The PGRMC1 Antagonist AG-205 Inhibits Synthesis of Galactosylceramide and Sulfatide
Abstract
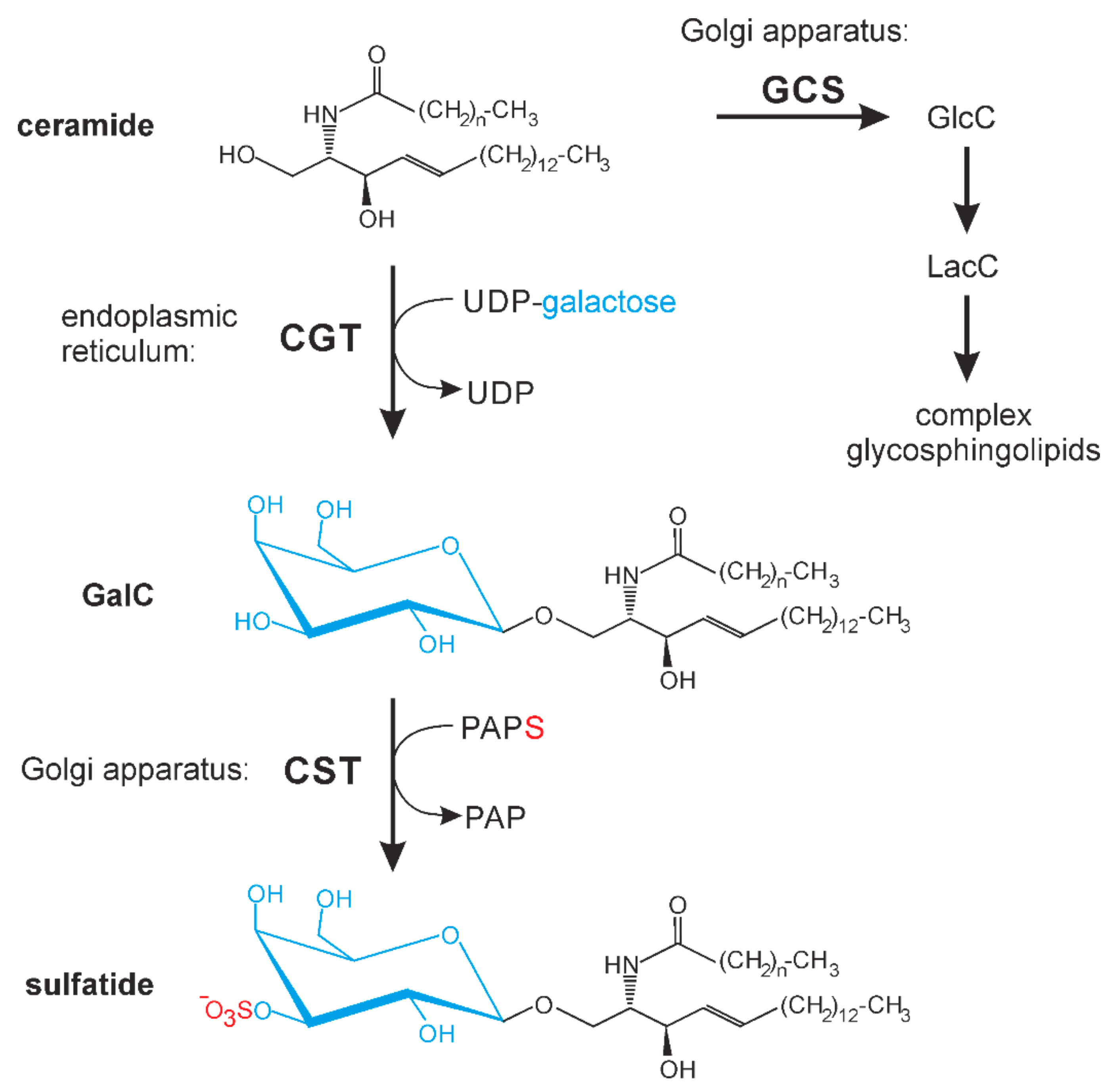
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Lines
2.3. Treatment of Cell Lines
2.4. MTT Cell Viability Assay
2.5. Lipid Extraction and Thin-Layer Chromatography
2.6. Metabolic Labelling of Cells
2.7. Western Blot Analysis
2.8. Indirect Immunofluorescence
2.9. Cerebroside Sulfotransferase (CST) and SULT1A1 Expression and Purification
2.10. Cerebroside Sulfotransferase Assay
2.11. Ceramide Galactosyltransferase (CGT) Assay
2.12. Statistics
3. Results
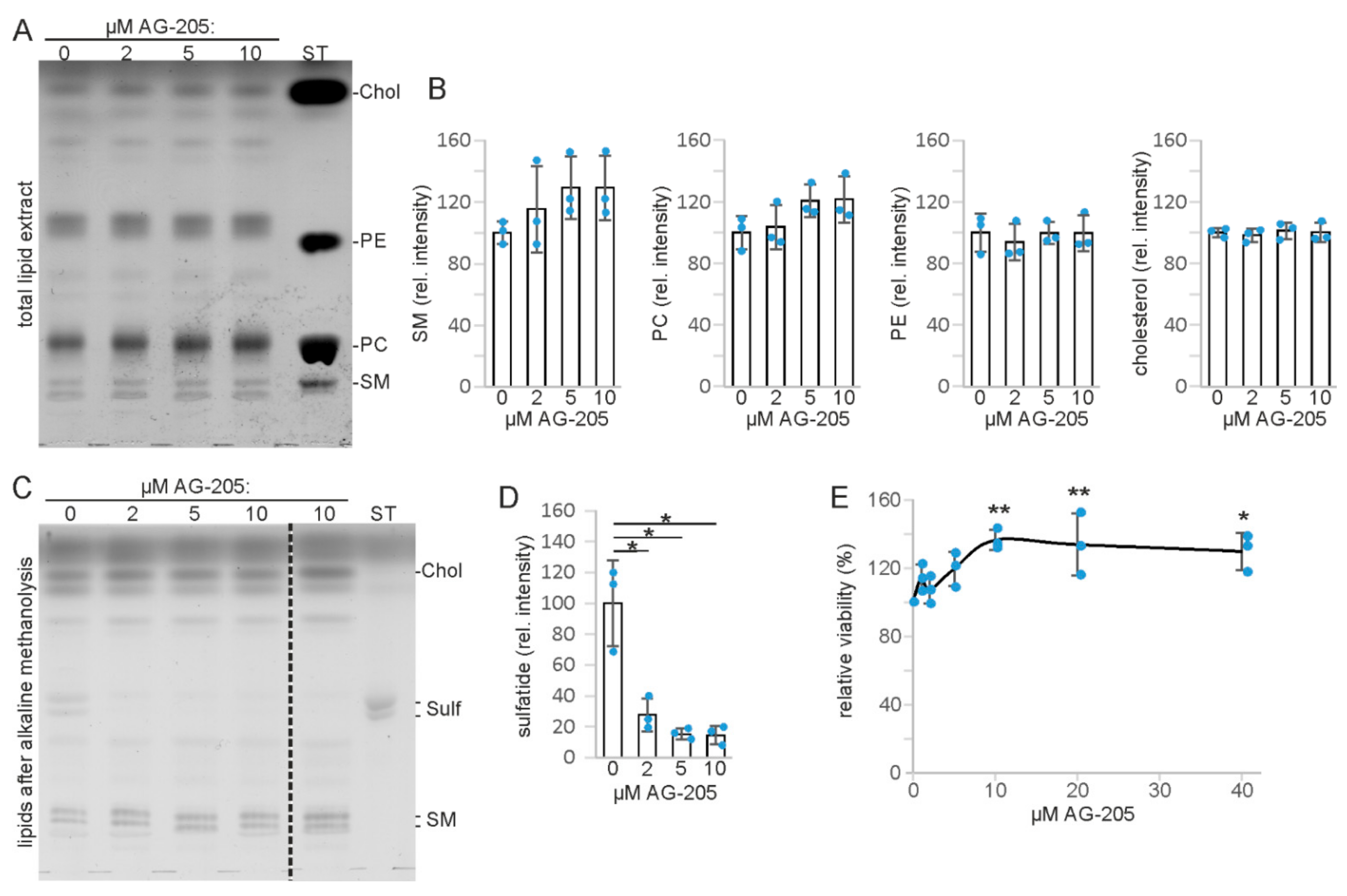
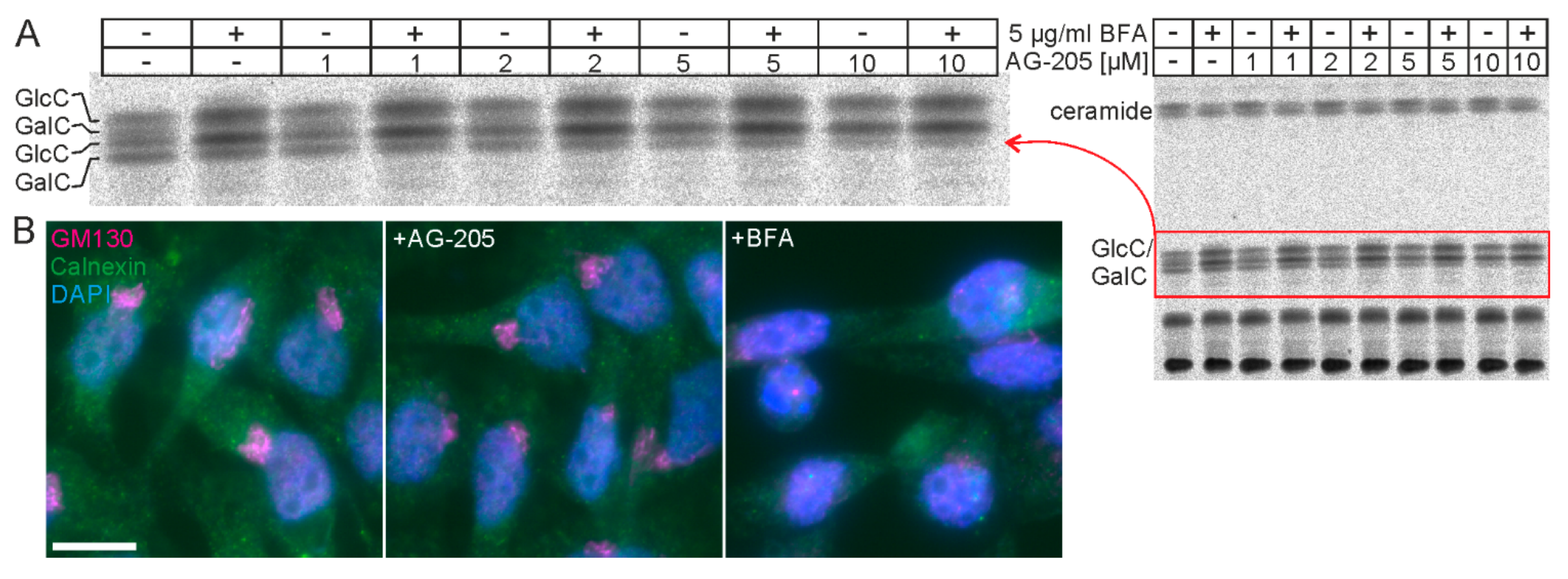
3.1. AG-205 Specifically Reduces Sulfatide Level in SMKT-R3 Cells
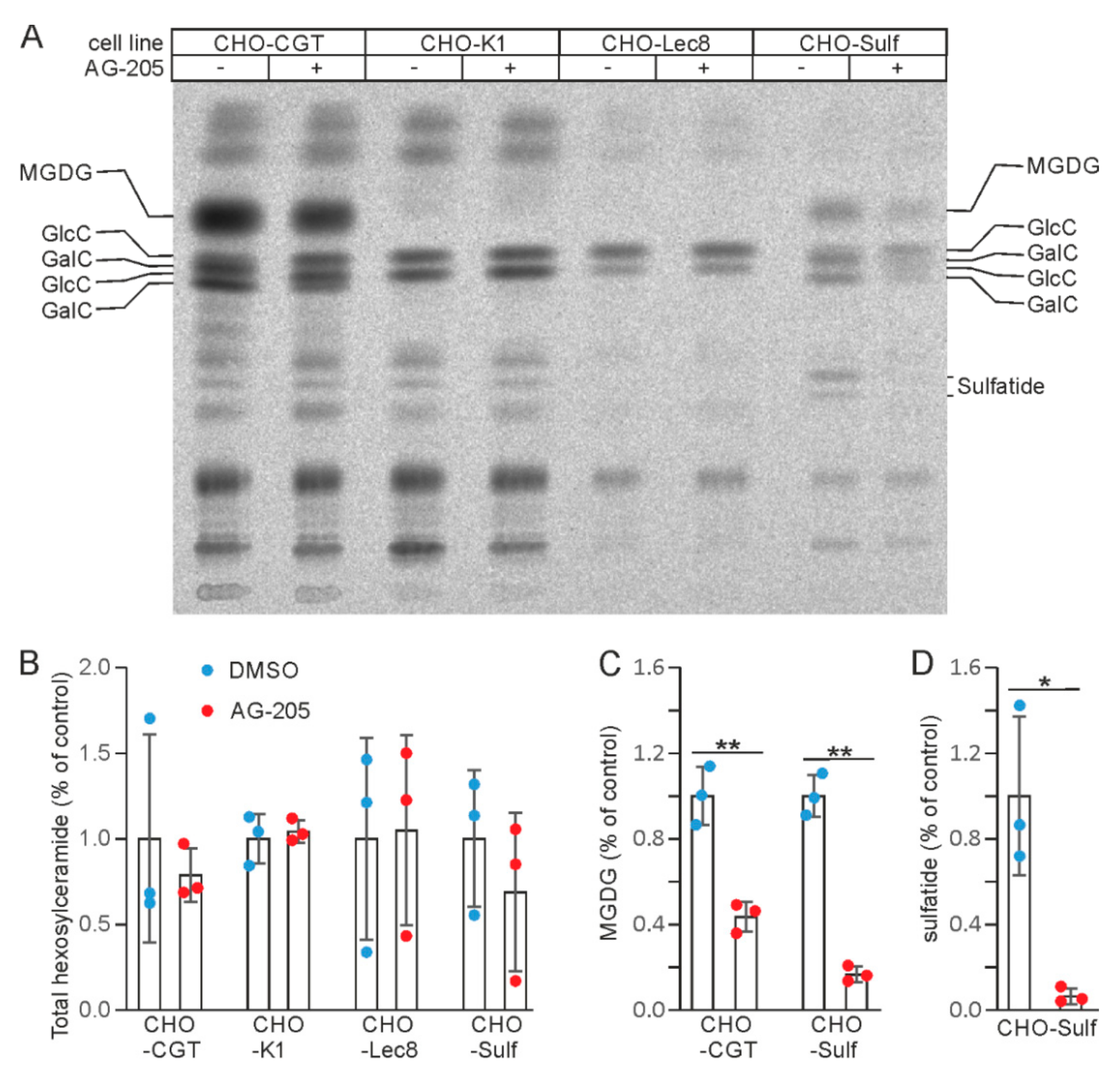
3.2. AG-205 Specifically Reduces Sulfatide and GalC Synthesis in CHO Cell Lines
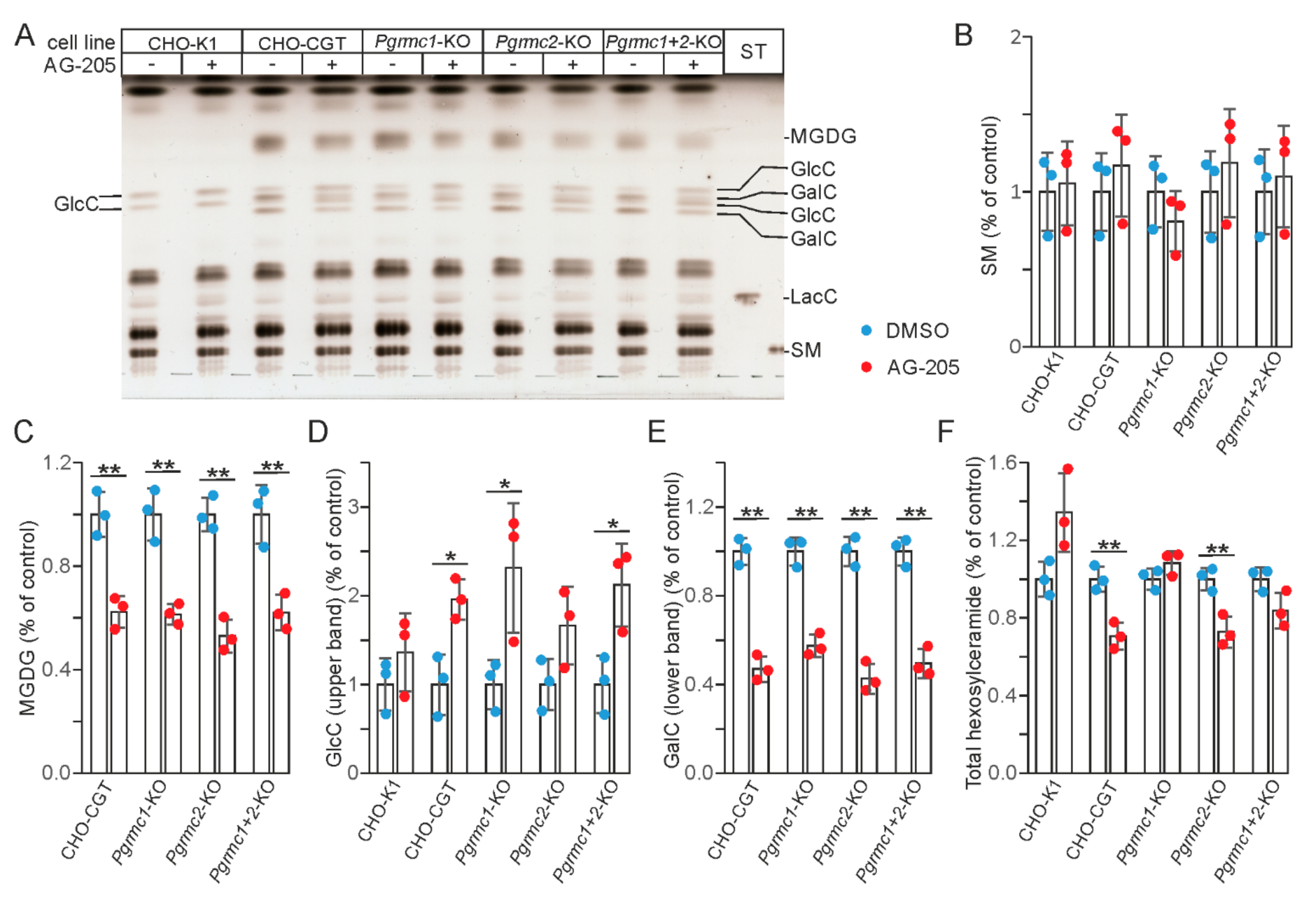
3.3. AG-205 Induced Inhibition of GalC Synthesis Is Independent of Pgrmc1 and Pgrmc2 Expression
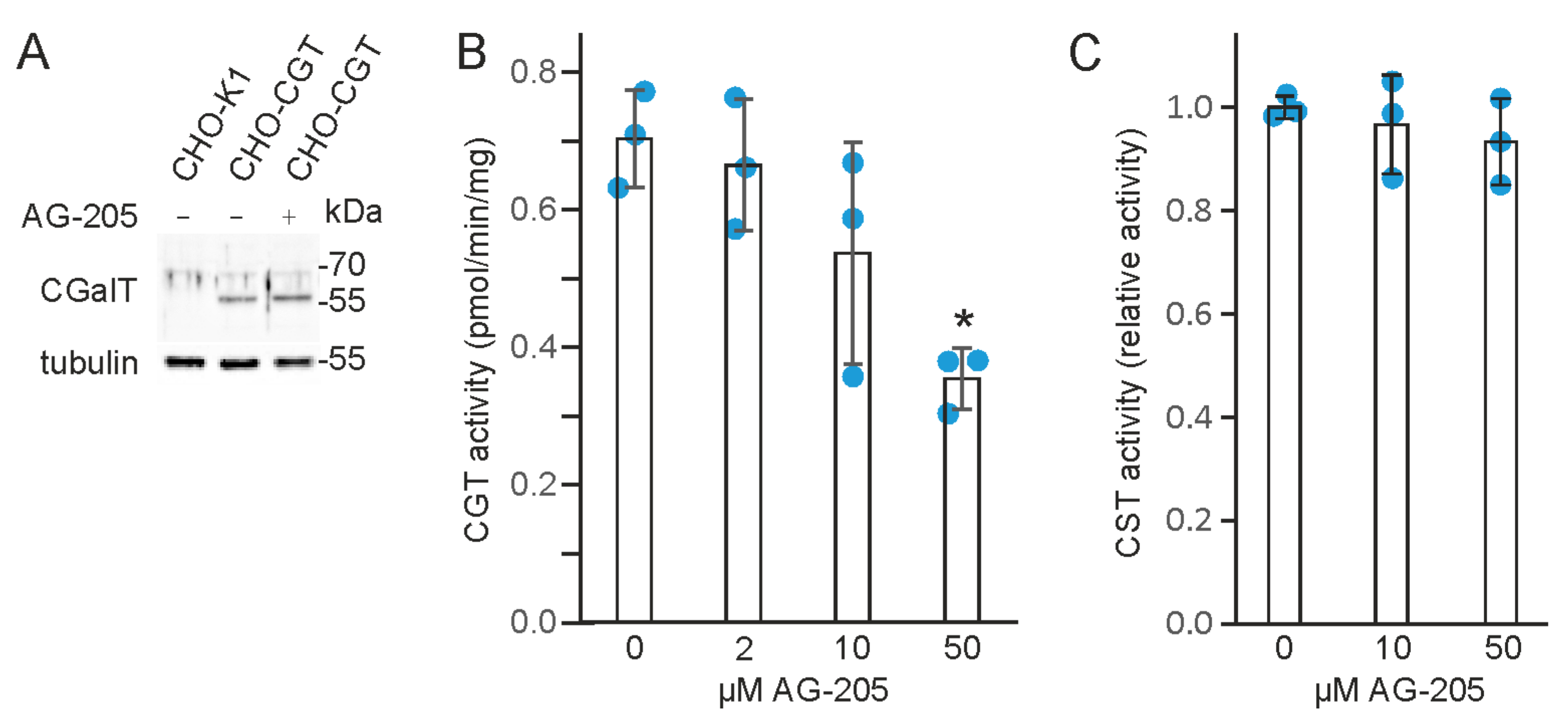
3.4. AG-205 Inhibits UDP-Galactose: Ceramide Galactosyltransferase (CGT)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cahill, M.A.; Jazayeri, J.A.; Catalano, S.M.; Toyokuni, S.; Kovacevic, Z.; Richardson, D.R. The emerging role of progesterone receptor membrane component 1 (PGRMC1) in cancer biology. Biochim. Biophys. Acta 2016, 1866, 339–349. [Google Scholar] [CrossRef]
- Kabe, Y.; Handa, H.; Suematsu, M. Function and structural regulation of the carbon monoxide (CO)-responsive membrane protein PGRMC1. J. Clin. Biochem. Nutr. 2018, 63, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Hehenberger, E.; Eitel, M.; Fortunato, S.A.V.; Miller, D.J.; Keeling, P.J.; Cahill, M.A. Early eukaryotic origins and metazoan elaboration of MAPR family proteins. Mol. Phylogenet. Evol. 2020, 148, 106814. [Google Scholar] [CrossRef]
