From Naturally-Sourced Protease Inhibitors to New Treatments for Fungal Infections
Abstract
:1. Introduction
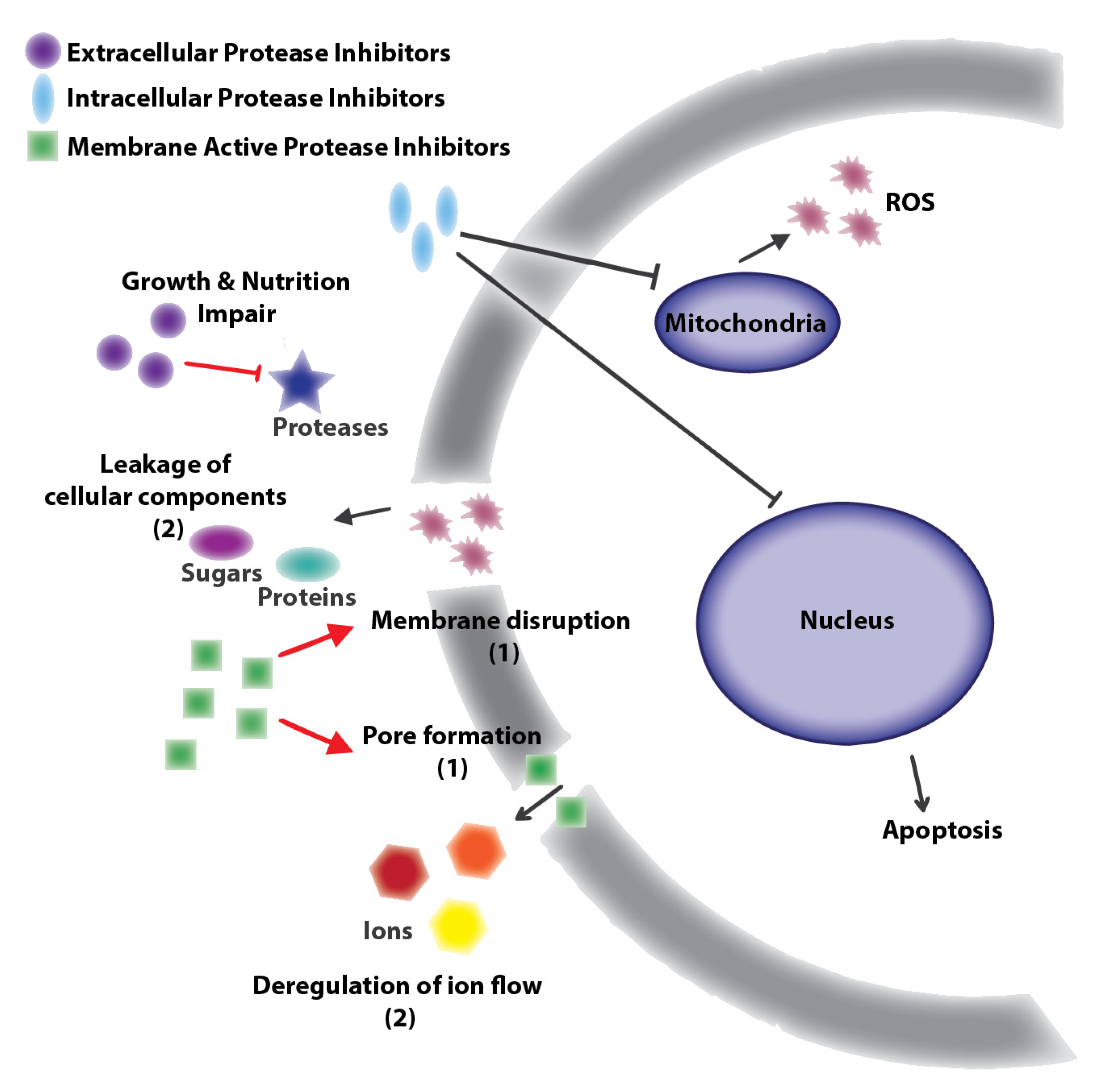
2. Protease Inhibition Exerts Anti-Virulence Effects on Fungal Pathogens
3. Classification of Naturally-Derived Protease Inhibitors
4. Plant-Derived Protease Inhibitors
4.1. Fabaceae (Leguminosae) Family
4.2. Solanaceae Family
4.3. Rhamnaceae Family
4.4. Rutaceae Family
4.5. Pinaceae Family
5. Invertebrate-Derived Protease Inhibitors
5.1. Arthropoda Phylum
5.2. Mollusk Phylum
6. Bacterial Protease Inhibitors
6.1. Actinomycetaceae Family
6.2. Bacillaceae Family
7. Future Directions and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrett, A.J. Bioinformatics of proteases in the MEROPS database. Curr. Opin. Drug Discov. 2004, 7, 334–341. [Google Scholar]
- Siezen, R.J.; Leunissen, J.A.M. Subtilases: The superfamily of subtilisin-like serine proteases. Protein Sci. 1997, 6, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.M.; Eckenroth, B.E.; Putnam, E.E.; Doublie, S.; Shen, A. Structural and Functional Analysis of the CspB Protease Required for Clostridium Spore Germination. PLoS Pathog. 2013, 9, e1003165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.C.; Pirofski, L.A.; Casadevall, A. Extracellular proteins of Cryptococcus neoformans and host antibody response. Infect. Immun. 1997, 65, 2599–2605. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Blank, E.S.; Casadevall, A. Extracellular Proteinase Activity of Cryptococcus neoformans. Clin. Diagn. Lab. Immunol. 1996, 3, 570–574. [Google Scholar] [CrossRef] [Green Version]
- Jansen, H.; Grenier, D.; Van der Hoeven, J.S. Characterization of immunoglobulin G-degrading proteases of Prevotella intermedia and Prevotella nigrescens. Oral Microbiol. Immunol. 1995, 10, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminishi, H.; Miyaguchi, H.; Tamaki, T.; Suenaga, N.; Hisamatsu, M.; Mihashi, I.; Matsumoto, H.; Maeda, H.; Hagihara, Y. Degradation of humoral host defense by Candida albicans proteinase. Infect. Immun. 1995, 63, 984–988. [Google Scholar] [CrossRef] [Green Version]
- Mielech, A.M.; Kilianski, A.; Baez-Santos, Y.M.; Mesecar, A.D.; Baker, S.C. MERS-CoV papain-like protease has deISGylating and deubiquitinating activities. Virology 2014, 450, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.; Tortorici, M.A.; Wall, A.; Mcguire, A.T.; Veesler, D.; Walls, A.C.; Park, Y.; Tortorici, M.A.; Wall, A.; et al. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Coutard, B.; Valle, C.; De Lamballerie, X.; Canard, B.; Seidah, N.G.; Decroly, E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin- like cleavage site absent in CoV of the same clade. Antivir. Res. 2020, 176, 104742. [Google Scholar] [CrossRef]
- Drag, M.; Salvesen, G.S. Emerging principles in protease-based drug discovery. Nat. Rev. Drug Discov. 2010, 9, 690–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Brik, A.; Wong, C.-H. HIV-1 protease: Mechanism and drug discovery. Org. Biomol. Chem. 2003, 1, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.S.; Braga-silva, L.A. Aspartic Protease Inhibitors: Effective Drugs against the Human Fungal Pathogen Candida albicans. Med. Chem. (Los. Angeles) 2013, 13, 155–162. [Google Scholar]
