Mechanism of Dy3+ and Nd3+ Ions Electrochemical Coreduction with Ni2+, Co2+, and Fe3+ Ions in Chloride Melts
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrochemical Cell and Electrodes
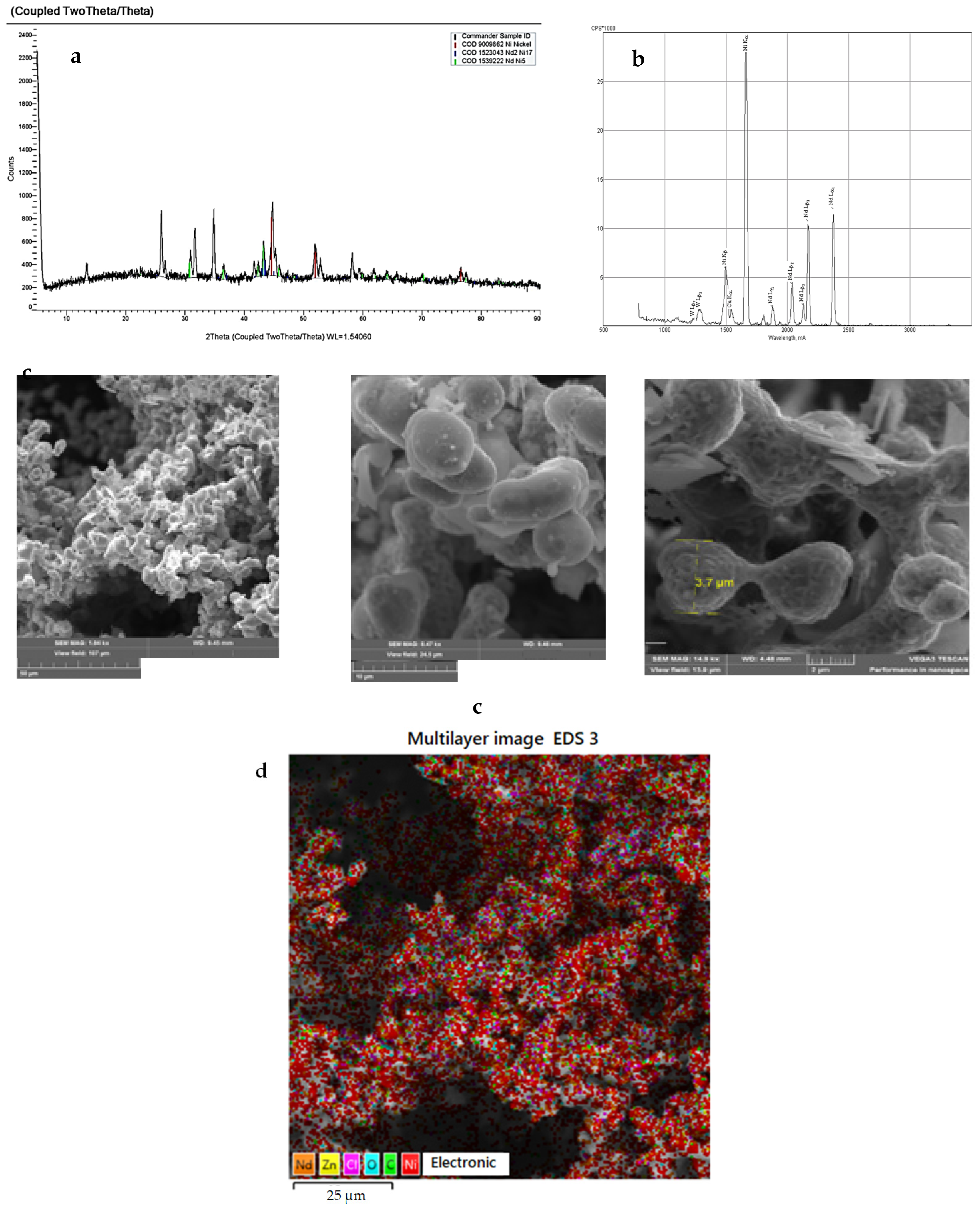