- Mir, S.U.; Ahmed, I.S.; Arnold, S.; Craven, R.J. Elevated progesterone receptor membrane component 1/sigma-2 receptor levels in lung tumors and plasma from lung cancer patients. Int. J. Cancer 2012, 131, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Hampton, K.K.; Stewart, R.; Napier, D.; Claudio, P.P.; Craven, R.J. PGRMC1 Elevation in Multiple Cancers and Essential Role in Stem Cell Survival. Adv. Lung Cancer 2015, 4, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Willibald, M.; Wurster, I.; Meisner, C.; Vogel, U.; Seeger, H.; Mueck, A.O.; Fehm, T.; Neubauer, H. High Level of Progesteron Receptor Membrane Component 1 (PGRMC 1) in Tissue of Breast Cancer Patients is Associated with Worse Response to Anthracycline-Based Neoadjuvant Therapy. Horm. Metab. Res. 2017, 49, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Kimura, I.; Nakayama, Y.; Konishi, M.; Terasawa, K.; Ohta, M.; Itoh, N.; Fujimoto, M. Functions of MAPR (membrane-associated progesterone receptor) family members as heme/steroid-binding proteins. Curr. Protein Pept. Sci. 2012, 13, 687–696. [Google Scholar] [CrossRef] [Green Version]
- Cahill, M.A.; Medlock, A.E. Thoughts on interactions between PGRMC1 and diverse attested and potential hydrophobic ligands. J. Steroid Biochem. Mol. Biol. 2017, 171, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.S.; Rohe, H.J.; Twist, K.E.; Craven, R.J. Pgrmc1 (progesterone receptor membrane component 1) associates with epidermal growth factor receptor and regulates erlotinib sensitivity. J. Biol. Chem. 2010, 285, 24775–24782. [Google Scholar] [CrossRef] [Green Version]
- Neubauer, H.; Clare, S.E.; Wozny, W.; Schwall, G.P.; Poznanovic, S.; Stegmann, W.; Vogel, U.; Sotlar, K.; Wallwiener, D.; Kurek, R.; et al. Breast cancer proteomics reveals correlation between estrogen receptor status and differential phosphorylation of PGRMC1. Breast Cancer Res. 2008, 10, R85. [Google Scholar] [CrossRef] [Green Version]
