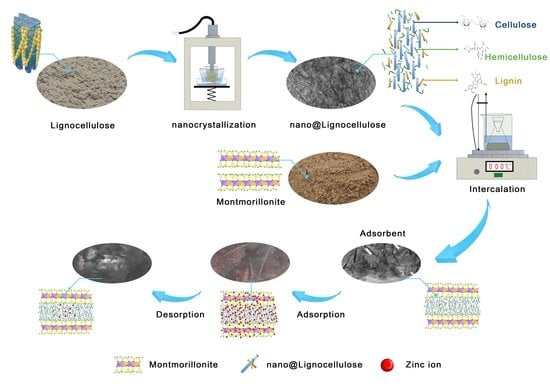
Lignocellulose-Based Superabsorbent Polymer Gel Crosslinked with Magnesium Aluminum Silicate for Highly Removal of Zn (II) from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the LCE-MOT Polymer Gel
2.3. Preparation of Nano-LCE
2.4. Preparation of the Nano-LCE-MOT Polymer Gel
2.5. Adsorption Experiments
2.6. Desorption Experiments
3. Results
3.1. Characterisation of the Materials
3.1.1. N2 Adsorption–Desorption Isotherms of the Nano-LCE-MOT Polymer Gel
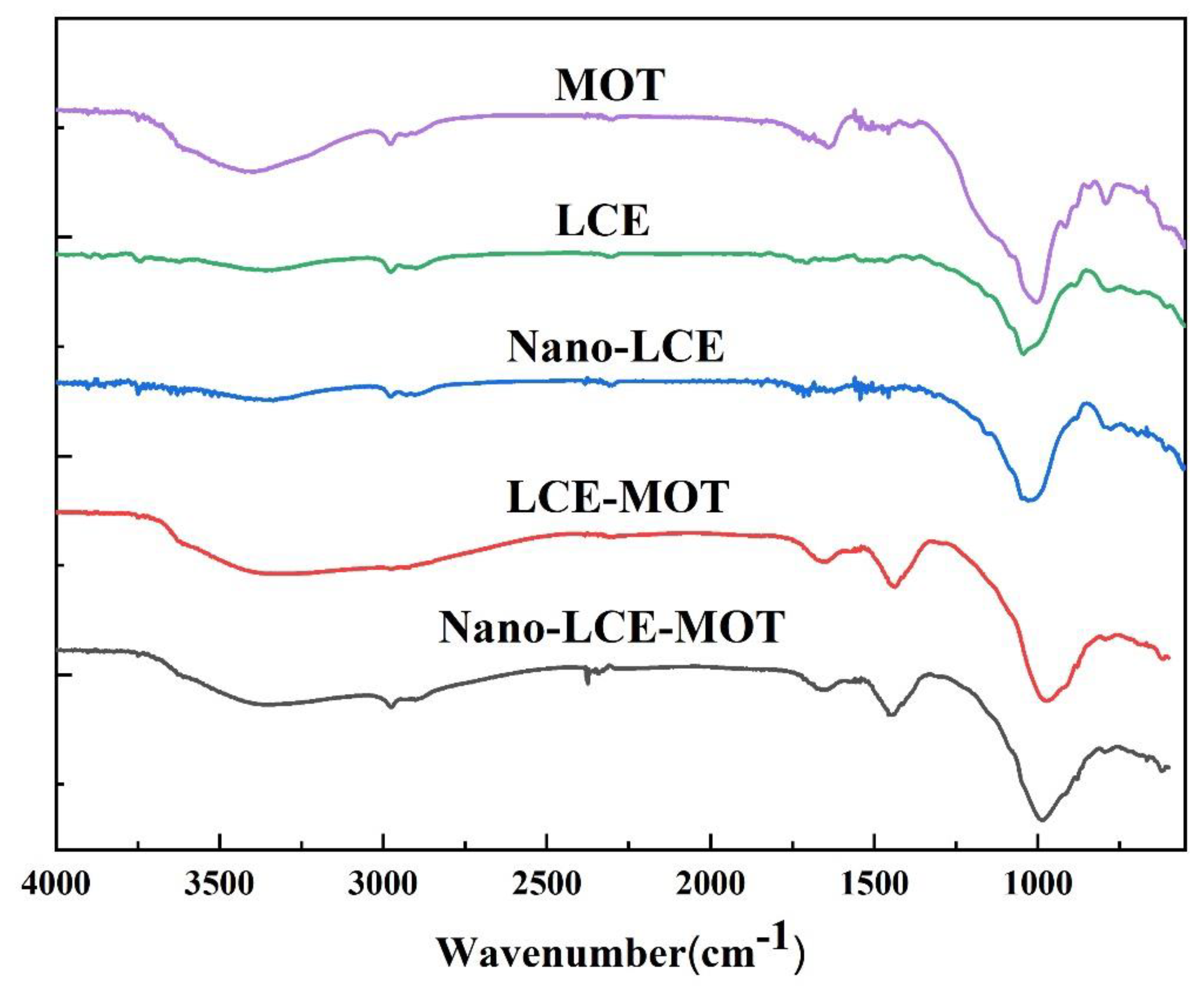
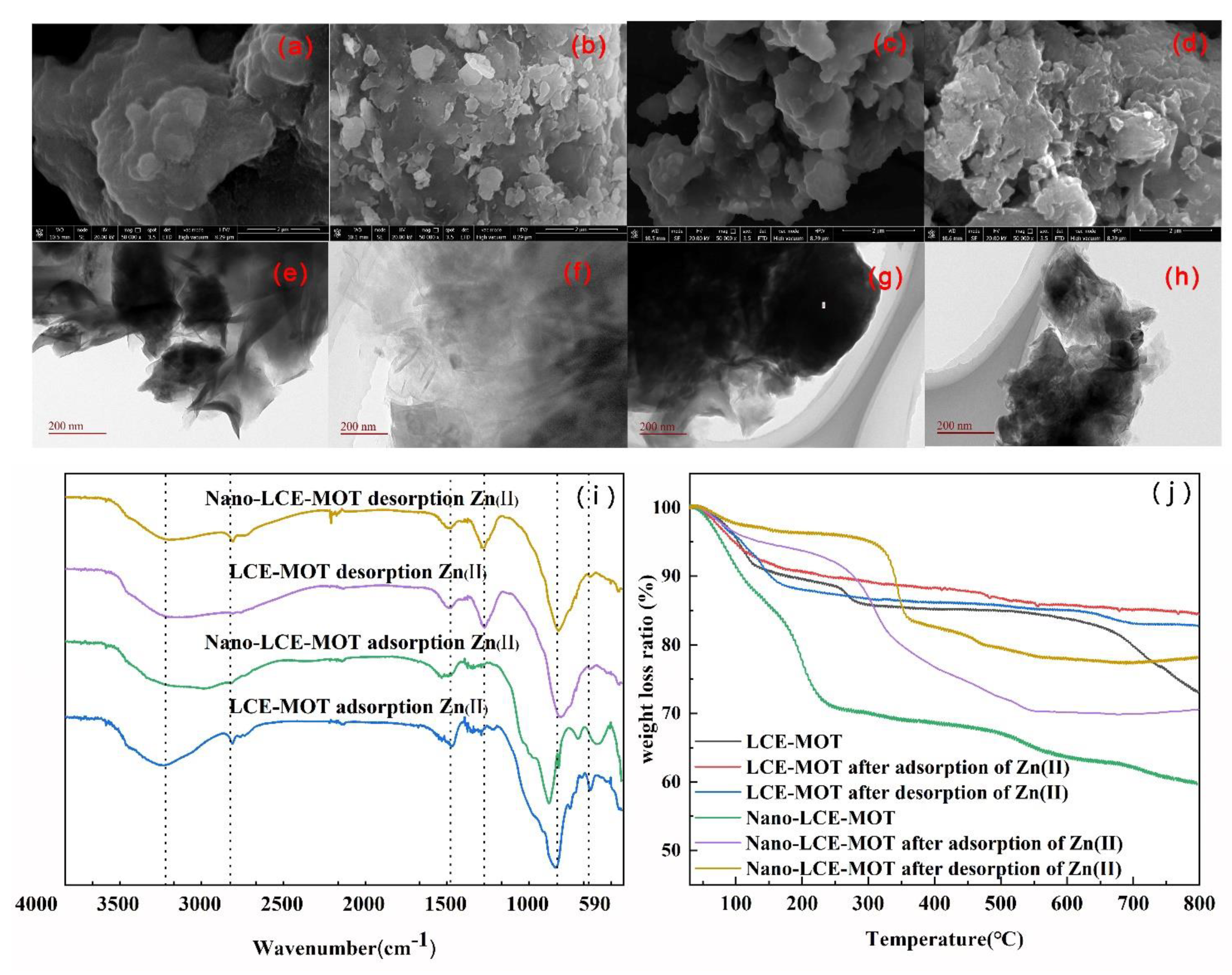
3.1.2. FTIR Analysis of the Materials
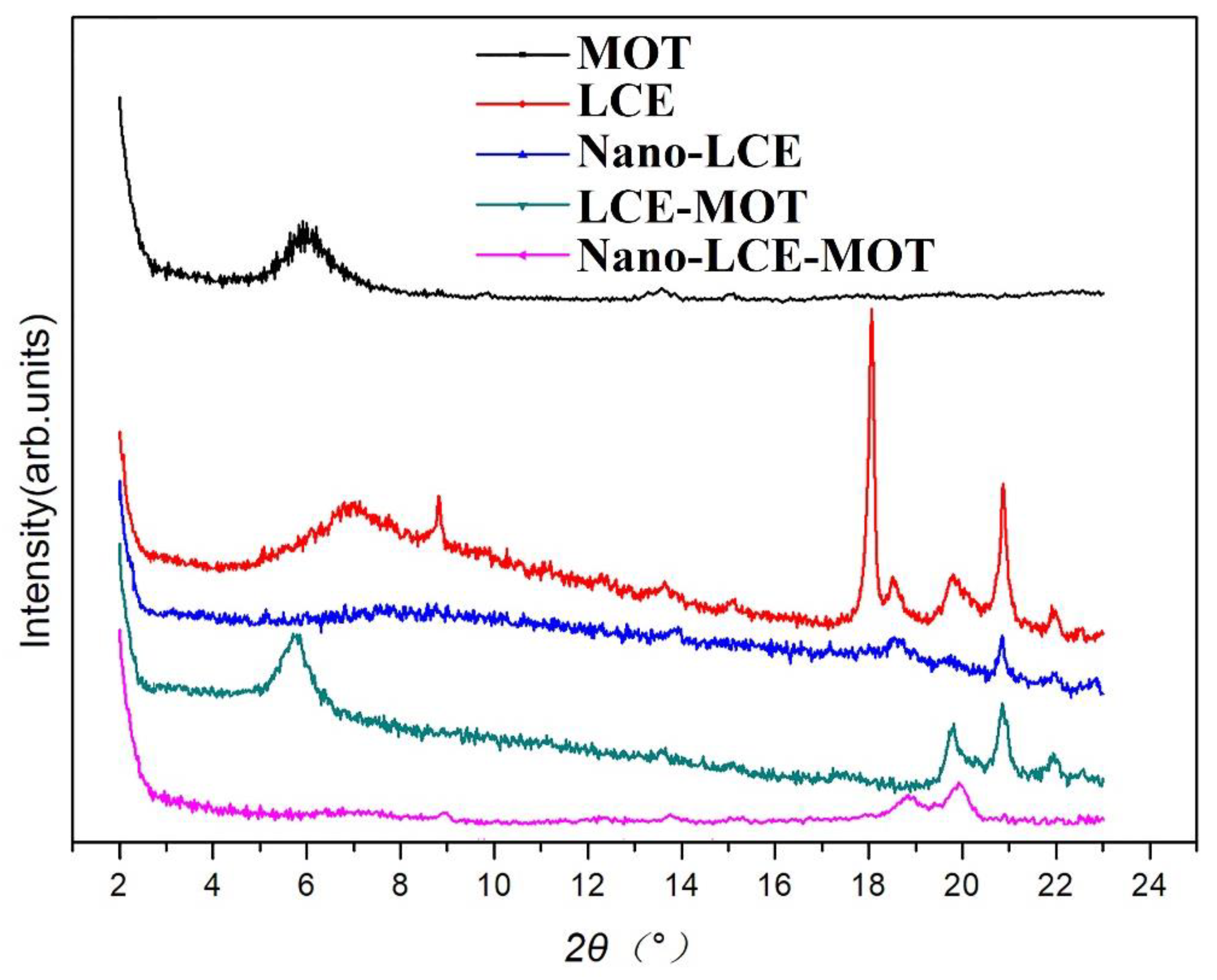
3.1.3. PXRD Analysis of the Materials
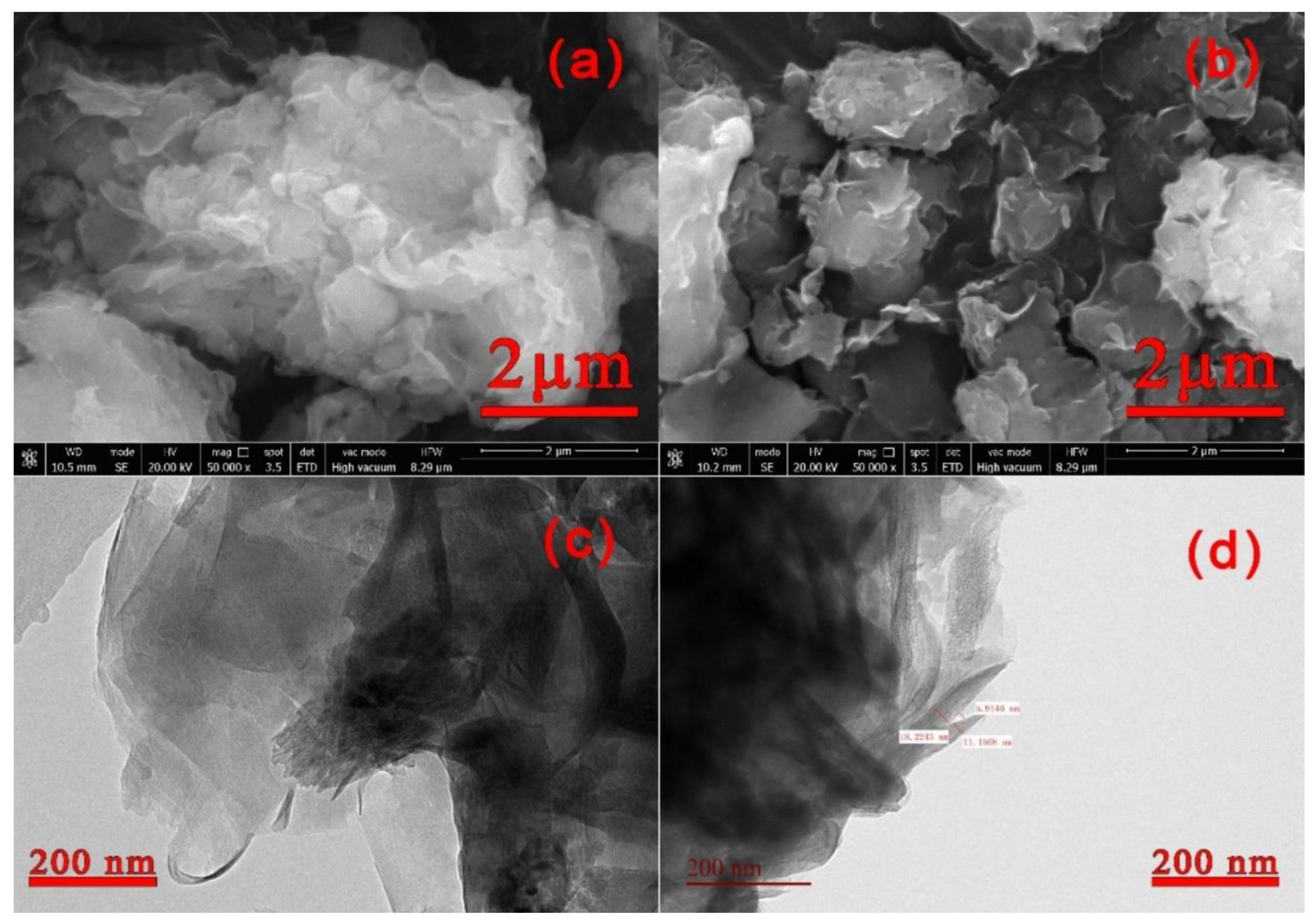
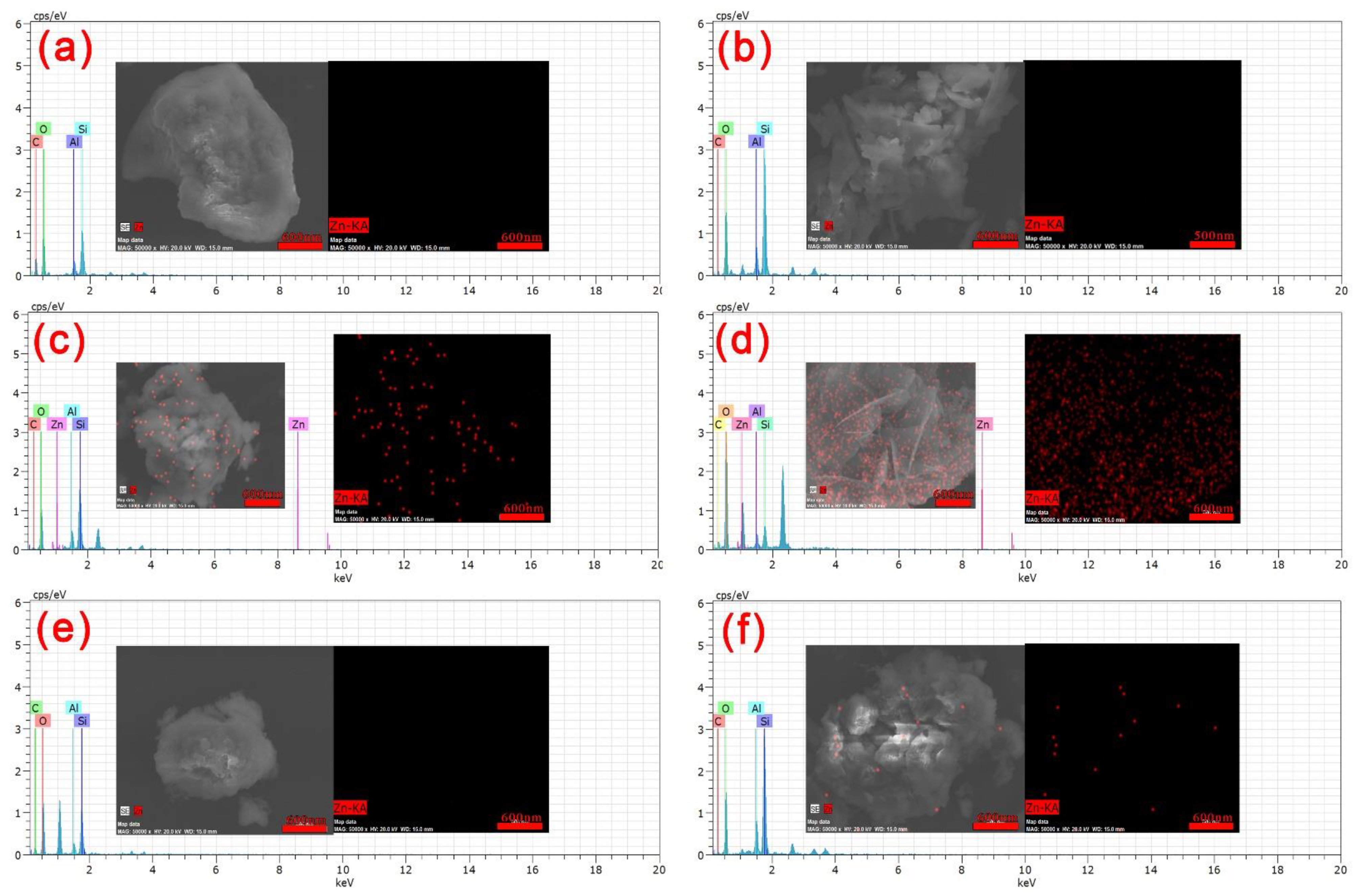
3.1.4. SEM Analysis of Nano-LCE-MOT and LCE-MOT
3.1.5. TEM Analysis of Nano-LCE-MOT and LCE-MOT
3.2. Adsorption Studies
3.2.1. Effect of the Initial Zn (II) Concentration
3.2.2. Effect of pH
3.2.3. Effect of Adsorption Temperature
3.2.4. Effect of Adsorption Time
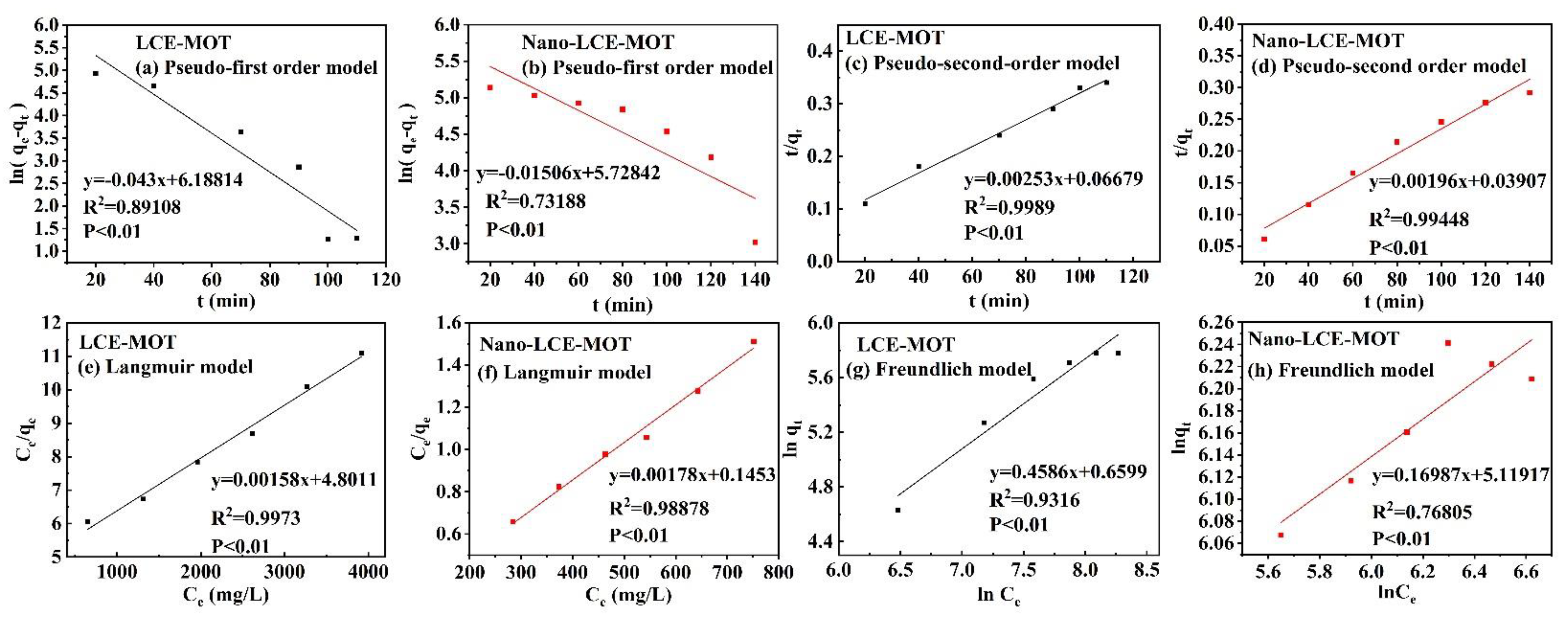
3.2.5. Adsorption Kinetics and Mechanism
3.2.6. Adsorption Modelling
3.3. Desorption and Regeneration Studies
3.3.1. Effects of Various Desorption Reagents
3.3.2. Effects of Desorption Agent Concentration
3.3.3. Effects of Desorption Temperature
3.3.4. Effects of Desorption Time
3.3.5. Regeneration and Reuse
3.3.6. Adsorption Capacity of Lignocellulose-Based Materials to Zn (II)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volesky, B.; Holan, Z.R. Biosorption of heavy metals. Biotechnol. Prog. 1995, 11, 235–250. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Bano, A.; Hussain, J.; Akbar, A.; Mehmood, K.; Anwar, M.; Hasni, M.S.; Ullah, S.; Sajid, S.; Ali, I. Biosorption of heavy metals by obligate halophilic fungi. Chemosphere 2018, 199, 218–222. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P. Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—an agricultural waste. Water Res. 2002, 36, 2304–2318. [Google Scholar] [CrossRef]
