas abrupta, sp. nov. and some rare species of Synurophyceae and Chrysophyceae, not formerly recorded from Denmark. Nord. J. Bot. 1994, 14, 473–480. [Google Scholar] [CrossRef]
- Ikävalko, J.; Thomsen, H.A. Scale-covered and loricate flagellates (Chrysophyceae and Synurophyceae) from Baltic Sea ice. Nova Hedwig. Beih. 1994, 114, 147–160. [Google Scholar]
- Ikävalko, J. Contribution to the flora of silica-scaled flagellates in Mikkeli, central Finland. Nova Hedwig. 1994, 58, 475–505. [Google Scholar]
- Finlay, B.J.; Clarke, K.J. Apparent global ubiquity of species in the protist genus Paraphysomonas. Protist 1999, 150, 419–430. [Google Scholar] [CrossRef]
- Takahashi, E. Studies on genera Mallomonas and Synura, and other plankton in fresh water with the electron microscope. VII. New genus Spiniferomonas of the Synuraceae (Chrysophyceae). Bot. Mag. Tok. 1973, 86, 75–88. [Google Scholar] [CrossRef]
- Nicholls, K.H. Spiniferomonas (Chrysophyceae) in Ontario lakes including a revision and descriptions of two new species. Can. J. Bot. 1981, 59, 107–117. [Google Scholar] [CrossRef]
- Jacobsen, V.A. Scale-bearing Chrysophyceae (Mallomonadaceae and Paraphysomonadaceae) from West Greenland. Nord. J. Bot. 1985, 5, 381–398. [Google Scholar] [CrossRef]
- Nicholls, K.H. Descriptions of Spiniferomonas silverensis sp. nov. and S. minuta sp. nov. and an assessment of form variation in their closest relative, S. trioralis (Chrysophyceae). Can. J. Bot. 1984, 62, 2329–2335. [Google Scholar] [CrossRef]
- Siver, P.A. The distribution and ecology of Spiniferomonas (Chrysophyceae) in Connecticut (U.S.A). Nord. J. Bot. 1988, 8, 205–212. [Google Scholar] [CrossRef]
- Němcová, Y.; Pusztai, M.; Škaloudová, M.; Neustupa, J. Silica-scaled chrysophytes (Stramenopiles, Ochrophyta) along a salinity gradient: A case study from the Gulf of Bothnia western shore (Northern Europe). Hydrobiologia 2016, 764, 187–197. [Google Scholar] [CrossRef]
- Siver, P.A.; Wujek, D.E. Scaled Chrysophyceae and Synurophyceae from Florida, U.S.A.: VI. Observations on the flora from waterbodies in the Ocala National Forest. Nova Hedwig. 1999, 68, 75–92. [Google Scholar] [CrossRef]
- Bessudova, A.Y.; Domysheva, V.M.; Firsova, A.D.; Likhoshway, Y.V. Silica-scaled chryso phytes of Lake Baikal. Acta Biol. Sib. 2017, 3, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Babich, D.B.; Korotaev, V.N.; Magritsky, D.V.; Mikhailov, V.N. Lower Indigirka: Estuarine and Riverbed Processes; GEOS: Moskow, Russia, 2001; p. 202. [Google Scholar]