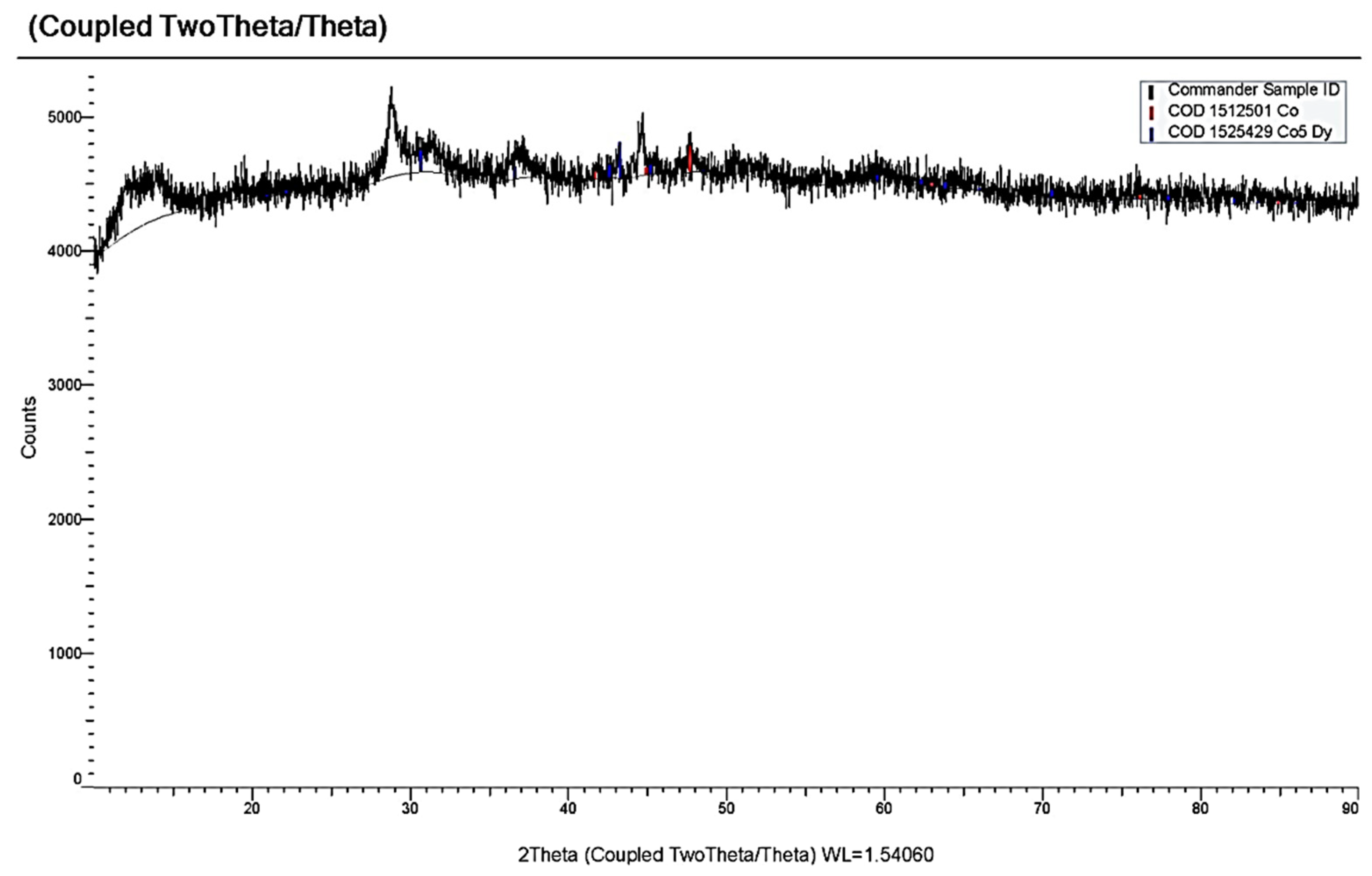
2.2. Methods for Analysis and Diagnostics of Cathode Deposits
2.3. Electrolyte Preparation
3. Results and Discussion
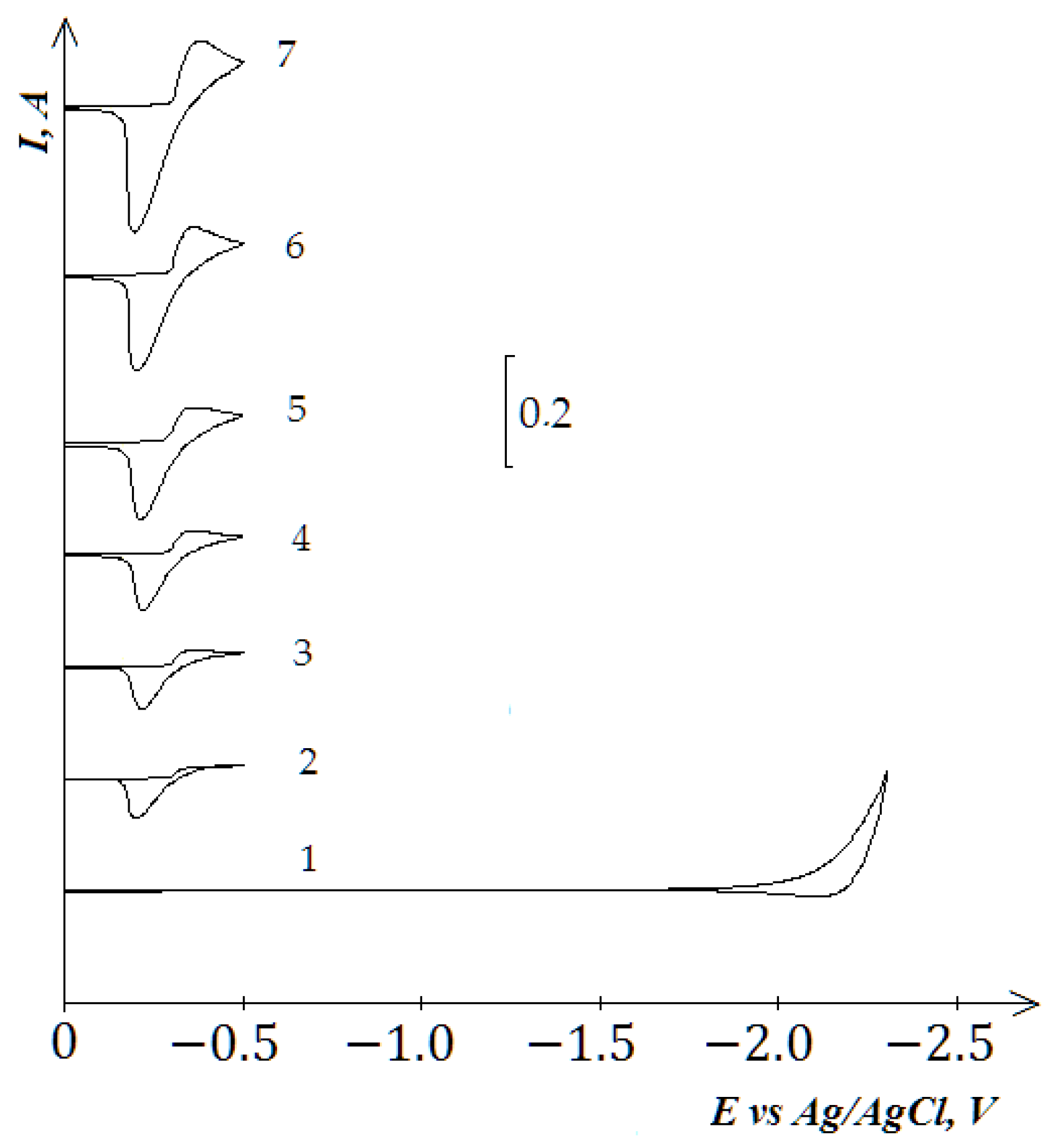
3.1. Electroreduction of Nd3+ and Dy3+ Ions in the KCl-NaCl Equimolar Melt at 973 K