- Kongsricharoern, N.; Polprasert, C. Chromium removal by a bipolar electro-chemical precipitation process. Water Sci. Technol. 1996, 34, 109–116. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, H.; Sun, Y.; Zhao, C.; Zhou, Y.; Tang, X.; Zhao, C. A combined process of chemical precipitation and flocculation for treating phosphating wastewater. Desalination Water Treat. 2016, 57, 25520–25531. [Google Scholar] [CrossRef]
- Chukwu, U.J.; Uzoukwu, B.A. Synergistic extraction of Zn2+ from aqueous buffer medium of chloride ions into 1- Phenyl -3- Methyl -4- trichloro acetyl pyrazolone -5 in benzene and hexane. Recent Patents Mater. Sci. 2010, 3, 2. [Google Scholar] [CrossRef]
- Tang, W.; Zeng, G.; Gong, J.; Liang, J.; Xu, P.; Zhang, C.; Huang, B. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total Environ. 2014, 468, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Cherif, A.T.; Elmidaoui, A.; Gavach, C. Separation of Ag+, Zn2+ and Cu2+ ions by electrodialysis with monovalent cation specific membrane and EDTA. J. Membr. Sci. 1993, 76, 39–49. [Google Scholar] [CrossRef]
- Cherif, A.T.; Gavach, C.; Molenat, J.; Elmidaoui, A. Transport and separation of Ag + and Zn 2+ by donnan dialysis through a monovalent cation selective membrane. Talanta 1998, 46, 1605–1611. [Google Scholar] [CrossRef]
- Pociecha, M.; Kastelec, D.; Lestan, D. Electrochemical EDTA recycling after soil washing of Pb, Zn and Cd contaminated soil. J. Hazard. Mater. 2011, 192, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Ipek, U. Removal of Ni (II) and Zn (II) from an aqueous solutionby reverse osmosis. Desalination 2005, 174, 161–169. [Google Scholar] [CrossRef]
- Ujang, Z.; Anderson, G.K. Application of low-pressure reverse osmosis membrane for Zn2+ and Cu2+ removal from wastewater. Water Sci. Technol. 1996, 34, 247–253. [Google Scholar] [CrossRef]
- Franco, P.E.; Veit, M.T.; Borba, C.E.; da Cunha Gonçalves, G.; Fagundes-Klen, M.R.; Bergamasco, R.; da Silva, E.A.; Suzaki, P.Y.R. Nickel (II) and zinc (II) removal using Amberlite IR-120 resin: Ion exchange equilibrium and kinetics. Chem. Eng. J. 2013, 221, 426–435. [Google Scholar] [CrossRef]