- Němcová, Y.; Neustupa, J.; Kvíderová, J.; Řezáčová-Škaloudová, M. Morphological plasticity of silica scales of Synura echinulate (Synurophyceae) in crossed gradients of light and temperature—A geometric morphometric approach. Nova Hedwig. Beih. 2010, 136, 21–32. [Google Scholar] [CrossRef]
- Němcová, Y.; Pichrtová, M. Shape dynamics of silica scales (Chrysophyceae, Stramenopiles) associated with pH. Fottea Olomouc 2012, 12, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Green, R.B. A new species of Spiniferomonas (Chrysophyceae) from an Alberta Lake. Can. J. Bot. 1979, 57, 557–560. [Google Scholar] [CrossRef]
- Eloranta, P. Biogeography of chrysophytes in Finnish lakes. In Chrysophyte Algae: Ecology, Phylogeny and Development; Sandgren, C.D., Smol, J.P., Kristiansen, J., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 214–231. [Google Scholar] [CrossRef]
- Siver, P.A. The distribution of chrysophytes along environmental gradients: Their use as biologicalindicators. In Chrysophyte Algae; Sandgren, C.D., Smol, J.P., Kristiansen, J., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 232–268. [Google Scholar]
- Kristiansen, J. Golden Algae: A Biology of Chrysophytes; A.R.G. Gantner Verlag: Königstein, Germany, 2005; p. 167. [Google Scholar]
- Siver, P.A.; Lott, A.M. The scaled chrysophyte flora in freshwater ponds and lakes from Newfoundland, Canada, and their relationship to environmental variables. Cryptogam. Algol. 2017, 38, 325–347. [Google Scholar] [CrossRef]
- Dodds, W.K.; Jones, J.R.; Welch, E.B. Suggested classification for stream trophic state: Distributions of temperate stream types by chlorophyll, total nitrogen and Phosphorus. Water Res. 1998, 32, 1455–1462. [Google Scholar] [CrossRef]
- Bessudova, A.Y.; Sorokovikova, L.M.; Sinyukovich, V.N.; Firsova, A.D.; Tomberg, I.V.; Likhoshway, Y.V. Effects of water levels on species diversity of silica-scaled chrysophytes in large tributaries of Lake Baikal. Acta Biol. Sib. 2020, 95, 1–24. [Google Scholar] [CrossRef]
- Rojo, C.; Mesquita-Joanes, F.; Monrós, J.S.; Armengol, J.; Sasa, M.; Bonilla, F.; Rueda, R.; Benavent-Corai, J.; Piculo, R.; Seguraet, M.M. Hydrology affects environmental and spatial structuring of microalgal metacommunities in tropical Pacific coast wetlands. PLoS ONE 2016, 11, e0149505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talbot, C.J.; Bennett, K.M.; Cassell, K.; Hanes, D.M.; Minor Hans, E.C.; Paerl, H.; Raymond, P.A.; Vargas, R.; Vidon, P.G.; Wollheim, W.; et al. The impact of flooding on aquatic ecosystem services. Biogeochemistry 2018, 141, 439–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Řezáčová-Škaloudová, M.; Neustupa, J.; Němcová, Y. Effect of temperature on the variability of silicate structures in Mallomonas kalinae and Synura curtispina (Synurophyceae). Nova Hedwig. Beih. 2010, 136, 55–69. [Google Scholar] [CrossRef]
