Flowers Characteristics of Selected Species of Lime-Tree (Tilia spp.) in Terms of miRNA-Based Markers Activity, Mannose Expression and Biological Compounds Content
Abstract
:1. Introduction
2. Materials and Methods
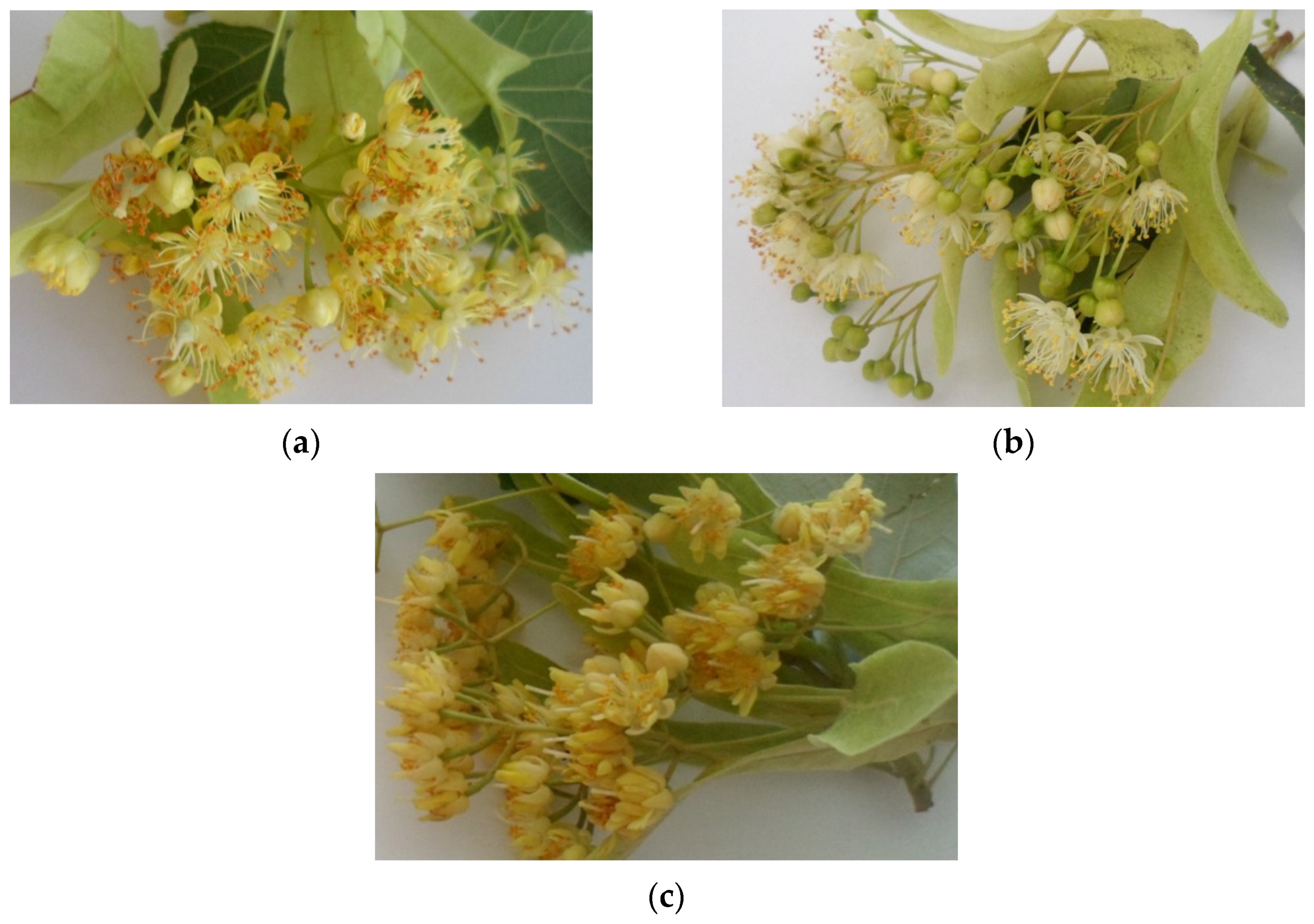
2.1. Plant Materials and Sample Preparation
2.2. Nucleic Acids Extraction
2.3. MiRNA-Marker Assay
2.4. Design of miRNA Primers
2.5. Primer Design for Mannose Expression Analysis and Real-Time PCR Analysis
2.6. Analyses of Antioxidant Activity
2.6.1. Free Radical Scavenging Activity
2.6.2. ABTS Radical Cation Decolorization Assay
2.6.3. Total Polyphenol Content
2.6.4. Total Flavonoid Content
3. Results
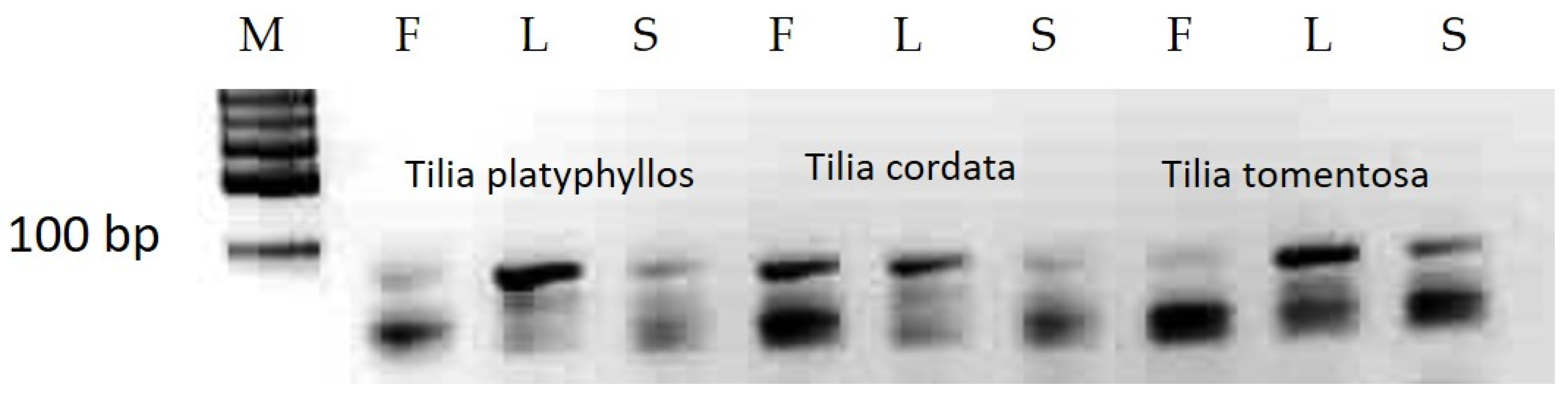
3.1. MicroRNA Analyses
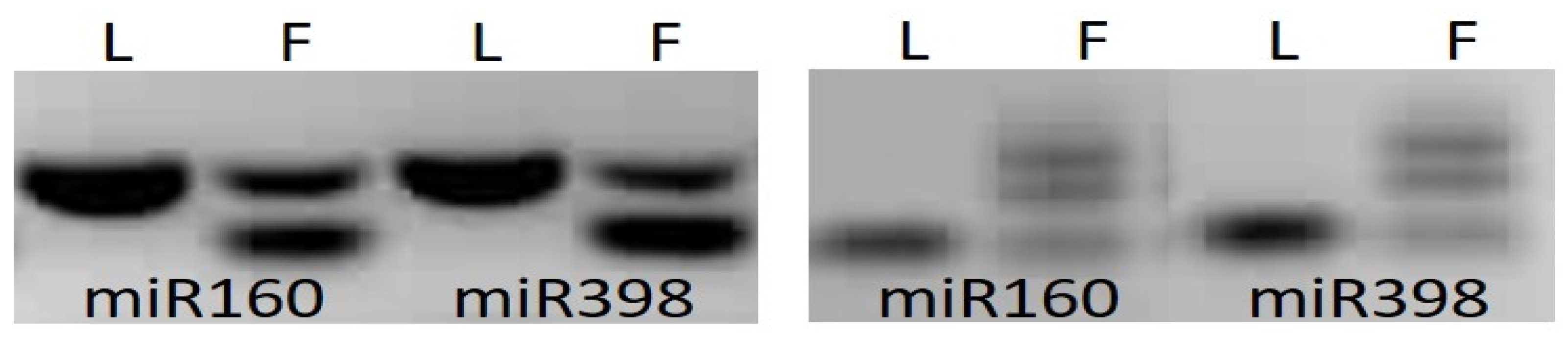
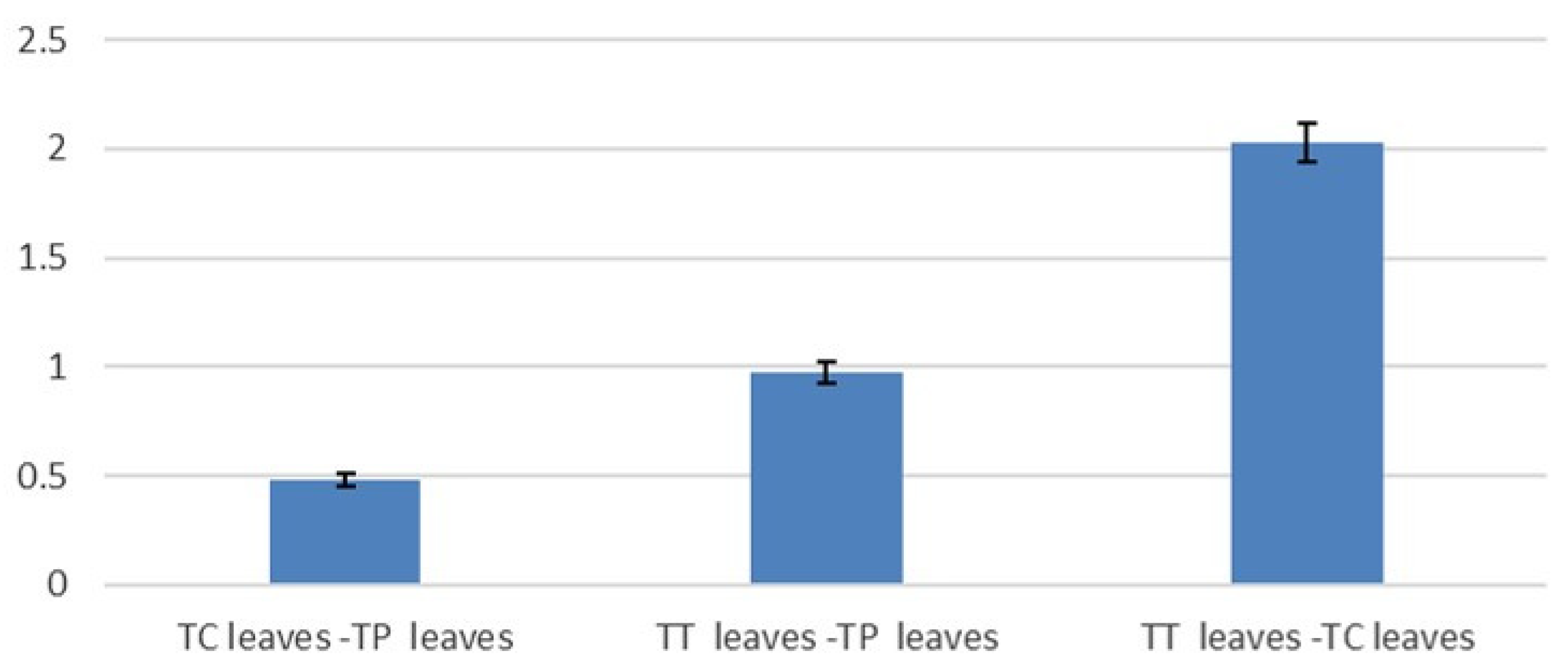
3.2. Mannose Pathway Regulator Expression Analysis
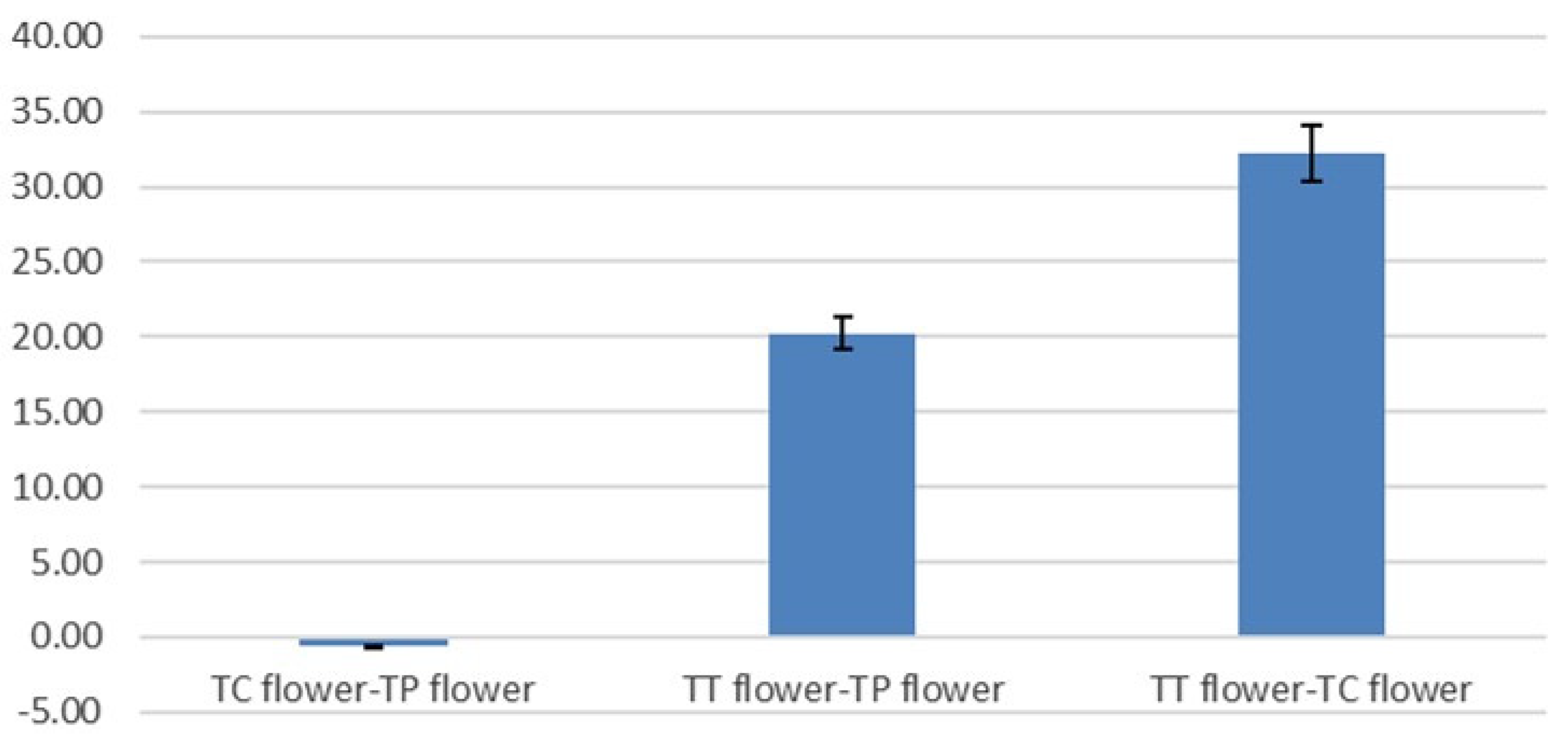
3.3. Antioxidant Activity, Total Polyphenol and Total Flavonoid Content
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Machovec, J.; Hrubík, P.; Vreštiak, P. Sadovnícka Dendrológia. (Hodnotenie Biologických Prvkov); Slovenská Poľnohospodárska Univerzita: Nitra, Slovakia, 2000; p. 228. ISBN 80-7137-702-3. [Google Scholar]
- Hrubík, P. Listnaté Dreviny v Sadovníckej Tvorbe; Slovenská Poľnohospodárska Univerzita: Nitra, Slovakia, 2002; p. 139. ISBN 80-8069-125-8. [Google Scholar]
- Hurych, V. Sadovníctvo, Sadovnícka Dendrológia 2, 1st ed.; PRÍRODA: Bratislava, Slovakia, 2000; p. 204. ISBN 80-07-01148-X. [Google Scholar]