- Ostroski, I.C.; Barros, M.A.S.D.; Silva, E.A.; Dantas, J.H.; Arroyo, P.A.; Lima, O.C.M. A comparative study for the ion exchange of Fe (III) and Zn (II) on zeolite NaY. J. Hazard. Mater. 2009, 161, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.K.; Mandal, S.N.; Das, S.K. Adsorption of Zn (II) from aqueous solution by using different adsorbents. Chem. Eng. J. 2006, 123, 43–51. [Google Scholar] [CrossRef]
- Bereket, G.; Arog, A.Z.; Özel, M.Z. Removal of Pb (II), Cd (II), Cu (II), and Zn (II) from Aqueous Solutions by Adsorption on Bentonite. J. Colloid Interface Sci. 1997, 187, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Kubilay, Ş.; Gürkan, R.; Savran, A.; Şahan, T. Removal of Cu (II), Zn (II) and Co (II) ions from aqueous solutions by adsorption onto natural bentonite. Adsorption 2007, 13, 41–51. [Google Scholar] [CrossRef]
- Juang, R.; Shao, H. Effect of pH on competitive adsorption of Cu(II), Ni(II), and Zn (II) from water onto chitosan beads. Adsorption 2002, 8, 71–78. [Google Scholar] [CrossRef]
- Pranata Putra, W.; Kamari, A.; Najiah Mohd Yusoff, S.; Fauziah Ishak, C.; Mohamed, A.; Hashim, N.; Md Isa, I. Biosorption of Cu (II), Pb (II) and Zn (II) ions from aqueous solutions using selected waste materials: Adsorption and characterisation studies. J. Encapsulation Adsorpt. Sci. 2014, 4, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Hoseinian, F.S.; Irannajad, M.; Nooshabadi, A.J. Ion flotation for removal of Ni (II) and Zn (II) ions from wastewaters. Int. J. Miner. Process. 2015, 143, 131–137. [Google Scholar] [CrossRef]
- Matarese Palmieri, R.; Pera, L.L.; Di Bella, G.; Dugo, G. Simultaneous determination of Cd (II), Cu (II), Pb (II) and Zn (II) by derivative stripping chronopotentiometry in Pittosporum tobira leaves: A measurement of local atmospheric pollution in Messina (Sicily, Italy). Chemosphere 2005, 59, 1161–1168. [Google Scholar] [CrossRef]
- Pang, F.M.; Kumar, P.; Teng, T.T.; Mohd Omar, A.K.; Wasewar, K.L. Removal of lead, zinc and iron by coagulation–flocculation. J. Taiwan Inst. Chem. Eng. 2011, 42, 809–815. [Google Scholar] [CrossRef]
- Alvarez, M.T.; Crespo, C.; Mattiasson, B. Precipitation of Zn (II), Cu (II) and Pb (II) at bench-scale using biogenic hydrogen sulfide from the utilization of volatile fatty acids. Chemosphere 2007, 66, 1677–1683. [Google Scholar] [CrossRef]