- Kristiansen, J. Cosmopolitan chrysophytes. Syst. Geogr. Plants 2000, 70, 291–300. [Google Scholar] [CrossRef]

| No | Site | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 11 | 12 | 14 | 20 | 21 | 26 | 27 | 30 | 31 | 32 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number Species | |||||||||||||||||||||
| 1. | Chrysosphaerella brevispina Korshikov | + | + | + | + | + | + | + | |||||||||||||
| 2. | Ch. coronacircumspina Wujek & Kristiansen in Wujek, Gretz & Wujek | + | + | + | + | + | + | + | + | ||||||||||||
| 3. | Ch. longispina Lauterborn | + | + | ||||||||||||||||||
| 4. | Ch. rotundata Škaloudová & Škaloud | + | |||||||||||||||||||
| 5. | Chrysosphaerella sp. | + | + | + | + | ||||||||||||||||
| 6. | Paraphysomonas acuminata acuminata Scoble & Cavalier-Smith | + | |||||||||||||||||||
| 7. | P. bandaiensis Takahashi | + | |||||||||||||||||||
| 8. | P. circumvallata Preisig & Hibberd | + | + | + | |||||||||||||||||
| 9. | P. gladiata Preisig & Hibberd | + | + | + | + | + | |||||||||||||||
| 10. | P. punctata Preisig & Hibberd | + | + | + | |||||||||||||||||
| 11. | P. cf. punctata ssp. simplicior Preisig & Hibberd | + | + | ||||||||||||||||||
| 12. | P. vulgaris Scoble & Cavalier-Smith | + | + | + | |||||||||||||||||
| 13. | P. uniformis hemiradia Scoble & Cavalier-Smith | + | + | + | + | + | + | + | + | ||||||||||||
| 14. | Paraphysomonas sp. 1 | + | + | + | |||||||||||||||||
| 15. | Paraphysomonas sp. 2 | + | |||||||||||||||||||
| 16. | Paraphysomonas sp. 3 | + | + | ||||||||||||||||||
| 17. | Paraphysomonas sp. 4 | + | |||||||||||||||||||
| 18. | Lepidochromonas butcheri (Pennick & Clarke) Kapustin & Guiry | + | |||||||||||||||||||
| 19. | L. coronata (Moestrup & Zimmerman) Kapustin & Guiry | + | |||||||||||||||||||
| 20. | L. diadernifera (Takahashi) Kristiansen | + | |||||||||||||||||||
| 21. | L. eiffelii (Thomsen) Kapustin & Guiry | + | |||||||||||||||||||
| 22. | L. elegantissima (Kling & Kristiansen) Kapustin & Guiry | + | |||||||||||||||||||
| 23. | L. quadrispina (Thomsen & Kristiansen) Kapustin & Guiry | + | |||||||||||||||||||
| 24. | L. quadrispina (Preisig & Hibberd) Kapustin & Guiry | + | |||||||||||||||||||
| 25. | L. undulata (Preisig & Hibberd) Kapustin & Guiry | + | |||||||||||||||||||
| 26. | Lepidochromonas sp. 1 | + | |||||||||||||||||||
| 27. | Lepidochromonas sp. 2 | + | |||||||||||||||||||
| 28. | Spiniferomonas abei Takahashi | + | |||||||||||||||||||
| 29. | S. bourrellyi Takahashi | + | + | + | + | + | |||||||||||||||