- Jacquemart, A.L.; Moquet, L.; Ouvrard, P.; Quentin-Leclerco, J.; Hérent, M.F.; Quinet, M. Tila trees: Toxic or valuable resources for pollinators? Apidologie 2018, 49, 538–550. [Google Scholar] [CrossRef] [Green Version]
- Manea, M.I.; Borlea, G.F.; Tenche-Constantinescu, A. Aspects of genetic diversity of silver lime (Tilia tomentosa Moench) populations in natural forests of western Romania. In Proceedings of the SGEM2017 GREEN Conference, 17th International Mul-Tidisciplinary Scientific GeoConference, Vienna, Austria, 27–29 November 2017; 51 Alexander Malinov blvd: Sofia, Bulgaria; Volume 17, pp. 473–480. [Google Scholar] [CrossRef]
- Banfi, E.; Consolinová, F. STROMY v Záhradách, v Parkoch a vo Voľnej Prírode; IKAR a.s.: Bratislava, Slovakia, 2001; ISBN 80-7118-995-2. [Google Scholar]
- Filiz, E.; Birbilener, S.; Ozyigit, I.I.; Kulac, S.; Orus, F.C.S. Assessment of genetic variations of silver lime (Tilia tomentosa Moench.) by RAPD markers in urban and forest ecosystems. Biotechnol. Biotechnol. Equip. 2015, 29, 631–636. [Google Scholar] [CrossRef]
- Gabur, I.; Lipşa, F.D.; Adumitresi, L.; Tanase, C.; Simioniuc, D.P. Assessment of genetic variation of Tilia tomentosa by RAPD markers. J. Plant Dev. 2019, 26, 85–91. [Google Scholar] [CrossRef]
- Erichsen, E.O.; Wolff, K.; Hansen, O.K. Genetic and clonal structures of the tree species Tilia cordata Mill. in remnants of ancient forests in Denmark. Popul. Ecol. 2019, 61, 243–255. [Google Scholar] [CrossRef]