- Trainor, T.P.; Brown, G.E.; Parks, G.A. Adsorption and precipitation of aqueous Zn (II) on alumina powders. J. Colloid Interface Sci. 2000, 231, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Ni, W.; Wu, Z.; Lai, Y. Removal of Mn (II) and Zn (II) ions from flue gas desulfurization wastewater with water-soluble chitosan. Sep. Purif. Technol. 2009, 65, 269–274. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Abdalla, M.A.; Ahamad, T.; Abdullah Alothman, Z.; Alshehri, S.M. Synthesis and characterization of Fe3O4@TSC nanocomposite: Highly efficient removal of toxic metal ions from aqueous medium. RSC Adv. 2016, 6, 22679–22689. [Google Scholar] [CrossRef]
- Li, S.; Song, X. Study on the preparation and production factors of a direct lignocellulose biomass fuel cell. J. Electroanal. Chem. 2018, 810, 55–61. [Google Scholar] [CrossRef]
- Guerra, A.; Filpponen, I.; Lucia, L.A.; Argyropoulos, D.S. Comparative Evaluation of three lignin isolation protocols for various wood species. J. Agric. Food Chem. 2006, 54, 9696–9705. [Google Scholar] [CrossRef]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crop. Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Saha, P.; Manna, S.; Chowdhury, S.R.; Sen, R.; Roy, D.; Adhikari, B. Enhancement of tensile strength of lignocellulosic jute fibers by alkali-steam treatment. Bioresour. Technol. 2010, 101, 3182–3187. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically ThinMoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [Green Version]
- Uetani, K.; Yano, H. Nano fibrillation of Wood Pulp Using a High-Speed Blender. Biomacromolecules 2010, 12, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Malainine, M.E.; Mahrouz, M.; Dufresne, A. Thermoplastic nanocomposites based on cellulose microfibrils from Opuntia Ficus-indica parenchyma cell. Compos. Sci. Technol. 2005, 65, 1520–1526. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Zhang, W.; An, Y.; Li, S.; Liu, Z.; Chen, Z.; Ren, Y.; Wang, S.; Zhang, X.; Wang, X. Enhanced heavy metal removal from an aqueous environment using an eco-friendly and sustainable adsorbent. Sci. Rep. 2020, 10, 16453. [Google Scholar] [CrossRef] [PubMed]