| 30. | S. conica Takahashi | + | |||||||||||||||||||
| 31. | S. cornuta Balonov | + | + | + | + | ||||||||||||||||
| 32. | S. minuta Nicholls | + | |||||||||||||||||||
| 33. | S. serrata Nicholls | + | + | + | + | + | + | + | |||||||||||||
| 34. | S. silverensis Nicholls | + | |||||||||||||||||||
| 35. | S. takahashii Nicholls | + | |||||||||||||||||||
| 36. | S. trioralis Takahashi | + | + | + | + | + | + | + | |||||||||||||
| 37. | S. trioralis f. cuspidata Balonov | + | + | + | |||||||||||||||||
| 38. | Mallomonas acaroides Perty | + | + | + | + | + | + | + | + | + | |||||||||||
| 39. | M. actinoloma Takahashi | + | + | ||||||||||||||||||
| 40. | M. akrokomos Ruttner | + | + | + | + | + | + | ||||||||||||||
| 41. | M. alpina Pascher & Ruttner | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| 42. | M. alata f. hualvensis Asmund, Cronberg & Dürrschmidt | + | + | ||||||||||||||||||
| 43. | M. areolata Nygaard | + | + | ||||||||||||||||||
| 44. | M. caudata Iwanoff | + | + | + | + | + | + | ||||||||||||||
| 45. | M. cratis Harris & Bradley | + | |||||||||||||||||||
| 46. | M. crassisquama (Asmund) Fott | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| 47. | M. elongata Reverdin | + | + | + | + | ||||||||||||||||
| 48. | M. eoa Takahashi in Asmund & Takahashi | + | |||||||||||||||||||
| 49. | M. kuzminii Gusev & Kulikovskiy | + | + | + | + | + | + | ||||||||||||||
| 50. | M. lychenensis Conrad | + | |||||||||||||||||||
| 51. | M. multiunca Asmund | + | |||||||||||||||||||
| 52. | M. papillosa Harris & Bradley | + | |||||||||||||||||||
| 53. | M. parvula Dürrschmidt | + | |||||||||||||||||||
| 54. | M. cf. pumilio Harris & Bradley | + | |||||||||||||||||||
| 55. | M. punctifera Korshikov | + | + | + | + | + | + | ||||||||||||||
| 56. | M. striata Asmund | + | |||||||||||||||||||
| 57. | M. tonsurata Teiling | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| 58. | Synura cf. americana Kynclová & Škaloud | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| 59. | S. borealis Škaloud & Škaloudová | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||
| 60. | S. cf. cornuta Škaloud, Škaloudová & Siver | + | + | ||||||||||||||||||
| 61. | S. conopea Kynclová & Škaloud | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| 62. | S. curtispina (Petersen & Hansen) Asmund | + | + | ||||||||||||||||||
| 63. | S. curtispina f. reticulata Asmund | + | + | + | + | + | |||||||||||||||
| 64. | S. echinulate Korshikov | + | + | + | + | + | + | + | + | ||||||||||||
| 65. | S. glabra (Korshikov) Škaloud & Kynclová | + | + | + | + | + | + | + | |||||||||||||