- Lobo, A.; Hansen, O.K.; Hansen, J.K.; Erichsen, E.O.; Jacobsen, B.; Kjaer, E.D. Local adaptation through genetic differentiation in highly fragmented Tilia cordata populations. Ecol. Evol. 2018, 8, 5968–5976. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.A.; Phuekvilai, P.; Wolff, K. Ancient woodlands in the limelight: Delineation and genetic structure of ancient woodland species Tilia cordata and Tilia platyphyllos (Tiliaceae) in the UK. Tree Genet. Genomes 2015, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Ma, P.F.; Li, H.T.; Li, D.Z. Complete plastid genome sequencing of four Tilia species (Malvaceae): A comparative analysis and phylogenetic implications. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Bej, S.; Basak, J. MicroRNAs: The Potential Biomarkers in Plant Stress Response. Am. J. Plant Sci. 2014, 5, 748–759. [Google Scholar] [CrossRef] [Green Version]
- Chiou, T.J. The role of microRNAs in sensing nutient stress. Plant Cell Environ. 2007, 30, 323–332. [Google Scholar] [CrossRef]
- Kruszka, K.; Pieczynski, M.; Windels, D.; Bielewicz, D.; Jarmolowski, A.; Szweykowska-Kulinska, Z.; Vazquez, F. Role of microRNAs and other sRNAs of plants in their changing environments. J. Plant Physiol. 2012, 169, 1664–1672. [Google Scholar] [CrossRef]