- Markaryan, A.; Morozova, I.; Yu, H.; Kolattukudy, P.E. Purification and characterization of an elastinolytic metalloprotease from Aspergillus fumigatus and immunoelectron microscopic evidence of secretion of this enzyme by the fungus invading the murine lung. Infect. Immun. 1994, 62, 2149–2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemunaitis, J.; Stanbery, L.; Senzer, N. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection: Let the virus be its own demise. Future Virol. 2020, 15, 381–395. [Google Scholar] [CrossRef]
- Neurath, H. Proteolytic enzymes, past and future. Proc. Natl. Acad. Sci. USA 1999, 96, 10962–10963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakao, Y.; Fusetani, N. Enzyme Inhibitors from Marine Invertebrates. J. Nat. Prod. 2007, 70, 689–710. [Google Scholar] [CrossRef] [PubMed]
- Sabotic, J.; Kos, J. Microbial and fungal protease inhibitors—Current and potential applications. Appl. Microbiol. Biotechnol. 2012, 93, 1351–1375. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.B.; Quigley, J.P. α2-macroglobulin: An evolutionarily conserved arm of the innate immune system. Dev. Comp. Immunol. 1999, 23, 375–390. [Google Scholar] [CrossRef]
- Xue, Q. Pathogen proteases and host protease inhibitors in molluscan infectious diseases. J. Invertebr. Pathol. 2019, 166, 107214. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Kumar, S.; Mittal, P.; Singh, I.K. Protease inhibitors: Recent advancement in its usage as a potential biocontrol agent for insect pest management. Insect Sci. 2020, 27, 186–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabotič, J.; Bleuler-Martinez, S.; Renko, M.; Caglič, P.A.; Kallert, S.; Štrukelj, B.; Turk, D.; Aebi, M.; Kos, J.; Künzler, M. Structural basis of trypsin inhibition and entomotoxicity of cospin, serine protease inhibitor involved in defense of Coprinopsis cinerea fruiting bodies. J. Biol. Chem. 2012, 287, 3898–3907. [Google Scholar] [CrossRef] [Green Version]
- Di Nisio, M.; Middeldorp, S.; Büller, H.R. Direct thrombin inhibitors. N. Engl. J. Med. 2005, 353, 1028–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agbowuro, A.A.; Huston, W.M.; Gamble, A.B.; Tyndall, J.D.A. Proteases and protease inhibitors in infectious diseases. Med. Res. Rev. 2017, 1–37. [Google Scholar] [CrossRef]
- Merin, N.M.; Kelly, K.R. Clinical use of proteasome inhibitors in the treatment of multiple myeloma. Pharmaceuticals 2015, 8, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Geddes, J.M.H.; Caza, M.; Croll, D.; Stoynov, N.; Foster, L.J.; Kronstad, J.W. Analysis of the protein kinase a-regulated proteome of Cryptococcus neoformans identifies a role for the ubiquitin-proteasome pathway in capsule formation. MBio 2016, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Geddes, J.M.H.; Croll, D.; Caza, M.; Stoynov, N.; Foster, L.J.; Kronstad, J.W. Secretome profiling of Cryptococcus neoformans reveals regulation of a subset of virulence-associated proteins and potential biomarkers by protein kinase A. BMC Microbiol. 2015, 15, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawar, S.D.; Freas, C.; Weber, I.T.; Harrison, R.W. Analysis of drug resistance in HIV protease. BMC Bioinform. 2018, 19, 362. [Google Scholar] [CrossRef]