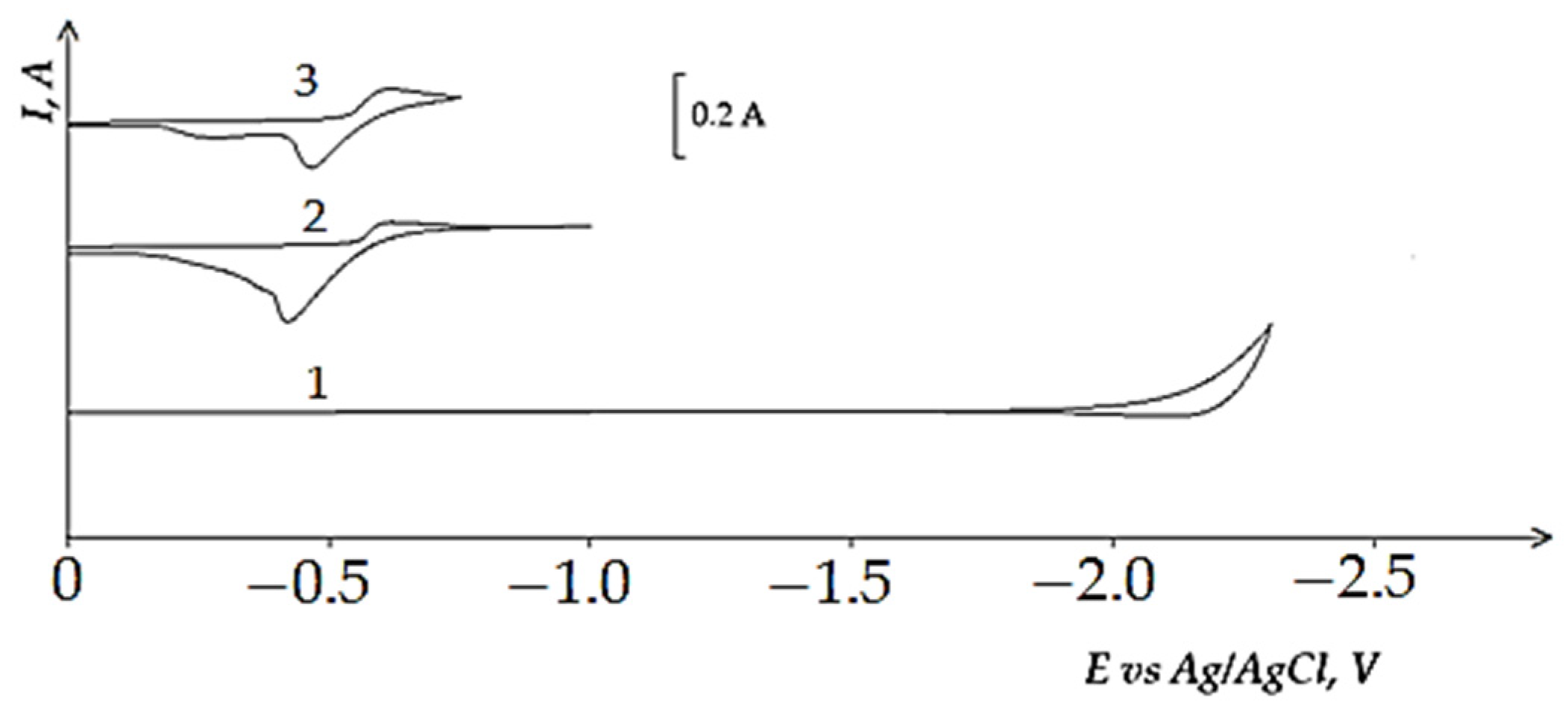
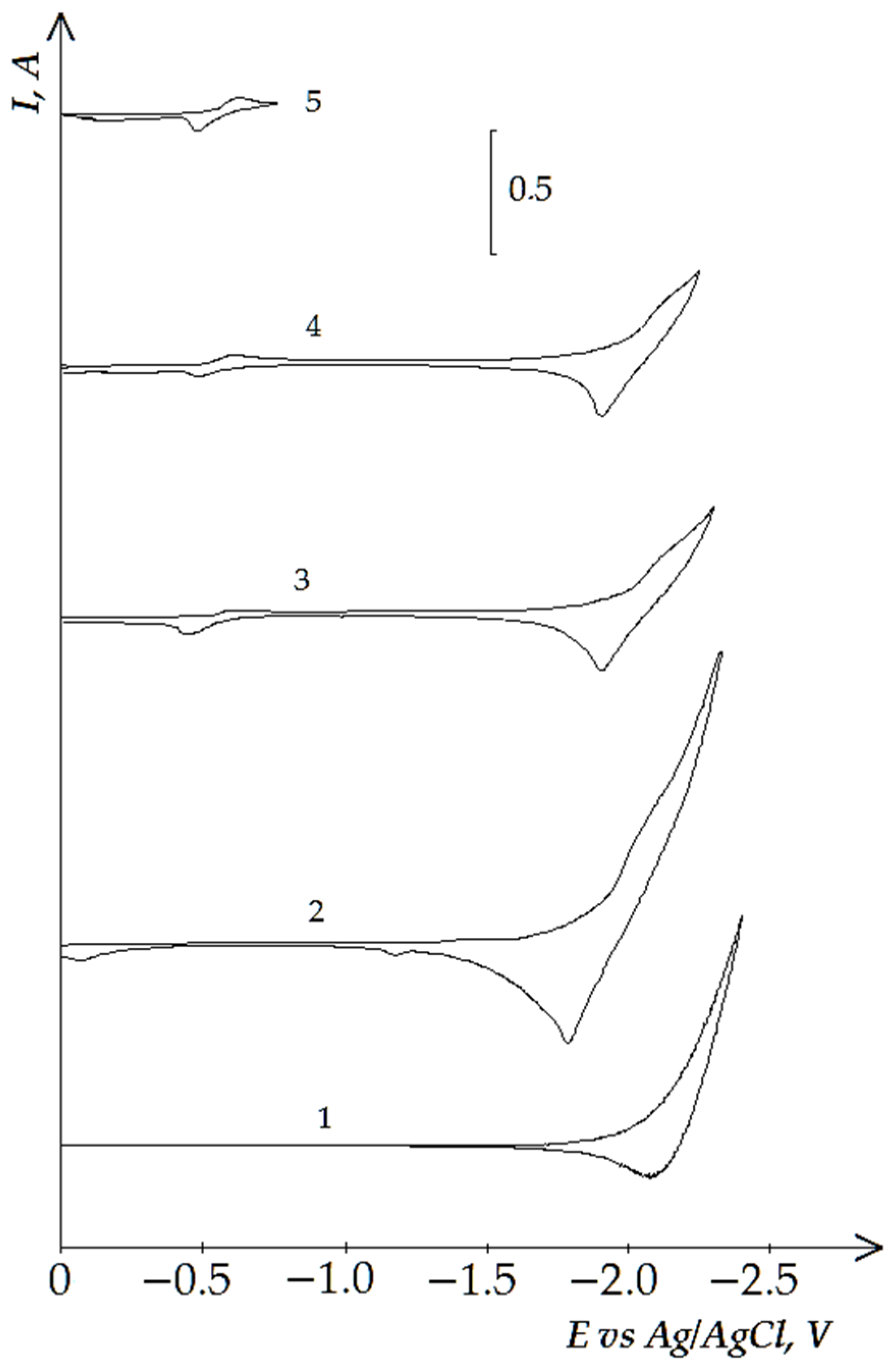
3.2. Electrochemical Reduction of the Iron Triad Metals in the KCl-NaCl Melt at 973 K
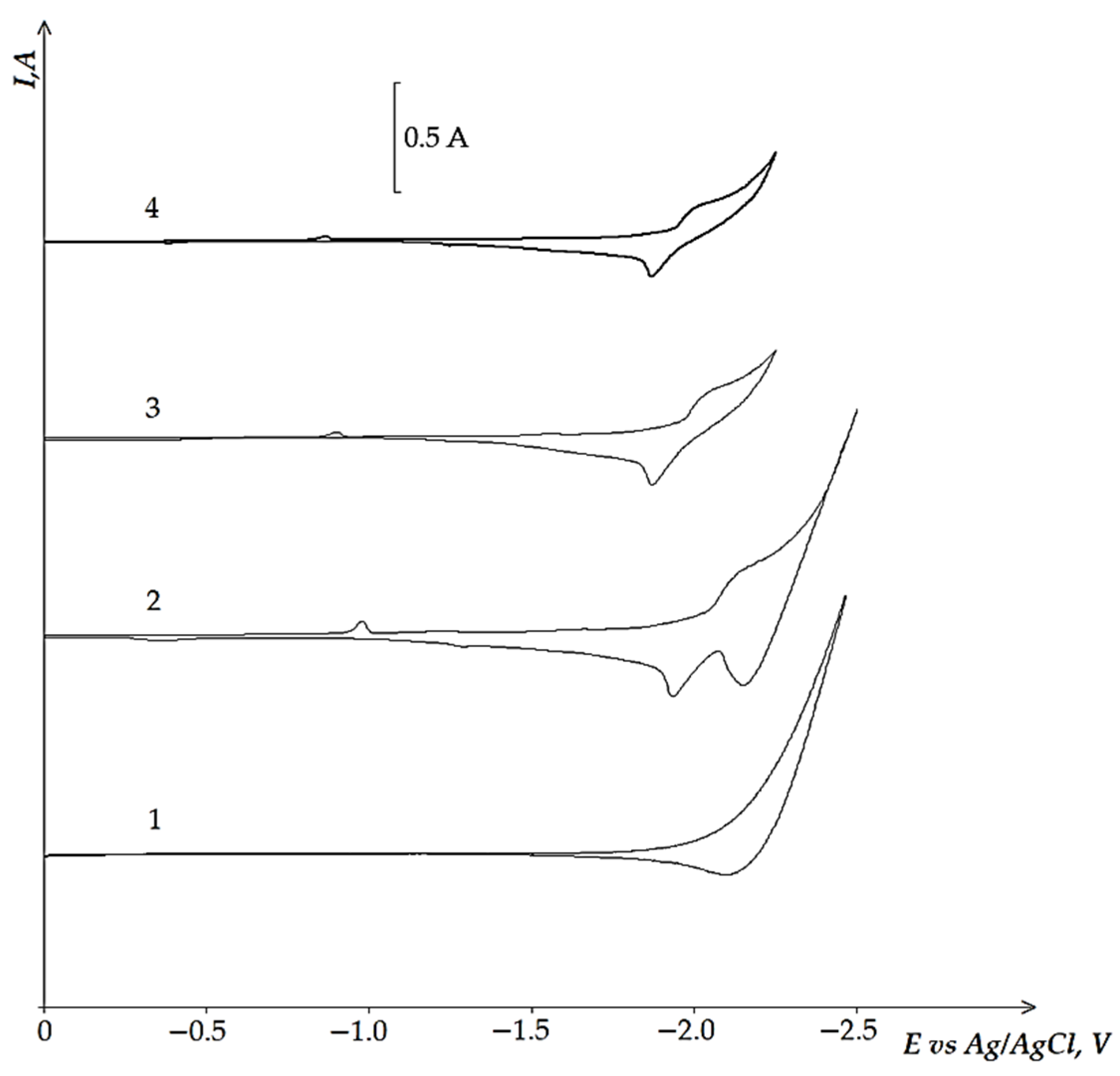