- Melnikova, N.V.; Dmitriev, A.A.; Belenikin, M.S.; Speranskava, A.S.; Krinitsina, A.A.; Rachinskaia, O.A.; Lakunina, V.A.; Krasnov, G.S.; Snezhkina, A.V.; Sadriidinova, A.F.; et al. Excess fertilizer responsive miRNAs revealed in Linum usitatissimum L. Biochemie 2015, 109, 36–41. [Google Scholar] [CrossRef]
- Melnikova, N.V.; Dmitriev, A.A.; Belenikin, M.S.; Koroban, N.V.; Speranskaya, A.S.; Krinitsina, A.A.; Krasnov, G.S.; Lakunina, V.A.; Snezhkina, A.V.; Sadritdinova, A.F.; et al. Identification, expression analysis, and target prediction of flax genotroph microRNAs under normal and nutrient stress sonditions. Plant Biotechnol. 2016, 7, 399. [Google Scholar] [CrossRef] [Green Version]
- Poczai, P.; Varga, I.; Laos, M.; Cseh, A.; Bell, N.; Valkonen, J.P.T.; Hyvönen, J. Advances in plant gene-targeted and functional markers: A review. Plant Methods 2013, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Fu, D.; Ma, B.; Mason, A.S.; Xiao, M.; Wei, L.; An, Z. MicroRNA-based molecular markers: A novel PCR-based genotyping technique in Brassica species. Plant Breed. 2013, 132, 375–381. [Google Scholar] [CrossRef]
- Yadav, C.B.Y.; Muthamilarasan, M.; Pandey, G.; Prasad, M. Development of novel microRNA-based genetic markers in foxtail millet for genotyping applications in related grass species. Mol. Breed. 2014, 34, 2219–2224. [Google Scholar] [CrossRef]
- Htwe, N.M.P.S.; Luo, Z.Q.; Jin, L.G.; Nadon, B.; Wang, K.J.; Qiu, L.J. Functional marker development of miR1511-InDel and allelic diversity within the genus Glycine. MBC Genom. 2015, 16, 467. [Google Scholar] [CrossRef] [Green Version]