- Macedo, M.L.; Ribeiro, S.F.F.; Taveira, G.B.; Gomes, V.; De Barros, K.M.C.A.; Maria-Neto, S. Antimicrobial Activity of ILTI, a Kunitz-Type Trypsin Inhibitor from Inga laurina (SW.) Willd. Curr. Microbiol. 2016, 72, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Dib, H.X.; De Oliveira, D.G.L.; De Oliveira, C.F.R.; Taveira, G.B.; Mello, E.D.O.; Verbisck, N.V.; Chang, M.; Junior, D.C.; Gomes, V.M.; Macedo, M.L.R. Biochemical characterization of a Kunitz inhibitor from Inga edulis seeds with antifungal activity against Candida spp. Arch. Microbiol. 2019, 201, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Lopes, J.L.S.; Valadares, N.F.; Moraes, D.I.; Rosa, J.C.; Araújo, H.S.S.; Beltramini, L.M. Physico-chemical and antifungal properties of protease inhibitors from Acacia plumosa. Phytochemistry 2009, 70, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Aaron, P.A.; Vu, K.; Gelli, A. An Antivirulence Approach for Preventing Cryptococcus neoformans from Crossing the Blood-Brain Barrier via Novel Natural Product Inhibitors of a Fungal Metalloprotease. MBio 2020, 11, e01249-20. [Google Scholar] [CrossRef]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans Secreted Aspartyl Proteinases in Virulence and Pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, S.C.; Dumesic, P.A.; Homer, C.M.; O’Donoghue, A.J.; La Greca, F.; Pallova, L.; Majer, P.; Madhani, H.D.; Craik, C.S. Integrated Activity and Genetic Profiling of Secreted Peptidases in Cryptococcus neoformans Reveals an Aspartyl Peptidase Required for Low pH Survival and Virulence. PLoS Pathog. 2016, 12, e1006051. [Google Scholar] [CrossRef] [PubMed]
- Eldesouky, H.E.; Salama, E.A.; Lanman, N.A.; Hazbun, T.R. Potent Synergistic Interactions between Lopinavir and Azole Antifungal Drugs against Emerging Multidrug-Resistant Candida auris. Antimicrob. Agents Chemother. 2021, 65, e00684-20. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Braga-Silva, L.; Gonçalves, D.; Ramos, L.; Oliveira, S.; Souza, L.; Oliveira, V.; Lins, R.; Pinto, M.; Muñoz, J.; et al. Repositioning Lopinavir, an HIV Protease Inhibitor, as a Promising Antifungal Drug: Lessons Learned from Candida albicans—In Silico, In Vitro and In Vivo Approaches. J. Fungi 2021, 7, 424. [Google Scholar] [CrossRef] [PubMed]
- Kryštůfek, R.; Ŝácha, P.; Starková, J.; Brynda, J.; Hradilek , M.; Tloušt’ová, E.; Grzymska, J.; Rut, W.; Boucher, M.J.; Drag, M.; et al. Re-emerging Aspartic Protease Targets: Examining Cryptococcus neoformans Major Aspartyl Peptidase 1 as a Target for Antifungal Drug Discovery. J. Med. Chem. 2021, 64, 6706–6719. [Google Scholar] [CrossRef]