| 66. | S. leptorhabda Nicholls in Nicholls & Gerrath | + | |||||||||||||||||||
| 67. | S. macropora Škaloud & Kynclová | + | + | + | + | + | |||||||||||||||
| 68. | S. mammillosa Takahashi | + | + | + | + | + | |||||||||||||||
| 69. | S. nygaardii (Petersen & Hansen) Kristiansen | + | + | + | |||||||||||||||||
| 70. | S. petersenii (Korshikov) Škaloud & Kynclová | + | + | + | + | + | + | + | + | + | + | + | |||||||||
| 71. | S. petersenii f. taymyrensis Kristiansen | + | |||||||||||||||||||
| 72. | S. praefracta (Asmund) Škaloud & Škaloudová | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||
| 73. | S. cf. soroconopea Jo, Shin, Kim & Siver | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||
| 74. | S. sphagnicola Korshikov | + | + | + | + | ||||||||||||||||
| 75. | S. spinosa Korshikov | + | + | + | + | + | + | + | + | ||||||||||||
| 76. | S. spinosa f. longispina Petersen & Hansen | + | + | + | + | + | + | + | + | ||||||||||||
| 77. | S. uvella Ehrenberg | + | + | ||||||||||||||||||
| 78. | S. cf. vinlandica Škaloud, Škaloudová & Siver | + | |||||||||||||||||||
| 79. | Synura sp. 1 | + | |||||||||||||||||||
| 80. | Synura sp. 2 | + | + | + | + | + | + | ||||||||||||||
| 81. | Synura sp. 3 | + | |||||||||||||||||||
| 82. | Synura sp. 4 | + | + | + | + | + | + | + | |||||||||||||
| Total | 14 | 22 | 26 | 40 | 52 | 21 | 21 | 6 | 15 | 23 | 31 | 5 | 3 | 1 | 16 | 16 | 7 | 9 | 24 | ||
| Site Number | Sampling Date | Coordinates | River | T, °C | pH, Epi | Water Transparency, m | Suspended Matter, mg/L | Σ Ions, mg/L | Si, mg/L | Ptotal, μg/L | O2, mg/L | CO2, mg/L | Number of Species | Total Number of Species in the River |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 August 2008 | 70°62′12′′ N, 122°55′654′′ E | Olenyok | 16.0 | 7.89 | 4.0 | 7.20 | 270.14 | 2.44 | 186.00 | 9.40 | 3.52 | 14 | 75 |
| 2 | 12 August 2008 | 71°30′749′′ N, 122°59′67′′ E | Olenyok | 16.4 | 22 | |||||||||
| 3 | 13 August 2008 | 71°78′533′′ N, 123°73′743′′ E | Olenyok | 16.4 | 7.93 | 4.0 | 11.20 | 228.15 | 2.30 | 26.00 | 9.19 | 3.96 | 26 | |
| 4 | 13 August 2008 | 71°78′935′′ N, 123°69′794′′ E | Olenyok | 17.4 | 6.78 | 4.0 | 6.40 | 67.46 | 1.34 | 33.00 | 9.81 | 2.64 | 40 | |
| 5 | 13 August 2008 | 71°81′098′′ N, 123°68′292′′ E | Olenyok | 16.8 | 7.52 | 4.0 | 7.20 | 180.67 | 1.80 | 38.00 | 9.26 | 4.40 | 52 | |
| 6 | 13 August 2008 | 72°13′966′′ N, 123°45′577′′ E | Olenyok | 17.1 | 21 | |||||||||
| 7 | 14 August 2008 | 72°55′621′′ N, 122°04′922′′ E | Olenyok | 15.0 | 7.80 | 4.0 | 5.60 | 205.52 | 2.48 | 26.00 | 9.83 | 3.96 | 21 | |
| 8 | 17 July 2009 | 70°63′703′′ N, 135°17′208′′ E | Yana | 14.6 | 6 | 40 | ||||||||