- Mondal, T.K.; Ganie, S.A. Identification and characterization of salt responsive miRNA-SSR. Gene 2014, 535, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.A.; Mondal, T.K. Genome-wide development of novel miRNA-based microsatellite markers of rice (Oryza sativa) for genotyping applications. Mol. Breed. 2015, 35, 51. [Google Scholar] [CrossRef]
- Min, X.; Zhang, Z.; Liu, Y.; Wei, X.; Liu, Z.; Wang, Y.; Liu, W. Genome-wide development of microRNA-based SSR markers in Medicago truncatula with their transferability analysis and utilization in related legume species. Int. J. Mol. Sci. 2017, 18, 2440. [Google Scholar] [CrossRef] [Green Version]
- Hlavačková, L.; Nôžková, J.; Porokhovinova, E.; Brutch, N.; Shelenga, T.; Bjelková, M.; Ražná, K. Analysis of miRNA polymorphism during the selected developmental processes of flax. J. Cent. Eur. Agric. 2016, 17, 707–724. [Google Scholar] [CrossRef]
- Ražná, K.; Sawinska, Z.; Ivanišová, E.; Vukovic, N.; Terentjeva, M.; Stričík, M.; Kowalczewski, P.L.; Hlavačková, L.; Rovná, K.; Žiarovská, J.; et al. Properties of Ginkgo biloba L.: Antioxidant characterization, antimicrobial activities, and genomic microRNA based marker fingerprints. Int. J. Mol. Sci. 2020, 21, 3087. [Google Scholar] [CrossRef]
- Johnson, R.M. Honey bee toxicology. Annu. Rev. Entomol. 2015, 60, 415–434. [Google Scholar] [CrossRef] [Green Version]