- Thejer, B.M.; Adhikary, P.P.; Kaur, A.; Teakel, S.L.; van Oosterum, A.; Seth, I.; Pajic, M.; Hannan, K.M.; Pavy, M.; Poh, P.; et al. PGRMC1 phosphorylation affects cell shape, motility, glycolysis, mitochondrial form and function, and tumor growth. BMC Mol. Cell. Biol. 2020, 21, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.R.; Lee, Y.H.; Jo, S.L.; Heo, J.H.; Kim, G.; Lee, G.S.; An, B.S.; Baek, I.J.; Hong, E.J. Absence of progesterone receptor membrane component 1 reduces migration and metastasis of breast cancer. Cell Commun. Signal. 2021, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Kabe, Y.; Koike, I.; Yamamoto, T.; Hirai, M.; Kanai, A.; Furuhata, R.; Tsugawa, H.; Harada, E.; Sugase, K.; Hanadate, K.; et al. Glycyrrhizin Derivatives Suppress Cancer Chemoresistance by Inhibiting Progesterone Receptor Membrane Component 1. Cancers 2021, 13, 3265. [Google Scholar] [CrossRef]
- Yoshitani, N.; Satou, K.; Saito, K.; Suzuki, S.; Hatanaka, H.; Seki, M.; Shinozaki, K.; Hirota, H.; Yokoyama, S. A structure-based strategy for discovery of small ligands binding to functionally unknown proteins: Combination of in silico screening and surface plasmon resonance measurements. Proteomics 2005, 5, 1472–1480. [Google Scholar] [CrossRef]
- Ahmed, I.S.; Rohe, H.J.; Twist, K.E.; Mattingly, M.N.; Craven, R.J. Progesterone receptor membrane component 1 (Pgrmc1): A heme-1 domain protein that promotes tumorigenesis and is inhibited by a small molecule. J. Pharmacol. Exp. Ther. 2010, 333, 564–573. [Google Scholar] [CrossRef] [Green Version]
- Piel, R.B., 3rd; Shiferaw, M.T.; Vashisht, A.A.; Marcero, J.R.; Praissman, J.L.; Phillips, J.D.; Wohlschlegel, J.A.; Medlock, A.E. A Novel Role for Progesterone Receptor Membrane Component 1 (PGRMC1): A Partner and Regulator of Ferrochelatase. Biochemistry 2016, 55, 5204–5217. [Google Scholar] [CrossRef] [Green Version]