- Sdiri, A.T.; Higashi, T.; Jamoussi, F. Adsorption of copper and zinc onto natural clay in single and binary systems. Int. J. Environ. Sci. Technol. 2014, 11, 1081–1092. [Google Scholar] [CrossRef]
- Diab, M.A.; Attia, N.F.; Attia, S.; El-Shahat, M.F. Green synthesis of cost-effective and efficient nano adsorbents based on zero- and two-dimensional nanomaterials for Zn2+ and Cr3+ removal from aqueous solutions. Synth. Met. 2020, 265, 116411. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nano porous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Xu, J.K.; Zhou, P.F.; Dai, L.; Gui, Y.; Yuan, L.; Shen, X.J.; Zhang, C.T.; Huo, K.F. A scalable waste-free biorefinery inspires revenue from holistic lignocellulose valorization. Green Chem. 2021, 23, 6008–6019. [Google Scholar] [CrossRef]
- Zhang, W.; Duo, H.; Li, S.; An, Y.; Chen, Z.; Liu, Z.; Ren, Y.; Wang, S.; Zhang, X.; Wang, X. An overview of the recent advances in functionalization biomass adsorbents for toxic metals removal. Colloid Interface Sci. Commun. 2020, 38, 100308. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Chen, Z. A novel nanocomposite as an efficient adsorbent for the rapid adsorption of Ni (II) from aqueous solution. Materials 2017, 10, 1124. [Google Scholar] [CrossRef]
- An, Y.; Zhang, X.; Wang, X.; Chen, Z.; Wu, X. Nano@lignocellulose intercalated montmorillonite as adsorbent for effective Mn (II) removal from aqueous solution. Sci. Rep. 2018, 8, 10863. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Lin, N.; Wang, Y.; Zhang, X. Highly efficient microwave-assisted Fenton degradation bisphenol A using iron oxide modified double perovskite intercalated montmorillonite composite nanomaterial as catalyst. J. Colloid Interface Sci. 2021, 594, 446–459. [Google Scholar] [CrossRef]
- Pei, X.; Gan, L.; Tong, Z.; Gao, H.; Meng, S.; Zhang, W.; Wang, P.; Chen, Y. Robust cellulose-based composite adsorption membrane for heavy metal removal. J. Hazard. Mater. 2020, 406, 124746. [Google Scholar] [CrossRef]
- Yousefi, N.; Jones, M.; Bismarck Mautner, A. Fungal chitin-glucan nanopapers with heavy metal adsorption properties for ultrafiltration of organic solvents and water. Carbohydr. Polym. 2020, 253, 117273. [Google Scholar] [CrossRef] [PubMed]