- Fahrenkrog, B.; Sauder, U.; Aebi, U. The S. cerevisiae HtrA-like protein Nma111p is a nuclear serine protease that mediates yeast apoptosis. J. Cell Sci. 2004, 117, 115–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona-Gutierrez, D.; Eisenberg, T.; Büttner, S.; Meisinger, C.; Kroemer, G.; Madeo, F. Apoptosis in yeast: Triggers, pathways, subroutines. Cell Death Differ. 2010, 17, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Bien, C.M.; Chang, Y.C.; Nes, W.D.; Kwon-chung, K.J.; Espenshade, P.J. Cryptococcus neoformans Site-2 protease is required for virulence and survival in the presence of azole drugs. Mol. Microbiol. 2009, 74, 672–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutfield, S.M.; Dodson, E.J.; Anderson, B.F.; Moody, P.C.E.; Marshall, C.J.; Sullivan, P.A.; Cutfield, J.F. The crystal structure of a major secreted aspartic proteinase from Candida albicans in complexes with two inhibitors. Curr. Biol. 1995, 3, 1261–1271. [Google Scholar] [CrossRef] [Green Version]
- Costa, H.P.S.; Oliveira, J.T.A.; Sousa, D.O.B.; Morais, J.K.S.; Moreno, F.B.; Monteiro-Moreira, A.C.O.; Viegas, R.A.; Vasconcelos, I.M. JcTI-I: A novel trypsin inhibitor from Jatropha curcas seed cake with potential for bacterial infection treatment. Front. Microbiol. 2014, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Park, S.-C.; Kim, J.-Y.; Lee, S.Y.; Lim, H.-T.; Cheong, H.; Hahm, K.-S.; Park, Y. Purification and characterization of a heat-stable serine protease inhibitor from the tubers of new potato variety “Golden Valley”. Biochem. Biophys. Res. Commun. 2006, 346, 681–686. [Google Scholar] [CrossRef]
- Torres-Castillo, J.A.; Jacobo, C.M.; Blanco-Labra, A. Characterization of a highly stable trypsin-like proteinase inhibitor from the seeds of Opuntia streptacantha (O. streptacantha Lemaire). Phytochemistry 2009, 70, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Monteiro Júnior, J.E.; Valadares, N.F.; Pereira, H.D.; Dyszy, F.H.; da Costa Filho, A.J.; Uchôa, A.F.; de Oliveira, A.S.; da Silveira Carvalho, C.P.; Grangeiro, T.B. Expression in Escherichia coli of cysteine protease inhibitors from cowpea (Vigna unguiculata): The crystal structure of a single-domain cystatin gives insights on its thermal and pH stability. Int. J. Biol. Macromol. 2017, 102, 29–41. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef]
- Losso, J.N. The Biochemical and Functional Food Properties of the Bowman-Birk. Crit. Rev. Food Sci. Nutr. 2008, 48, 94–118. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Song, Z.; Chi, C. Structural features and molecular evolution of Bowman-Birk protease inhibitors and their potential application. Acta Biochim. Biophys. Sin. (Shanghai) 2005, 37, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Habib, H.; Fazili, K.M. Plant protease inhibitors: A defense strategy in plants. Biotechnol. Mol. Biol. Rev. 2007, 2, 68–85. [Google Scholar]
- Mistry, R.; Snashall, P.D.; Totty, N.; Briskin, S.; Guz, A.; Tetley, T.D. Purification and characterization of a novel Kazal-type serine proteinase inhibitor of neutrophil elastase from sheep lung. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1997, 1342, 51–61. [Google Scholar] [CrossRef]