- Will, E.A.; Liu, X.; Peluso, J.J. AG 205, a progesterone receptor membrane component 1 antagonist, ablates progesterone’s ability to block oxidative stress-induced apoptosis of human granulosa/luteal cells. Biol. Reprod. 2017, 96, 843–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Higashisaka, K.; Shintani, T.; Maki, A.; Hanamuro, S.; Haga, Y.; Maeda, S.; Tsujino, H.; Nagano, K.; Fujio, Y.; et al. Progesterone receptor membrane component 1 leads to erlotinib resistance, initiating crosstalk of Wnt/β-catenin and NF-κB pathways, in lung adenocarcinoma cells. Sci. Rep. 2020, 10, 4748. [Google Scholar] [CrossRef]
- Pedroza, D.A.; Rajamanickam, V.; Subramani, R.; Bencomo, A.; Galvez, A.; Lakshmanaswamy, R. Progesterone receptor membrane component 1 promotes the growth of breast cancers by altering the phosphoproteome and augmenting EGFR/PI3K/AKT signalling. Br. J. Cancer 2020, 123, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L.; Powell, D.W.; Bard, M.; Eckstein, J.; Barbuch, R.; Link, A.J.; Espenshade, P.J. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell Metab. 2007, 5, 143–149. [Google Scholar] [CrossRef] [Green Version]
- McGuire, M.R.; Mukhopadhyay, D.; Myers, S.L.; Mosher, E.P.; Brookheart, R.T.; Kammers, K.; Sehgal, A.; Selen, E.S.; Wolfgang, M.J.; Bumpus, N.N.; et al. Progesterone receptor membrane component 1 (PGRMC1) binds and stabilizes cytochromes P450 through a heme-independent mechanism. J. Biol. Chem. 2021, 297, 101316. [Google Scholar] [CrossRef]
- Suchanek, M.; Radzikowska, A.; Thiele, C. Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells. Nat. Methods 2005, 2, 261–267. [Google Scholar] [CrossRef]
- Yang, T.; Espenshade, P.J.; Wright, M.E.; Yabe, D.; Gong, Y.; Aebersold, R.; Goldstein, J.L.; Brown, M.S. Crucial step in cholesterol homeostasis: Sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell 2002, 110, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Asperger, H.; Stamm, N.; Gierke, B.; Pawlak, M.; Hofmann, U.; Zanger, U.M.; Marton, A.; Katona, R.L.; Buhala, A.; Vizler, C.; et al. Progesterone receptor membrane component 1 regulates lipid homeostasis and drives oncogenic signaling resulting in breast cancer progression. Breast Cancer Res. 2020, 22, 75. [Google Scholar] [CrossRef]