- Koch, H.; Stevenson, P.C. Do linden trees kill bees? Reviewing the causes of bee deaths on silver linden (Tilia tomentosa). Biol. Lett. 2017, 13, 20170484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staudenmayer, T. Die Giftigkeit der Mannose fur Bienen und andere Insekten. J. Comp. Physiol. A Neuroethol. Sen. Neural. Behav. Physiol. 1939, 26, 644–668. [Google Scholar]
- Madel, G. Vergiftungen von Hummeln durch den Nektar der Silberlinde Tilia timentosa. Bonn. Zool. Beitr. 1977, 28, 149–154. [Google Scholar]
- Ball, T.; Denker, B.; Muhlen, W.; Surholt, B. DieUrsachen des Massensterbens von Hummeln unter spätblühenden Linden. Nat. Landsch. 1994, 69, 412–418. [Google Scholar]
- Petanidou, T. Sugars in Mediterranean floral nectars: An ecological and evolutionary approach. J. Chem. Ecol. 2005, 31, 1065–1087. [Google Scholar] [CrossRef]
- Ražná, K.; Nôžková, J.; Hlavačková, L.; Brutch, N.; Porokhovinova, E.; Shelenga, T.; Pavlov, A. Genotyping of flax genetic resources by miRNA-based molecular markers and morphology. Agriculture 2015, 61, 129–138. [Google Scholar] [CrossRef]
- Griffiths-Jones, S. The microRNA Registry. Nucleic Acids Res. 2004, 32, D109–D111. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Willett, W.C. Balancing life-style and genomics research for disease prevention. Science 2002, 296, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Assessment Report on Tilia tomentosa Moench, Flos. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-tilia-tomentosa-moench-flos_en.pdf (accessed on 18 May 2020).