| 9 | 18 July 2009 | 70°75′302′′ N, 136°17′058′′ E | Yana | 14.5 | 7.13 | 0.8 | 14.80 | 101.00 | 1.90 | 40.00 | 10.33 | 2.64 | 15 | |
| 10 | 18 July 2009 | 70°74′704′′ N, 136°20′373′′ E | Yana | 15.1 | 6.69 | 0.9 | 4.80 | 45.96 | 1.10 | 20.00 | 10.60 | 2.64 | 0 | |
| 11 | 18 July 2009 | 70°76′075′′ N, 136°23′337′′ E | Yana | 14.2 | 7.13 | 0.9 | 8.40 | 92.06 | 1.60 | 50.00 | 10.28 | 2.64 | 23 | |
| 12 | 18 July 2009 | 71°14′201′′ N, 136°12′924′′ E | Yana | 14 | 7.13 | 0.9 | 14.00 | 104.26 | 1.90 | 50.00 | 10.30 | 2.64 | 31 | |
| 13 | 26 June 2009 | 68°33′803′′ N, 146°03′186′′ E | Indigirka | 14.2 | 7.77 | 0.2 | 79.20 | 97.65 | 2.40 | 69.00 | 9.86 | 3.52 | 0 | 7 |
| 14 | 26 June 2009 | 68°34′601′′ N, 146°01′735′′ E | Indigirka | 14.9 | 7.24 | 0.1 | 8.00 | 52.21 | 2.00 | 33.00 | 9.45 | 5.72 | 5 | |
| 15 | 26 June 2009 | 68°35′042′′ N, 145°99′79′′ E | Indigirka | 14.3 | 7.29 | 0.1 | 21.60 | 60.91 | 2.20 | 33.00 | 9.47 | 4.40 | 0 | |
| 16 | 27 June 2009 | 68°56′854′′ N, 146°74′313′′ E | Indigirka | 14.2 | 0 | |||||||||
| 17 | 28 June 2009 | 68°85′087′′ N, 147°38′221′′ E | Indigirka | 14.7 | 7.43 | 0.1 | 10.00 | 90.12 | 1.90 | 33.00 | 9.61 | 3.52 | 0 | |
| 18 | 29 June 2009 | 69°19′623′′ N, 147°49′045′′ E | Indigirka | 15.1 | 0 | |||||||||
| 19 | 29 June 2009 | 69°55′775′′ N, 147°62′09′′ E | Indigirka | 15.5 | 7.55 | 0.1 | 44.80 | 90.65 | 0.50 | 33.00 | 9.00 | 4.40 | 0 | |
| 20 | 29 June 2009 | 69°57′149′′ N, 147°62′26′′ E | Indigirka | 17.7 | 7.09 | 0.1 | 6.40 | 51.14 | 2.50 | 13.00 | 9.60 | 7.48 | 3 | |
| 21 | 29 June 2009 | 69°58′76′′ N, 147°59′92′′ E | Indigirka | 17.7 | 6.99 | 0.1 | 7.60 | 52.60 | 2.50 | 13.00 | 9.36 | 6.16 | 1 | |
| 22 | 30 June 2009 | 70°52′957′′ N, 147°76′007′′ E | Indigirka | 15.5 | 0 | |||||||||
| 23 | 2 July 2009 | 70°51′058′′ N, 147°68′349′′ E | Indigirka | 12.5 | 7.49 | 0.1 | 130.40 | 97.22 | 2.50 | 100.00 | 9.59 | 2.64 | 0 | |
| 24 | 2 July 2009 | 70°53′011′′ N, 147°70′653′′ E | Indigirka | 14.1 | 7.47 | 0.1 | 88.40 | 80.66 | 2.40 | 168.00 | 9.42 | 3.08 | 0 | |
| 25 | 2 July 2009 | 70°52′957′′ N, 147°76′007′′ E | Indigirka | 14.1 | 7.44 | 0.1 | 84.80 | 80.71 | 2.50 | 168.00 | 9.36 | 3.08 | 0 | |
| 26 | 5 August 2010 | 68°31′505′′ N, 157°74′07′′ E | Kolyma | 16.7 | 16 | 31 | ||||||||
| 27 | 6 August 2010 | 68°71′893′′ N, 158°66′36′′ E | Kolyma | 15.9 | 7.60 | 1.9 | 5.20 | 126.06 | 1.34 | 80.00 | 9.84 | 5.28 | 16 | |
| 28 | 6 August 2010 | 68°70′169′′ N, 158°70′129′′ E | Kolyma | 12.0 | 7.64 | 3.0 | 5.20 | 68.35 | 1.64 | 40.00 | 10.52 | 3.96 | 0 | |
| 29 | 6 August 2010 | 68°70′217′′ N, 158°72′184′′ E | Kolyma | 14.4 | 7.48 | 1.9 | 5.20 | 92.00 | 2.04 | 380.00 | 10.63 | 3.52 | 0 | |