- Hardt, R.; Winter, D.; Gieselmann, V.; Eckhardt, M. Identification of progesterone receptor membrane component-1 as an interaction partner and possible regulator of fatty acid 2-hydroxylase. Biochem. J. 2018, 475, 853–871. [Google Scholar] [CrossRef] [PubMed]
- Sandhoff, R.; Sandhoff, K. Emerging concepts of ganglioside metabolism. FEBS Lett. 2018, 592, 3835–3864. [Google Scholar] [CrossRef] [Green Version]
- Eckhardt, M. The role and metabolism of sulfatide in the nervous system. Mol. Neurobiol. 2008, 37, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Suzuki, T. Role of sulfatide in normal and pathological cells and tissues. J. Lipid Res. 2012, 53, 1437–1450. [Google Scholar] [CrossRef] [Green Version]
- Honke, K.; Tsuda, M.; Hirahara, Y.; Miyao, N.; Tsukamoto, T.; Satoh, M.; Wada, Y. Cancer-associated expression of glycolipid sulfotransferase gene in human renal cell carcinoma cells. Cancer Res. 1998, 58, 3800–3805. [Google Scholar] [PubMed]
- Eckhardt, M.; Fewou, S.N.; Ackermann, I.; Gieselmann, V. N-glycosylation is required for full enzymic activity of the murine galactosylceramide sulphotransferase. Biochem. J. 2002, 368, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Miyao, N.; Tsukamoto, T.; Kumamoto, Y. Establishment of three human renal cell carcinoma cell lines (SMKT-R-1, SMKT-R-2, and SMKT-R-3) and their characters. Urol. Res. 1989, 17, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Puck, T.T.; Cieciura, S.J.; Robinson, A. Genetics of somatic mammalian cells. III. Long-term cultivation of euploid cells from human and animal subjects. J. Exp. Med. 1958, 108, 945–956. [Google Scholar] [CrossRef] [Green Version]