- Rustgi, S.; Boex-Fontvieille, E.; Reinbothe, C.; von Wettstein, D.; Reinbothe, S. The complex world of plant protease inhibitors Insights into a Kunitz type cysteine protease inhibitor of Arabidopsis thaliana. Commun. Integr. Biol. 2018, 11, e1368599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Shea, R.; Moser, H.E. Physicochemical properties of antibacterial compounds: Implications for drug discovery. J. Med. Chem. 2008, 51, 2871–2878. [Google Scholar] [CrossRef]
- Hussain, I.; Hawkins, J.; Harrison, D.; Hille, C.; Wayne, G.; Cutler, L.; Buck, T.; Walter, D.; Demont, E.; Howes, C.; et al. Oral administration of a potent and selective non-peptidic BACE-1 inhibitor decreases β-cleavage of amyloid precursor protein and amyloid-β production in vivo. J. Neurochem. 2007, 100, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Dabhade, A.R.; Mokashe, N.U.; Patil, U.K. Purification, characterization, and antimicrobial activity of nontoxic trypsin inhibitor from Albizia amara Boiv. Process Biochem. 2016. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Gopal, R.; Kim, S.Y.; Seo, C.H.; Lee, H.B.; Cheong, H.; Park, Y. PG-2, a potent AMP against pathogenic microbial strains, from potato (Solanum tuberosum L cv. Gogu Valley) tubers not cytotoxic against human cells. Int. J. Mol. Sci. 2013, 14, 4349–4360. [Google Scholar] [CrossRef]
- Bacha, A.B.; Jemel, I.; Moubayed, N.M.S.; Ben, I. Purification and characterization of a newly serine protease inhibitor from Rhamnus frangula with potential for use as therapeutic drug. 3 Biotech 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manojlovic, N.T.; Solujic, S.; Sukdolak, S.; Milosev, M. Antifungal activity of Rubia tinctorum, Rhamnus frangula and Caloplaca cerina. Fitoterapia 2005, 76, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Opportunities for natural products in 21st century for antibiotic disease. Nat. Prod. Reports 2017, 34, 694–701. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Hwang, I.; Cheong, H. Protease Inhibitors from Plants with Antimicrobial Activity. Int. J. Mol. Sci. 2009, 10, 2860–2872. [Google Scholar] [CrossRef] [Green Version]
- Valueva, T.A.; Mosolov, V. V Role of inhibitors of proteolytic enzymes in plant defense against phytopathogenic microorganisms. Biochemical 2004, 69, 1305–1309. [Google Scholar] [CrossRef]
- Lawrence, P.K.; Koundal, K.R. Plant protease inhibitors in control of phytophagous insects. Electron. J. Biotechnol. 2002, 5, 5–6. [Google Scholar] [CrossRef]
- Ryan, C.A. Protease inhibitors in plants: Genes for improving defenses against insects and pathogens. Annu. Rev. Phytopathol. 1990, 28, 425–449. [Google Scholar] [CrossRef]