- Boughrara, L.; Sebba, F.Z.; Sebti, H.; Choukchou-Braham, E.; Bounaceur, B.; Kada, S.O.; Zaoui, F. Removal of Zn (II) and Ni (II) heavy metal ions by new alginic acid-ester derivatives materials. Carbohydr. Polym. 2021, 272, 118439. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, Z.; Zhu, Y.; Zhu, Y.; Jiang, Y.; Wang, Y.; Gao, H. Adsorption characteristics of modified rice straw biochar for Zn and in-situ remediation of Zn contaminated soil. Environ. Technol. Innov. 2021, 22, 101388. [Google Scholar] [CrossRef]
- He, X.; Zhang, T.; Xue, Q.; Zhou, Y.; Wang, H.; Bolan, N.S.; Jiang, R.; Tsang, D.C. Enhanced adsorption of Cu (II) and Zn (II) from aqueous solution by polyethyleneimine modified straw hydrochar. Sci. Total Environ. 2021, 778, 146116. [Google Scholar] [CrossRef] [PubMed]
- Ighalo, J.O.; Arowoyele, L.T.; Ogunniyi, S.; Adeyanju, C.A.; Oladipo-Emmanuel, F.M.; Belgore, O.R.; Omisore, M.O.; Adeniyi, A.G. Recent innovations in chemical engineering (formerly recent patents on chemical engineering). Bentham Sci. 2021, 14, 148–159. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, Y.; Lin, Y.; Yang, D.; Zheng, H. Adsorption behaviors of paper mill sludge biochar to remove Cu, Zn and As in wastewater. Environ. Technol. Innov. 2021, 23, 101616. [Google Scholar] [CrossRef]
- Wang, Z.; Li, T.; Liu, D.; Fu, Q.; Hou, R.; Li, Q.; Cui, S.; Li, M. Research on the adsorption mechanism of Cu and Zn by biochar under freeze-thaw conditions. Sci. Total Environ. 2021, 774, 145194. [Google Scholar] [CrossRef]
- Deng, H.; Li, Y.F.; Kang, C.Y.; Yan, L.; Li, S.; Li, S. Adsorption characteristics of Cu (II) and Zn (II) in water on sulfhydryl modified cassava straw. Water Treat. Technol. 2021, 47, 22–27. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, Y.; Chen, Z.; An, Y.; Zhang, W.; Wang, X. Lignocellulose @activated clay nanocomposite with hierarchical nanostructure enhancing the removal of aqueous Zn (II). Polymers 2019, 11, 1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, J.; Wang, H.; Wang, C. Adsorption of toxic zinc by functionalized lignocellulose derived from waste biomass: Kinetics, isotherms and thermodynamics. Sustainability 2021, 13, 10673. [Google Scholar] [CrossRef]