- Deutscher, S.L.; Hirschberg, C.B. Mechanism of galactosylation in the Golgi apparatus. A Chinese hamster ovary cell mutant deficient in translocation of UDP-galactose across Golgi vesicle membranes. J. Biol. Chem. 1986, 261, 96–100. [Google Scholar] [CrossRef]
- Wang-Eckhardt, L.; Eckhardt, M. A progesterone receptor membrane component 1 antagonist induces large vesicles independent of progesterone receptor membrane component 1 expression. Biol. Chem. 2020, 401, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Bodennec, J.; Koul, O.; Aguado, I.; Brichon, G.; Zwingelstein, G.; Portoukalian, J. A procedure for fractionation of sphingolipid classes by solid-phase extraction on aminopropyl cartridges. J. Lipid Res. 2000, 41, 1524–1531. [Google Scholar] [CrossRef]
- Yao, J.K.; Rastetter, G.M. Microanalysis of complex tissue lipids by high-performance thin-layer chromatography. Anal. Biochem. 1985, 150, 111–116. [Google Scholar] [CrossRef]
- Becker, I.; Wang-Eckhardt, L.; Lodder-Gadaczek, J.; Wang, Y.; Grünewald, A.; Eckhardt, M. Mice deficient in the NAAG synthetase II gene Rimkla are impaired in a novel object recognition task. J. Neurochem. 2021, 157, 2008–2023. [Google Scholar] [CrossRef]
- Sprong, H.; Kruithof, B.; Leijendekker, R.; Slot, J.W.; van Meer, G.; van der Sluijs, P. UDP-galactose: Ceramide galactosyltransferase is a class I integral membrane protein of the endoplasmic reticulum. J. Biol. Chem. 1998, 273, 25880–25888. [Google Scholar] [CrossRef] [Green Version]
- Harlow, E.; Lane, D. Antibodies: A Laboratory Manual, 1st ed.; Cold Spring Harbor Laboratory: New York, NY, USA, 1988. [Google Scholar]
- Tom, R.; Bisson, L.; Durocher, Y. Purification of his-tagged proteins using fractogel-cobalt. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot4980. [Google Scholar] [CrossRef] [Green Version]
- Carey, M.F.; Peterson, C.L.; Smale, S.T. PCR-mediated site-directed mutagenesis. Cold Spring Harb. Protoc. 2013, 2013, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Zech, I. Substratreduktionstherapie der Metachromatischen Leukodystrophie. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2013. [Google Scholar]