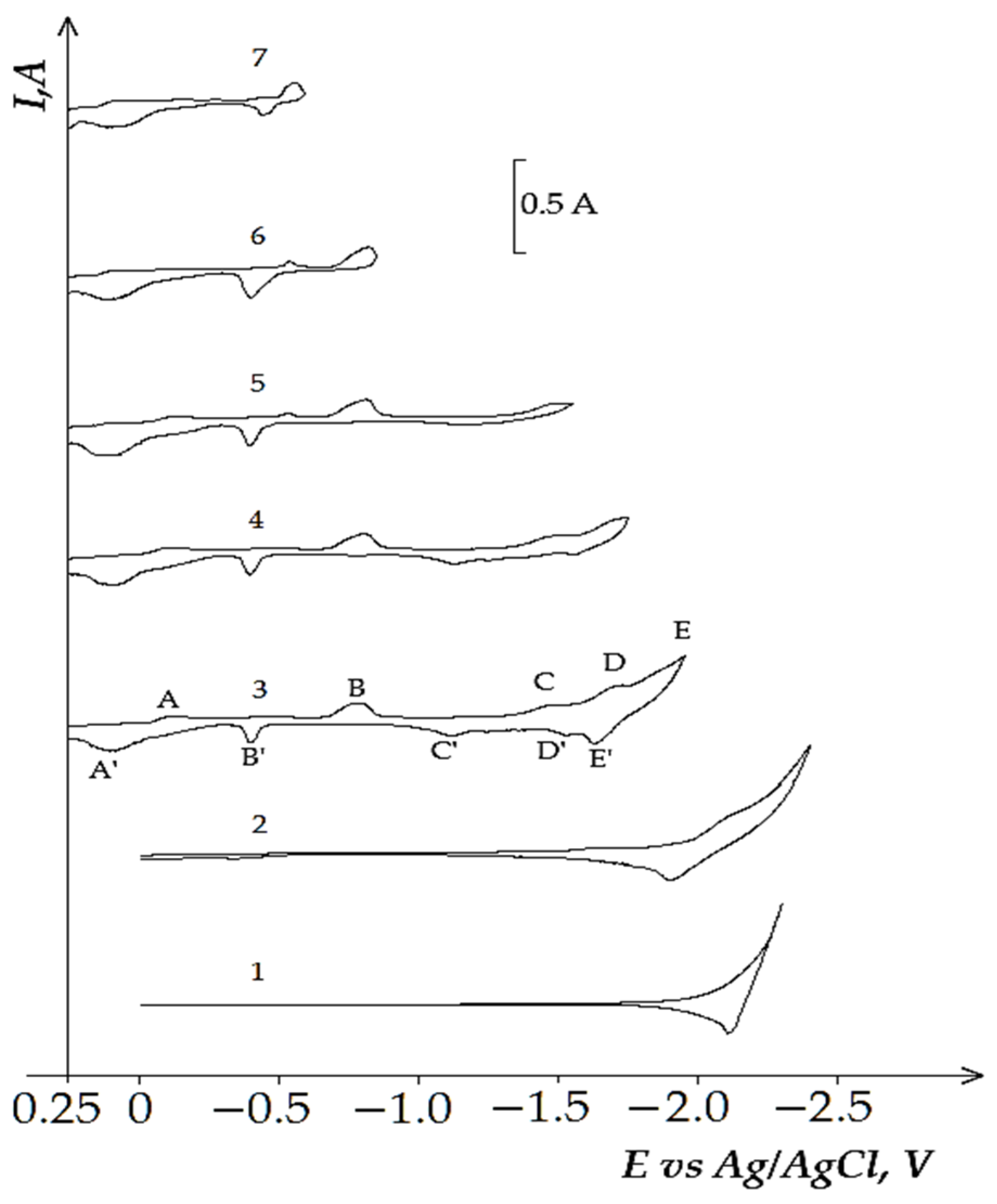
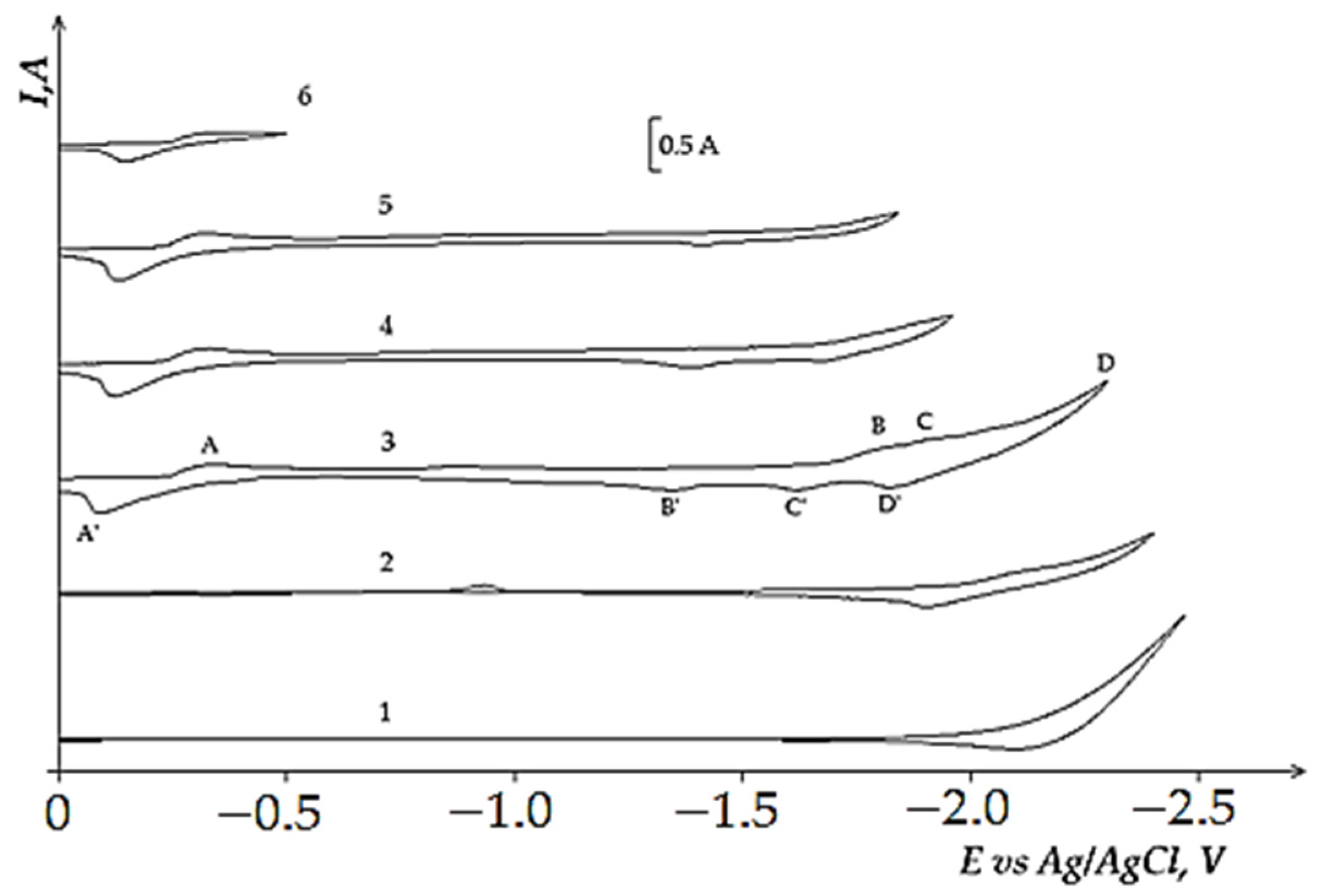
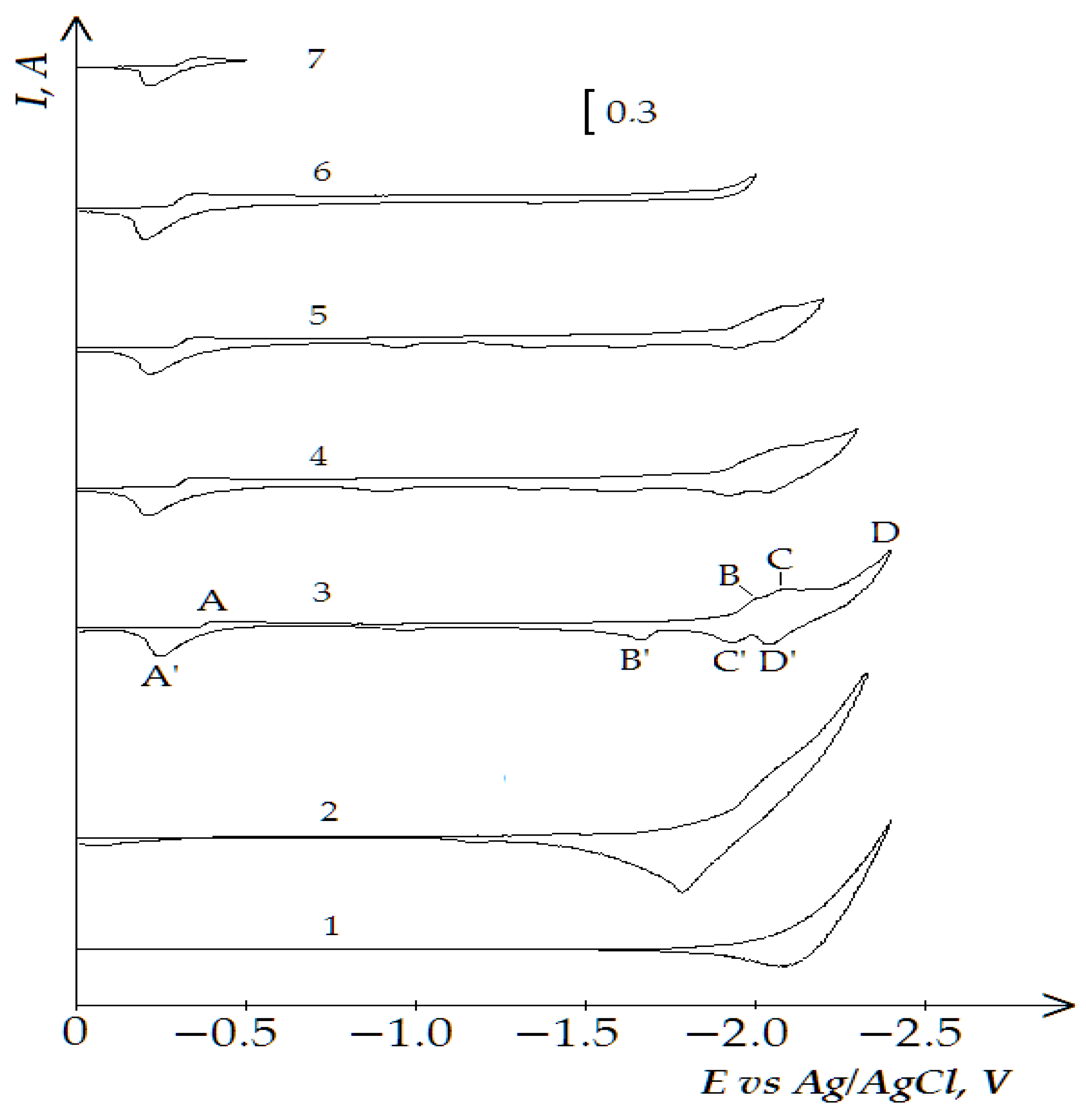
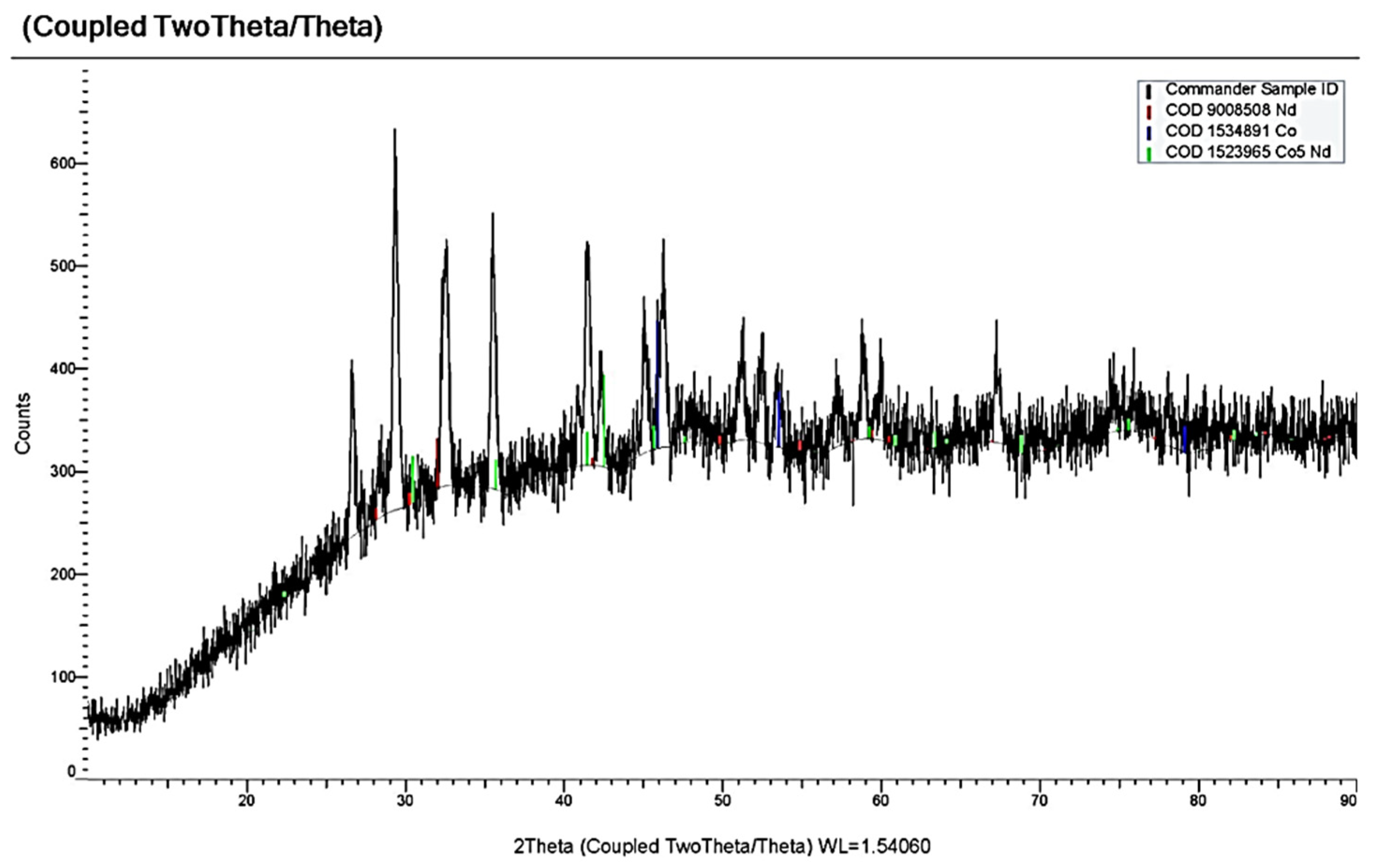
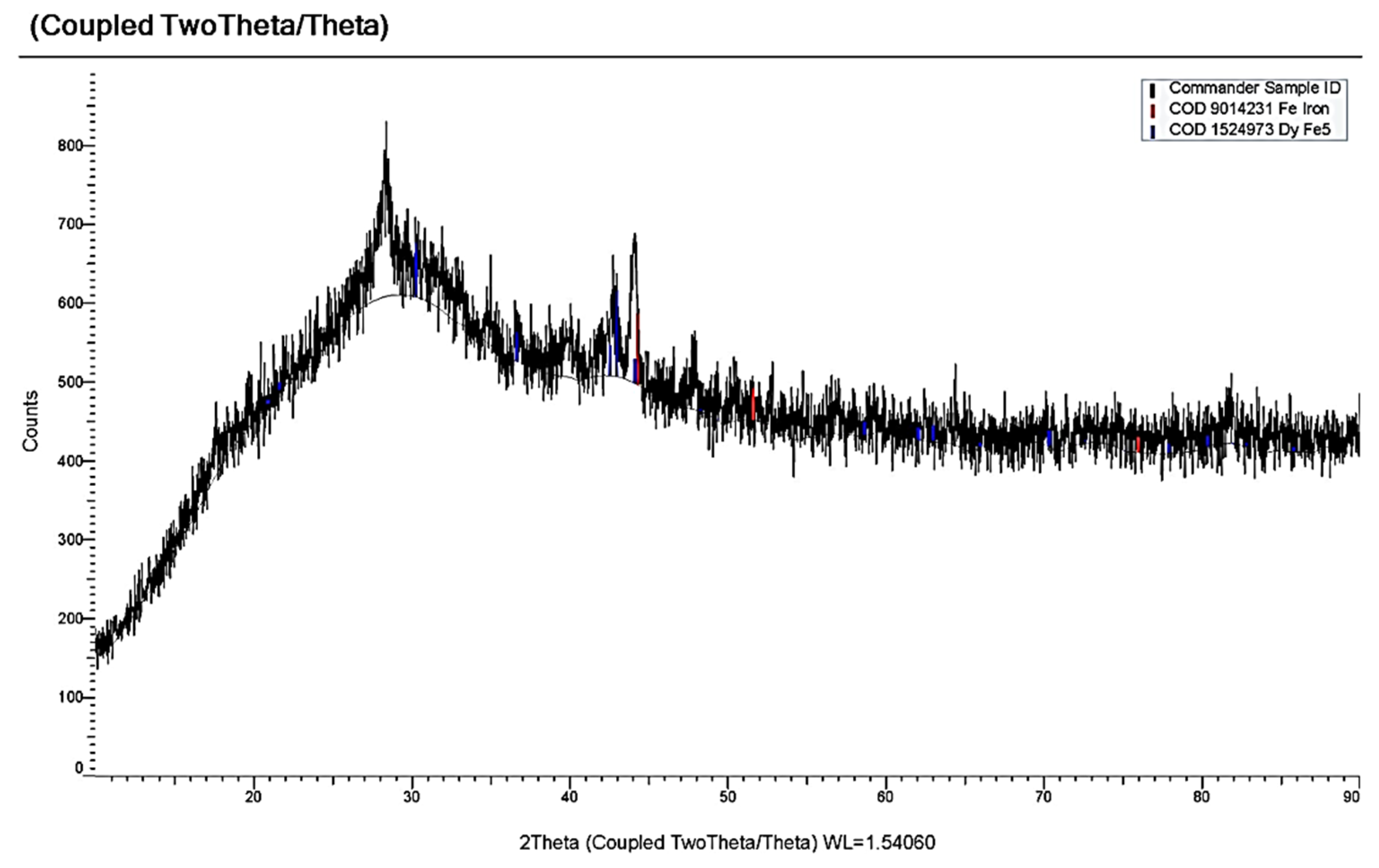
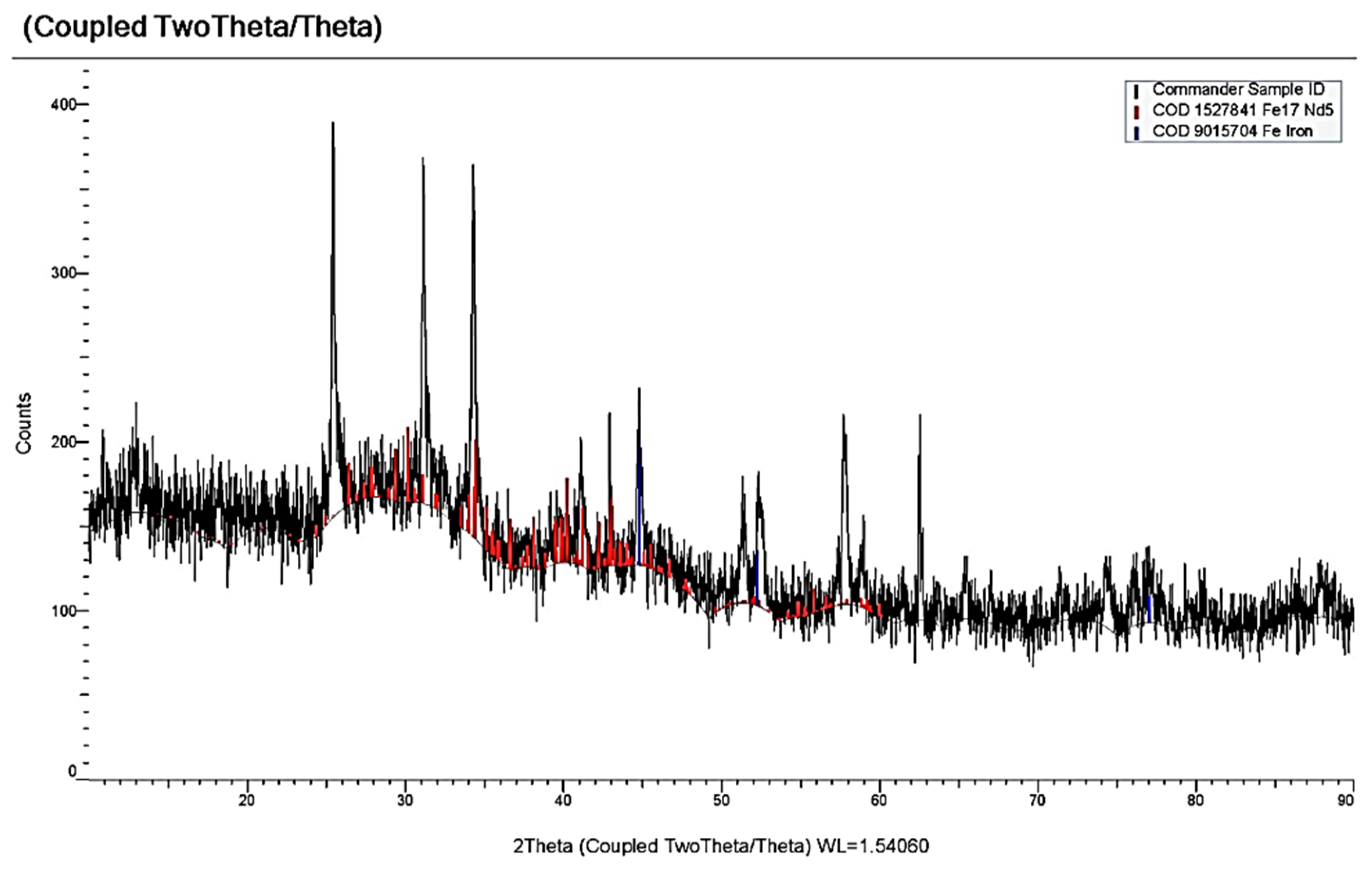
3.3. Electrochemical Coreduction of Nd and Dy Ions with Iron Triad Ions in the KCl-NaCl Melt at 973 K
- (i)
- First, measured portions of the NiCl2, CoCl2, and FeCl3 were added to the equimolar melt and the voltammetry dependencies of the electrochemical reduction process were recorded. Then, NdCl3 and DyCl3 were added to the iron triad containing KCl-NaCl melt and the voltammetry dependence was recorded considering their bulk concentration in the background electrolyte.