- Park, Y.; Choi, B.H.; Kwak, J.-S.; Kang, C.-W.; Lim, H.-T.; Cheong, H.-S.; Hahm, K.-S. Kunitz-type serine protease inhibitor from potato (Solanum tuberosum L. cv. Jopung). J. Agric. Food Chem. 2005, 53, 6491–6496. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Lam, S.K.; Fong, W.P. A Homodimeric Sporamin-Type Trypsin Inhibitor with Antiproliferative, HIV Reverse Transcriptase-Inhibitory and Antifungal Activities from Wampee (Clausena lansium) Seeds. Biol. Chem. 2003, 384, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.; Perez-Guaita, D.; Correa-Royero, J.; Zapata, B.; Agudelo, L.; Mesa-Arango, A.B.-G.L. Synthesis and biological evaluation of dehydroabietic acid derivatives. Eur. J. Med. Chem. 2010, 45, 811–816. [Google Scholar] [CrossRef]
- Madeo, F.; Fröhlich, E.; Ligr, M.; Grey, M.; Sigrist, S.J.; Wolf, D.H.; Fröhlich, K.U. Oxygen stress: A regulator of apoptosis in yeast. J. Cell Biol. 1999, 145, 757–767. [Google Scholar] [CrossRef]
- Vu, K.; Tham, R.; Uhrig, J.; Thompson, G.R.; Na Pombejra, S.; Jamklang, M.; Bautos, J.M.; Gelli, A. Invasion of the Central Nervous System by Cryptococcus neoformans Requires a Secreted Fungal Metalloprotease. mBio 2014, 5, e01101-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond, C.S.; Eastwood, R.J.; Miotto, S.T.S.; Hughes, C.E. Multiple continental radiations and correlates of diversification in Lupinus (Leguminosae): Testing for key innovation with incomplete taxon sampling. Syst. Biol. 2012, 61, 443–460. [Google Scholar] [CrossRef] [Green Version]
- Sethi, G.; Shanmugam, M.K.; Warrier, S.; Merarchi, M.; Arfuso, F.; Kumar, A.P.; Bishayee, A. Pro-apoptotic and anti-cancer properties of diosgenin: A comprehensive and critical review. Nutrients 2018, 10, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, K.; Takino, T.; Miyamori, H.; Kinsen, H.; Yoshizaki, T.; Furukawa, M.; Sato, H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J. Biol. Chem. 2003, 278, 40764–40770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Shih, Y.; Huang, H.; Cheng, H. Diosgenin, a steroidal saponin, inhibits migration and invasion of human prostate cancer PC-3 cells by reducing matrix metalloproteinases expression. PLoS ONE 2011, 6, e20164. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Aoki, T.; Mori, Y.; Ahmad, M.; Miyamori, H.; Takino, T.; Sato, H. Cleavage of lumican by membrane-type matrix metalloproteinase-1 abrogates this proteoglycan-mediated suppression of tumor cell colony formation in soft agar. Cancer Res. 2004, 64, 7058–7064. [Google Scholar] [CrossRef] [Green Version]
- Koshikawa, N.; Giannelli, G.; Cirulli, V.; Miyazaki, K.; Quaranta, V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J. Cell Biol. 2000, 148, 615–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratnikov, B.I.; Rozanov, D.V.; Postnova, T.I.; Baciu, P.G.; Zhang, H.; DiScipio, R.G.; Chestukhina, G.G.; Smith, J.W.; Deryugina, E.I.; Strongin, A.Y. An alternative processing of integrin αv subunit in tumor cells by membrane type-1 matrix metalloproteinase. J. Biol. Chem. 2002, 277, 7377–7385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bártová, V. Antifungal and antimicrobial proteins and peptides of potato (Solanum tuberosum L.) tubers and their applications. Appl. Microbiol. Biotechnol. 2019, 103, 5533–5547. [Google Scholar] [CrossRef] [PubMed]