- Li, Q.; Tang, L.; Hu, J.; Jiang, M.; Shi, X.; Zhang, T.; Li, Y.; Pan, X. Removal of toxic metals from aqueous solution by biochar’s derived from long-root Eichhornia crassipes. R. Soc. Open Sci. 2018, 5, 180966. [Google Scholar] [CrossRef] [Green Version]
- Kayranli, B.; Gok, O.; Yilmaz, T.; Gok, G.; Celebi, H.; Seckin, I.Y.; Kalat, D. Zinc removal mechanisms with recycled lignocellulose: From fruit residual to biosorbent then soil conditioner. Water Air Soil Pollut. 2021, 232, 8. [Google Scholar] [CrossRef]
- Ahmad, S.Z.N.; Wan, S.; Wan, N.; Ismail, A.F.; Yusof, N.; Mohd, Y.; Mohd, Z.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef] [PubMed]
- AnĐukić, A.B.; Kumrić, K.; Vukelić, N.S.; Dimitrijević, M.; Baščarević, Z.D.; Kurko, S.; Matovic, L. Simultaneous removal of Pb2+, Cu2+, Zn2+ and Cd2+ from highly acidic solutions using mechanochemically synthesized montmorillonite–kaolinite/TiO2 composite. Appl. Clay Sci. 2015, 103, 20–27. [Google Scholar] [CrossRef]



| Sample | SBET (m2/g) | SLangmuir (m2/g) | VMic (cm3/g) | RMic (nm) | Rmes (nm) | RAve (nm) |
|---|---|---|---|---|---|---|
| MOT | 61.4 | 137.52 | 0.024 | 204.05 | ||
| LCE-MOT | 213.50 | 378.06 | 1.051 | 1.98 | 45.93 | 31.82 |
| Nano-LCE-MOT | 532.74 | 701.80 | 0.25 | 0.68 | 173.02 | 6.17 |
| Materials | Parameters | Pseudo-First Order | Pseudo-Second Order | ||
|---|---|---|---|---|---|
| LCE-MOT | R2 | 0.8912 | 0.9989 | ||
| Constants | k1 | 0.0045 min−1 | k2 | 0.2876 min−1 | |
| qe | 297.01 mg/g | qe | 327.11 mg/g | ||
| Nano-LCE-MOT | R2 | 0.7319 | 0.9945 | ||
| Constants | k1 | 0.015 min−1 | k2 | 0.000098 min−1 | |
| qe | 541.10 mg/g | qe | 510.20 mg/g | ||
| Materials | Parameters | Langmuir | Freundlich | ||
|---|---|---|---|---|---|
| LCE-MOT | R2 | 0.9973 | 0.9316 | ||
| Constants | KL | 0.0311 L/mg | Kf | 69.11 L/mg | |
| qmax | 326.08 mg/g | 1/n | 0.135 | ||
| Nano-LCE-MOT | R2 | 0.9888 | 0.7681 | ||
| Constants | KL | 0.0012 L/mg | Kf | 167.28 L/mg | |
| qmax | 561.80 mg/g | 1/n | 0.1699 | ||
| Materials | Adsorption-Desorption Capacity | Adsorption/Desorption Cycles | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| LCE-MOT | Adsorption capacity | 324.02 mg/g | 317.05 mg/g | 280.23 mg/g |