- (ii)
- NdCl3 and DyCl3 were first added to the equimolar KCl-NaCl melt, the voltammetry dependence of the molten KCl-NaCl-NdCl3 (DyCl3) system was recorded; then, iron triad ions were added to the previously formed mixture and the voltammograms of electrochemical coreduction were recorded.
- (iii)
- The calculated amount of the background electrolyte compounds KCl and NaCl (1:1), NdCl3 and DyCl3, as well as NiCl2, CoCl2, and FeCl3 were mixed in a dry powder form, put into a glassy carbon crucible and melted in the three-electrode cell in argon atmosphere of a dry box with continuously increasing temperature up to 973 K.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tamamura, H.; Aoyagi, M.; Wakamatsu, S. Technology of Molten Salts Electrolysis to Produce Lantanoid Metals and Alloys for Magnet Application on Industrial Manufacturing Basis. In Proceedings of the 6th International Symposium on Molten Salt Chemistry and Technology, Shanghai, China, 08–13 October 2001; pp. 36–43. [Google Scholar]
- Bolshakov, K.A. Khimiya y Technologiya redkikh y rasseyanykh elementov. In Chast 2 (Chemistry and technology of rare and Trace Elements. Part II); Vysshaya shkola: Moscow, Russia, 1976; 360p. [Google Scholar]
- Itin, V.I.; Nayborodenko, Y.S. Vysokotemperaturnye Syntez Intermetallicheskikh Soedineniy (High-Temperature Synthesis of Intermetallic Compounds); Tomskyi Universitet: Tomsk, Russia, 1989; 214p. [Google Scholar]
- Shapoval, V.I.; Malyshev, V.V.; Novoselova, I.A.; Kushkhov, K.B. Sovremenniye problem vysokotemperaturnogo sysnteza soedineniy perekhodnykh metallov IV-VI grup (Synthesis of high-temperature electrochemical synthesis of transient IV-VI group metals). Uspekhi Khimii 1989, 64, 133–141. [Google Scholar]
- Kovalevskyi, A.V.; Kondratyev, D.A. Obtaining of nickel and cobalt coating alloys by diffusion saturation with dysprosium in LiCl–KCl–DyCl3 melt. Izv. Vuzov. Poroshkovaya Metall. i Funktsional’nye Pokrytiya 2016, 3, 51–57. [Google Scholar] [CrossRef][Green Version]
- Kovalevskiy, A.V.; Ilushenko, N.G.; Varkin, V.N.; Sorokina, V.V. Difusionnoye nasysheniye nickely y kobalta circoniyem, lantanom y itriyem v galogenidnykh rasplavah (Diffusion saturation of nickel and cobalt by zirconium, lanthanum and yttrium in halide melts). Izv. Vyzov. Tsvetnaya Metall. 1988, 5, 20–22. [Google Scholar]
- Konishi, H.; Nohira, T.; Ito, Y. Formation of Dy-Fe alloys films by molten salt electrochemical process. Electrochim. Acta 2002, 47, 3533–3539. [Google Scholar] [CrossRef]
- Guankun, L.; Yexiang, T.; Huichan, H. Eelectrochemical investigation on the Formation of Dy-Fe Alloy in molten chlorides. J. Rare Earths 1997, 4, 271–275. [Google Scholar]
- Konishi, H.; Nishikiori, T.; Nohira, T. Thermodynamic properties of Dy –Ni intermetallic compounds. Electrochim. Acta 2003, 48, 1403–1408. [Google Scholar] [CrossRef]
- Konishi, H.; Nohira, T. Morphology control of Dy-Ni alloy films by electrochemical displantation. Electrochem. Solid-State Lett. 2002, 5, 37–39. [Google Scholar] [CrossRef]
- Konishi, H.; Nohira, T.; Ito, Y. Kinetics of DyNi2 film growth by electrochemical implantation. Electrochimica Acta 2003, 48, 563–568. [Google Scholar] [CrossRef]
- Konishi, H.; Usui, T.; Nohira, T. Electrochemical formation of Dy alloy films in a molten LiCl-KCl-DyCl3 system. J. Phys. Conf. Ser. 2009, 165, 012060. [Google Scholar] [CrossRef]
- Yasuda, K.; Kobayashi, S.; Nohira, T. Electrochemical formation of Dy-Ni alloys in molten NaCl-KCl-DyCl3. Electrochim. Acta 2013, 106, 293–300. [Google Scholar] [CrossRef]
- Edwar, M. Molten salts electrowinning of rare-earth and yttrium metals and alloys. In Proceedings of the International Conference on Rare Earth-Development and Applications, Bejing, China, 10–14 September 1985; pp. 1099–1106. [Google Scholar]
- Vindigeva, M.K.; Mukozheva, R.A.; Tlenkopachev, M.R.; Kushkhov, H.B. Electrokhimicheskiy sintez intermetallidaov na osnove samariya y kobalta v ionnykh rasplavah (Electrochemical synthesis of samarium and cobalt based intermetallic compounds in ionic melts). Perspect. Mater. 2010, 9, 255–257. [Google Scholar]
- Kushkhov, H.B.; Vindizheva, M.K.; Mukozheva, R.A.; Kalibatova, M.N. Electrochemical synthesis of ultrafine intermetallic powders based on lanthanum and cobalt from chloride melts. Izv. Vyzov. Poroshkovaya Metall. y Funktsionalnye Pokrytiya 2014, 2, 11–16. [Google Scholar] [CrossRef][Green Version]
- Kushkhov, H.B.; Vindigeva, M.K.; Mukozheva, R.A.; Abazova, A.K.; Kyrova, Z.H. Issledovaniya processa sovmestnogo electrovosstanovleniya ionov tseriya s ionami cobalta y bora y electrokhimicheskiy sintez dvoinykh boridov tseriya y cobalta iz galogenidnykh rasplavov (Study of the process of mutual electroreduction of cerium, cobalt and boron ions and electrochemical synthesis of the double cerium and cobalt borides from halide melts). Izv. Kabard. -Balkar. Gos. Univ. 2016, 1, 52–59. [Google Scholar]
- Liu, L.; Tong, Y.; Yang, Q. Electroreduction Co(II), Ni(II), and codeposition with La(III) in in urea-NaBr melt. Rare Metals 2000, 19, 237–241. [Google Scholar]