- Yaghootfam, A.; Gieselmann, V.; Eckhardt, M. Delay of myelin formation in arylsulphatase A-deficient mice. Eur. J. Neurosci. 2005, 21, 711–720. [Google Scholar] [CrossRef]
- Kobayashi, T.; Honke, K.; Kamio, K.; Sakakibara, N.; Gasa, S.; Miyao, N.; Tsukamoto, T.; Ishizuka, I.; Miyazaki, T.; Makita, A. Sulfolipids and glycolipid sulfotransferase activities in human renal cell carcinoma cells. Br. J. Cancer 1993, 67, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, K.; Hanada, K. Structure, functions and regulation of CERT, a lipid-transfer protein for the delivery of ceramide at the ER-Golgi membrane contact sites. FEBS Lett. 2019, 593, 2366–2377. [Google Scholar] [CrossRef] [Green Version]
- Teakel, S.L.; Ludescher, M.; Thejer, B.M.; Poschmann, G.; Forwood, J.K.; Neubauer, H.; Cahill, M.A. Protein complexes including PGRMC1 and actin-associated proteins are disrupted by AG-205. Biochem. Biophys. Res. Commun. 2020, 524, 64–69. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc. Natl. Acad. Sci. USA 2004, 101, 14949–14954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pituch, K.C.; Moyano, A.L.; Lopez-Rosas, A.; Marottoli, F.M.; Li, G.; Hu, C.; van Breemen, R.; Månsson, J.E.; Givogri, M.I. Dysfunction of platelet-derived growth factor receptor α (PDGFRα) represses the production of oligodendrocytes from arylsulfatase A-deficient multipotential neural precursor cells. J. Biol. Chem. 2015, 290, 7040–7053. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Han, M.; Park, I.H.; Park, C.H.; Kwak, M.S.; Shin, J.S. Sulfatide Inhibits HMGB1 Secretion by Hindering Toll-Like Receptor 4 Localization Within Lipid Rafts. Front. Immunol. 2020, 11, 1305. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Finkielstein, C.V.; Capelluto, D.G. The enigmatic role of sulfatides: New insights into cellular functions and mechanisms of protein recognition. Adv. Exp. Med. Biol. 2013, 991, 27–40. [Google Scholar] [CrossRef]