| Desorption capacity | 296.11 mg/g | 267.11 mg/g | 145.37 mg/g | |
| Nano-LCE-MOT | Adsorption capacity | 520.33 mg/g | 503.73 mg/g | 497.54 mg/g |
| Desorption capacity | 507.29 mg/g | 497.38 mg/g | 490.63 mg/g | |
| Sample | Zn (II) (mg/g) | Author |
|---|---|---|
| nano-LCE-MOT | 520.33 | This work |
| Thiol-lignocellulose sodium bentonite nanocomposites | 357.29 | Zhang [37] |
| Smectite clay | 102.04 | Sdiri [38] |
| Silica nanoparticles | 49.75 | Diab [39] |
| Sulfhydryl-modified cassava straw | 60.24 | Deng [54] |
| Lignocellulose@ activated clay nanocomposite | 315.90 | Zhang [55] |
| Functionalized lignocellulose derived from waste biomass | 46.49 | Dang [56] |
| Biochars derived from long-root Eichhornia crassipes | 45.40 | Li [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Y.; Zhang, W.; Liu, H.; Zhong, Y.; Hu, Z.; Shao, Y.; Chen, Z.; Ren, Y.; Wang, B.; Wang, S.; et al. Lignocellulose-Based Superabsorbent Polymer Gel Crosslinked with Magnesium Aluminum Silicate for Highly Removal of Zn (II) from Aqueous Solution. Polymers 2021, 13, 4161. https://doi.org/10.3390/polym13234161
An Y, Zhang W, Liu H, Zhong Y, Hu Z, Shao Y, Chen Z, Ren Y, Wang B, Wang S, et al. Lignocellulose-Based Superabsorbent Polymer Gel Crosslinked with Magnesium Aluminum Silicate for Highly Removal of Zn (II) from Aqueous Solution. Polymers. 2021; 13(23):4161. https://doi.org/10.3390/polym13234161
Chicago/Turabian StyleAn, Yuhong, Wanqi Zhang, Hui Liu, Yuan Zhong, Zichu Hu, Yali Shao, Zhangjing Chen, Yukun Ren, Boyun Wang, Sunguo Wang, and et al. 2021. "Lignocellulose-Based Superabsorbent Polymer Gel Crosslinked with Magnesium Aluminum Silicate for Highly Removal of Zn (II) from Aqueous Solution" Polymers 13, no. 23: 4161. https://doi.org/10.3390/polym13234161
APA StyleAn, Y., Zhang, W., Liu, H., Zhong, Y., Hu, Z., Shao, Y., Chen, Z., Ren, Y., Wang, B., Wang, S., Zhang, X., & Wang, X. (2021). Lignocellulose-Based Superabsorbent Polymer Gel Crosslinked with Magnesium Aluminum Silicate for Highly Removal of Zn (II) from Aqueous Solution. Polymers, 13(23), 4161. https://doi.org/10.3390/polym13234161