- Kushkhov, H.B.; Kardanova, R.A. Electrochemical Sunthesis of Nanosized Powders of Holmium and Nickel Intermetallic Compounds in Halide Melts. RF Patent № RU2621508C2, 6 June 2017. [Google Scholar]
- Su, L.L.; Liu, K.; Liu, Y.L.; Wang, L.; Yuan, L.Y.; Wang, L.; Li, Z.J.; Zhao, X.L.; Chai, Z.F.; Shi, W.Q. Electrochemical behaviors of Dy(III) and its co-reduction with Al(III) in molten LiCl−KCl salts. Electrochim. Acta 2014, 147, 87–95. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.L.; Yuan, L.Y.; Wang, L.; Wang, L.; Li, Z.J.; Chai, Z.F.; Shi, W.Q. Thermodynamic and electrochemical properties of holmium and HoxAly intermetallic compounds in the LiCl−KCl eutectic. Electrochim. Acta 2015, 174, 15–25. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Yan, Y.-D.; Zhang, M.-L.; Zheng, J.-N.; Zhao, Y.; Wang, P.; Yin, T.-Q.; Yun Xue, Y.; Jing, X.-Y.; Han, W. Electrochemical syntheses of Sm-Ni alloy magnetic materials by co-reduction of Sm(III) and Ni(II) in LiCl-KCl -SmCl3-NiCl2 melt. J. Electrochem. Soc. 2016, 163, D672–D681. [Google Scholar] [CrossRef]
- Kushkhov, K.B.; Tlenkopachev, M.R. Electrochemical synthesis of intermetallic and refractory compounds based on rare-earth metals in ionic melts: Achievements and prospects. Curr. Top. Electrochem. 2020, 22, 57–77. [Google Scholar] [CrossRef]
- Kushkhov, K.B.; Tlenkopachev, M.R. Electrochemical Synthesis of Magnetic Materials Based on Intermetallic and Refractory Compounds of Rare-Earth Metals in Ionic Melts: Current State of Research and Directions of Development. Newest Updates in Physical Science Research. 2021, 12, 137–165. [Google Scholar]
- Yin, T.-Q.; Xue, Y.; Yan, Y.; Ma, Z.C.; Ma, F.-Q.; Zhang, M.-L.; Wang, G.-L.; Qiu, M. Recovery and separation of rare earth elements by molten salt electrolysis. Int. J. Miner. Metall. Mater. 2021, 28, 899–914. [Google Scholar] [CrossRef]
- Kushkhov, K.B.; Zhanikaeva, Z.A.; Chuksin, S.I. Electroreduction of ions of neodymium in chloride melts. Rasplavy 2009, 3, 50–59. [Google Scholar]
- Kushkhov, K.B.; Chuksin, S.I.; Zhanikaeva, Z.A. Electroreduction of neodymium and praseodymium ions in equimolar KCl-NaCl and eutectic KCl-NaCl-CsCl melts at a tungsten electrode. Rasplavy 2013, 8, 610–616. [Google Scholar] [CrossRef]
- Kushkhov, K.B.; Uzdenova, A.S.; Saleh, M.M.A.; Qahtan, A.M.F.; Uzdenova, L.A. The Electroreducion of Gadolinium and Dysprosium Ions in Eqiumolar NaCl-KCl Melt. Am. J. Analyt. Chem. 2013, 4, 39–46. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980; 850p. [Google Scholar]
- Scholz, F. Electroanalytical Methods: Guide to Experiments and Applications, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; 359p. [Google Scholar]
- Volkov, S.V.; Grishenko, V.F.; Delimarskyi, Y.K. Koordinatsionnaya Khimiya Solevykh Rasplavov (Coordination Chemistry of Molten Salts; Naukova Dumka: Kyiv, Ukraine, 1977; 332p. (In Russian) [Google Scholar]
- Sytchev, J.; Kushkhov, H.; Sychev, J. Voltammetric investigation of the reduction processes of nickel cobalt and iron ions in chloride and chloro—fluoride melts. In Proceedings of the International Computer Science Conference, Miskolc, Hungary, 23–24 February 2000; p. 69. [Google Scholar]
- Kushkhov, K.B.; Supatashvili, D.G.; Shapoval, V.I.; Novoselova, V.I.; Gasviana, N.A. Sovmestnoye electrovosstanovleniye molybdat iona s kationamy Ni y Co v khloridnykh rasplavakh (Mutual electroreduction of molybdat-ion with Ni and Co cations in chloride melts. Electrokhimiya 1990, 26, 300–304. (In Russian) [Google Scholar]
- Lyakishev, N.P. Diagramma sostoyaniya dvoynykh metallicheskihk sistem. In Tom 2 (Diagrams of Double Metallic Systems: Reference Book); Mashinostroyeniye: Moscow, Russia, 1997; Volume 2, p. 1024. (In Russian) [Google Scholar]
- Massalski, T.B. Binary Alloy. Phase Diagrams, 2nd ed.; Okamoto, H., Subramanian, P.R., Kacprzak, L., Eds.; ASM International: Materials Park, OH, USA, 1990; Volume 2, 3589p. [Google Scholar]
- Nourry, C.; Massot, L.; Chemelot, P.; Taxil, P. Formation of Ni-Nd alloys by Nd(|||) Electrochemical Reduction Molten Fluoride. J. Mater. Electrochem. Syst. 2007, 10, 117–122. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushkhov, K.; Ali, Z.; Khotov, A.; Kholkina, A. Mechanism of Dy3+ and Nd3+ Ions Electrochemical Coreduction with Ni2+, Co2+, and Fe3+ Ions in Chloride Melts. Materials 2021, 14, 7440. https://doi.org/10.3390/ma14237440
Kushkhov K, Ali Z, Khotov A, Kholkina A. Mechanism of Dy3+ and Nd3+ Ions Electrochemical Coreduction with Ni2+, Co2+, and Fe3+ Ions in Chloride Melts. Materials. 2021; 14(23):7440. https://doi.org/10.3390/ma14237440
Chicago/Turabian StyleKushkhov, Khasbi, Zhubagi Ali, Astemir Khotov, and Anna Kholkina. 2021. "Mechanism of Dy3+ and Nd3+ Ions Electrochemical Coreduction with Ni2+, Co2+, and Fe3+ Ions in Chloride Melts" Materials 14, no. 23: 7440. https://doi.org/10.3390/ma14237440
APA StyleKushkhov, K., Ali, Z., Khotov, A., & Kholkina, A. (2021). Mechanism of Dy3+ and Nd3+ Ions Electrochemical Coreduction with Ni2+, Co2+, and Fe3+ Ions in Chloride Melts. Materials, 14(23), 7440. https://doi.org/10.3390/ma14237440