- Shroff, S.M.; Pomicter, A.D.; Chow, W.N.; Fox, M.A.; Colello, R.J.; Henderson, S.C.; Dupree, J.L. Adult CST-null mice maintain an increased number of oligodendrocytes. J. Neurosci. Res. 2009, 87, 3403–3414. [Google Scholar] [CrossRef] [Green Version]
- Kajigaya, H.; Tanaka, K.F.; Hayashi, A.; Suzuki, A.; Ishibashi, T.; Ikenaka, K.; Baba, H. Increased numbers of oligodendrocyte lineage cells in the optic nerves of cerebroside sulfotransferase knockout mice. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 415–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckhardt, M. Pathology and current treatment of neurodegenerative sphingolipidoses. Neuromol. Med. 2010, 12, 362–382. [Google Scholar] [CrossRef] [PubMed]
- Platt, F.M.; d’Azzo, A.; Davidson, B.L.; Neufeld, E.F.; Tifft, C.J. Lysosomal storage diseases. Nat. Rev. Dis. Primers 2018, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Babcock, M.C.; Mikulka, C.R.; Wang, B.; Chandriani, S.; Chandra, S.; Xu, Y.; Webster, K.; Feng, Y.; Nelvagal, H.R.; Giaramita, A.; et al. Substrate reduction therapy for Krabbe disease and metachromatic leukodystrophy using a novel ceramide galactosyltransferase inhibitor. Sci. Rep. 2021, 11, 14486. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang-Eckhardt, L.; Becker, I.; Eckhardt, M. The PGRMC1 Antagonist AG-205 Inhibits Synthesis of Galactosylceramide and Sulfatide. Cells 2021, 10, 3520. https://doi.org/10.3390/cells10123520
Wang-Eckhardt L, Becker I, Eckhardt M. The PGRMC1 Antagonist AG-205 Inhibits Synthesis of Galactosylceramide and Sulfatide. Cells. 2021; 10(12):3520. https://doi.org/10.3390/cells10123520
Chicago/Turabian StyleWang-Eckhardt, Lihua, Ivonne Becker, and Matthias Eckhardt. 2021. "The PGRMC1 Antagonist AG-205 Inhibits Synthesis of Galactosylceramide and Sulfatide" Cells 10, no. 12: 3520. https://doi.org/10.3390/cells10123520