- Lande, C.; Rao, S.; Morré, J.T.; Galindo, G.; Kirby, J.; Reardon, P.N.; Bobe, G.; Stevens, J.F. Linden (Tilia cordata) associated bumble bee mortality: Metabolomic analysis of nectar and bee muscle. PLoS ONE 2019, 14, e0218406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viola, H.; Wolfman, C.; Levide Stein, M.; Wasowski, C.; Pena, C.; Medina, J.H.; Paladini, A.C. Isolation of pharmacologically active benzodiazepine receptor ligands from Tilia tomentosa (Tiliaceae). J. Ethnopharm. 1994, 44, 47–53. [Google Scholar] [CrossRef]
- Cárdenas-Rodríguez, N.; González-Trujano, M.E.; Aguirre-Hernández, E.; Ruíz-García, M.; Sampieri, A.; Coballase-Urrutia, E.; Carmona-Aparicio, L. Anticonvulsant and Antioxidant Effects of Tilia americana var. mexicana and Flavonoids Constituents in the Pentylenetetrazole-Induced Seizures. Oxidative Med. Cell. Longev. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Cordenunsi, B.R.; Oliveira do Nascimento, J.R.; Genovese, M.I.; Lajolo, F.M. Influence of Cultivar on Quality Parameters and Chemical Composition of Strawberry Fruits Grown in Brazil. J. Agric. Food Chem. 2002, 50, 2581–2586. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Influence of light on health-promoting phytochemicals of broccoli sprouts. J. Sci. Food Agric. 2008, 88, 904–910. [Google Scholar] [CrossRef]
- Khalili, R.M.A.; Shafekh, S.E.; Norhayati, A.H.; Fatahudin, I.M.; Rahimah, R.; Norkamalia, H.; Azimah, A.N. Total Phenolic Content and in vitro Antioxidant Activity of Winged Bean (Psophocarpus tetragonolobus). Pak. J. Nutr. 2013, 12, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Wissam, Z.; Nour, A.A.; Jarkas, B.; Nukari, Z.; Dunia, S. Extracting and studying the antioxidant capacity of polyphenols in dry linden leaves (Tilia cordata). J. Pharmacogn. Phytochem. 2017, 6, 258–262. [Google Scholar]
- Buřičová, L.; Réblová, Z. Czech Medicinal Plants as Possible Sources of Antioxidants. Czech J. Food Sci. 2008, 26, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, A.; Mavi, A.; Oktay, M.; Kara, A.A.; Algur, O.F.; Bilaloglu, V. Comparison of antioxidant and antimicrobial activities of tilia (Tilia argentea Desf ex DC), sage (Salvia triloba L.), and black tea (Camellia sinensis) extracts. J. Agric Food Chem. 2000, 48, 5030–5034. [Google Scholar] [CrossRef]
- Cittan, M.; Altuntaş, E.; Çelik, A. Evaluation of antioxidant capacities and phenolic profiles in Tilia cordata fruit extracts: A comparative study to determine the efficiency of traditional hot water infusion method. Ind. Crop. Prod. 2018, 122, 553–558. [Google Scholar] [CrossRef]
- Jia, X.; Yan, J.; Tang, G. MicroRNA-mediated DNA methylation in plants. Front. Biol. 2011, 6, 133–139. [Google Scholar] [CrossRef]
- Pickaard, C.S.; Scheid, O.M. Epigenetic Regulation in Plants. Cold Spring Harb. Perspect. Biol. 2014, 6, a019315. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Burd, S.; Lers, A. miR408 is involved in abiotic stress responses in Arabidopsis. Plant J. 2015, 84, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, G.; Ng, T.B.; Lin, J.; Ye, X. Laccase Production and Differential Transcription of Laccase Genes in Cerrena sp. in Response to Metal Ions, Aromatic Compounds, and Nutrients. Front. Microbiol. 