| 30 | 6 August 2010 | 68°57′6098′′ N 159°60′2341′′ E | Kolyma | 7 | ||||||||||
| 31 | 7 August 2010 | 68°51′189′′ N, 160° 88′912′′ E | Kolyma | 13.9 | 7.63 | 1.4 | 5.60 | 124.01 | 1.34 | 120.00 | 10.30 | 5.28 | 9 | |
| 32 | 7 August 2010 | 68°46′522′′ N, 160°80′042′′ E | Kolyma | 14.7 | 7.55 | 2.5 | 6.00 | 94.49 | 1.44 | 180.00 | 10.60 | 3.96 | 24 | |
| 33 | 7 August 2010 | 68°50′719′′ N, 160°97′508′′ E | Kolyma | 14.4 | 7.26 | 1.4 | 5.60 | 90.32 | 1.44 | 180.00 | 9.89 | 3.96 | 0 |
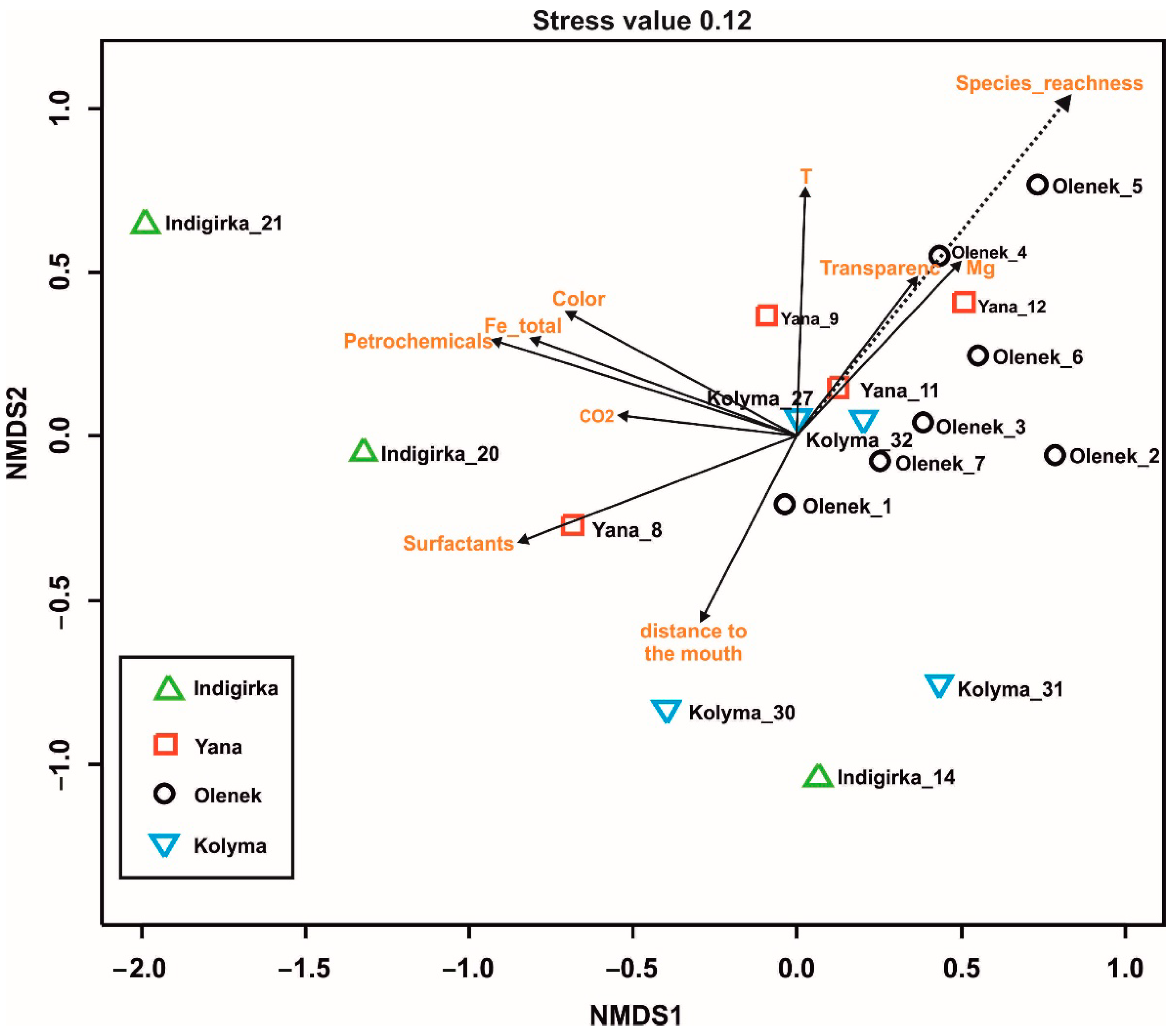
| Factor Names | R2 Covariance Coefficient | p Value |
|---|---|---|
| Sampling river | 0.290 | 0.001 |
| Distance to the mouth, km | 0.098 | 0.049 |
| T, °C | 0.090 | 0.042 |
| Water transparency, m | 0.097 | 0.031 |
| pH, Epi | 0.065 | 0.275 |
| Suspended matter, mg/L | 0.062 | 0.373 |
| Color, Pt-Co | 0.105 | 0.019 |
| O2, mg/L | 0.068 | 0.228 |
| Oxygen saturation, % | 0.068 | 0.232 |
| CO2, mg/L | 0.097 | 0.048 |
| Σions, mg/L | 0.065 | 0.300 |
| Water hardness, mg-equ/L | 0.069 | 0.249 |
| Ca2+, mg/L | 0.056 | 0.505 |
| Mg2+, mg/L | 0.099 | 0.025 |
| Na+, mg/L | 0.052 | 0.564 |
| K+, mg/L | 0.060 | 0.415 |
| HCO3−, mg/L | 0.063 | 0.342 |
| Cl−, mg/L | 0.045 | 0.727 |
| SO42−, mg/L | 0.062 | 0.364 |
| NH4, mg/L | 0.075 | 0.204 |
| NO2, mg/L | 0.062 | 0.199 |
| NO3, mg/L | 0.077 | 0.138 |
| PO4, mg/L | 0.071 | 0.205 |
| Ptotal, μg/L | 0.055 | 0.515 |
| Si, mg/L | 0.059 | 0.419 |
| COD, mg/L | 0.074 | 0.177 |
| BOD5, mg/L | 0.063 | 0.328 |
| Fetotal, mg/L | 0.102 | 0.025 |
| Phenols, mg/L | 0.066 | 0.298 |
| Surfactants, mg/L | 0.133 | 0.007 |
| Petrochemicals, mg/L | 0.121 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessudova, A.; Gabyshev, V.; Firsova, A.; Gabysheva, O.; Bukin, Y.; Likhoshway, Y. Diversity Variation of Silica-Scaled Chrysophytes Related to Differences in Physicochemical Variables in Estuaries of Rivers in an Arctic Watershed. Sustainability 2021, 13, 13768. https://doi.org/10.3390/su132413768
Bessudova A, Gabyshev V, Firsova A, Gabysheva O, Bukin Y, Likhoshway Y. Diversity Variation of Silica-Scaled Chrysophytes Related to Differences in Physicochemical Variables in Estuaries of Rivers in an Arctic Watershed. Sustainability. 2021; 13(24):13768. https://doi.org/10.3390/su132413768
Chicago/Turabian StyleBessudova, Anna, Viktor Gabyshev, Alena Firsova, Olga Gabysheva, Yurij Bukin, and Yelena Likhoshway. 2021. "Diversity Variation of Silica-Scaled Chrysophytes Related to Differences in Physicochemical Variables in Estuaries of Rivers in an Arctic Watershed" Sustainability 13, no. 24: 13768. https://doi.org/10.3390/su132413768