2016, 6, 1558. [Google Scholar] [CrossRef]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Li, S.B.; Xie, Z.Z.; Hu, C.G.; Zhang, J.Z. A Review of Auxin Response Factors (ARFs) in Plants. Front. Plant Sci. 2016, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Lai, Z.; Tian, Q.; Lin, L.; Lai, R.; Yang, M.; Zhang, D.; Chen, Y.; Zhang, Z. Endogenous target mimics down-regulate miR160 mediation of ARF10, -16, and -17 cleavage during somatic embryogenesis in Dimocarpus longan Lour. Front. Plant Sci. 2015. [Google Scholar] [CrossRef]
- Yao, X.; Chen, J.; Zhou, J.; Yu, H.; Ge, C.; Zhang, M.; Gao, X.; Dai, X.; Yang, Z.N.; Zhaob, Y. An Essential Role for miRNA167 in Maternal Control of Embryonic and Seed Development. Plant Physiol. 2019, 180, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, K.; Chen, L.; Zou, Y.; Liu, H.; Tian, Y.; Li, D.; Wang, R.; Zhao, F.; Ferguson, B.J.; et al. MicroRNA167-Directed Regulation of the Auxin Response Factors GmARF8a and GmARF8b Is Required for Soybean Nodulation and Lateral Root Development. Plant Physiol. 2015, 168, 101–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruszka, K.; Pacak, A.; Swida-Barteczka, A.; Nuc, P.; Alaba, S.; Wroblewska, Z.; Karlowski, W.; Jarmolowski, A.; Szweykowska-Kulinska, Z. Transcriptionally and post-transcriptionally regulated microRNAs in heat stress response in barley. J. Exp. Bot. 2014, 65, 6123–6135. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Sample | DPPH (mg TEAC/g FM) | ABTS (mg TEAC/g FM) | TPC (mg GAE/g FM) | TFC (mg QE/g FM) | TPAC (mg CAE/g FM) |
|---|---|---|---|---|---|
| Tilia platyphyllos | 0.593 | 6.952 | 4.274 | 1.904 | 1.538 |
| Tilia cordata | 0.561 | 6.716 | 3.968 | 2.296 | 1.576 |
| Tilia tomentosa | 0.657 | 9.620 | 5.759 | 3.419 | 1.909 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ražná, K.; Žiarovská, J.; Ivanišová, E.; Urbanová, L.; Harenčár, Ľ.; Kováčik, A.; Kučka, M.; Hrubík, P. Flowers Characteristics of Selected Species of Lime-Tree (Tilia spp.) in Terms of miRNA-Based Markers Activity, Mannose Expression and Biological Compounds Content. Forests 2021, 12, 1748. https://doi.org/10.3390/f12121748
Ražná K, Žiarovská J, Ivanišová E, Urbanová L, Harenčár Ľ, Kováčik A, Kučka M, Hrubík P. Flowers Characteristics of Selected Species of Lime-Tree (Tilia spp.) in Terms of miRNA-Based Markers Activity, Mannose Expression and Biological Compounds Content. Forests. 2021; 12(12):1748. https://doi.org/10.3390/f12121748
Chicago/Turabian StyleRažná, Katarína, Jana Žiarovská, Eva Ivanišová, Lucia Urbanová, Ľubomír Harenčár, Adam Kováčik, Matúš Kučka, and Pavel Hrubík. 2021. "Flowers Characteristics of Selected Species of Lime-Tree (Tilia spp.) in Terms of miRNA-Based Markers Activity, Mannose Expression and Biological Compounds Content" Forests 12, no. 12: 1748. https://doi.org/10.3390/f12121748
APA StyleRažná, K., Žiarovská, J., Ivanišová, E., Urbanová, L., Harenčár, Ľ., Kováčik, A., Kučka, M., & Hrubík, P. (2021). Flowers Characteristics of Selected Species of Lime-Tree (Tilia spp.) in Terms of miRNA-Based Markers Activity, Mannose Expression and Biological Compounds Content. Forests, 12(12), 1748. https://doi.org/10.3390/f12121748