- Wakamiya, I.; Newton, R.J.; Johnston, J.S.; Price, H.J. Genome size and environmental factors in the genus Pinus. Am. J. Bot. 1993, 80, 1235–1241. [Google Scholar] [CrossRef]
- Guan, R.; Mariuzza, R.A. Peptidoglycan recognition proteins of the innate immune system. Trends Microbiol. 2007, 15, 127–134. [Google Scholar] [CrossRef]
- Summer, K.; Browne, J.; Liu, L. Molluscan Compounds Provide Drug Leads for the Treatment and Prevention of Respiratory Disease. Mar. Drugs 2020, 18, 570. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. 2008, 8, 69–85. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta (BBA)-General Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, V.T.; Benkendorff, K.; Green, T. Marine Snails and Slugs: A Great Place To Look for Antiviral Drugs. J. Virol. 2015, 89, 8114–8118. [Google Scholar] [CrossRef] [Green Version]
- González, L.; Sánchez, R.E.; Rojas, L.; Pascual, I.; García-fernández, R.; Chávez, M.A.; Betzel, C. Screening of Protease Inhibitory Activity in Aqueous Extracts of Marine Invertebrates from Cuban Coast. Am. J. Anal. Chem. 2016, 7, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Xu, Y.; Jiang, H.; Xu, S.; Zhao, X.; Wang, J. Antibacterial activity of serine protease inhibitor 1 from kuruma shrimp Marsupenaeus japonicus. Dev. Comp. Immunol. 2014, 44, 261–269. [Google Scholar] [CrossRef]
- Amparyup, P.; Donpudsa, S.; Tassanakajon, A. Shrimp single WAP domain (SWD)-containing protein exhibits proteinase inhibitory and antimicrobial activities. Dev. Comp. Immunol. 2008, 32, 1497–1509. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, K.; Dong, Z.; Chen, Z.; Zhu, H.; Zhang, Y.; Xia, Q.; Zhao, P. Kunitz-type protease inhibitor BmSPI51 plays an antifungal role in the silkworm cocoon. Insect Biochem. Mol. Biol. 2020, 116, 103258. [Google Scholar] [CrossRef]
- Lee, T.; Maruyama, S. Isolation of HIV-1 Protease-Inhibiting Peptides from Thermolysin Hydrolysate of Oyster Proteins. Biochem. Biophys. Res. Commun. 1998, 253, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Gongora, D.G.; Perez, L.R.; Muñoz, A.C.; del Rivero Antigua, M.A. Biomedical and Biological relevance of subtilases from the S8 family, diverse and widely distributed serine peptidases. Rev. Cuba. Cienc. Biológicas 2021, 9, 1–13. [Google Scholar]
- Eigenheer, R.A.; Lee, Y.J.; Blumwald, E.; Phinney, B.S.; Gelli, A. Extracellular glycosylphosphatidylinositol-anchored mannoproteins and proteases of Cryptococcus neoformans. FEMS Yeast Res. 2007, 7, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Homer, C.M.M.; Summers, D.K.; Goranov, A.I.; Nielsen, K.; Craik, C.S.S.; Madhani, H.D.D. Intracellular Action of a Secreted Peptide Required for Fungal Virulence. Cell Host Microbe 2016, 19, 849–864. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Ni, Y.; Guo, K.; Dong, Z.; Chen, Y.; Zhu, H.; Xia, Q.; Zhao, P. The mutation of SPI51, a protease inhibitor of silkworm, resulted in the change of antifungal activity during domestication. Int. J. Biol. Macromol. 2021, 178, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kurioka, A.; Yamazaki, M.; Hirano, H. Primary structure and possible functions of a trypsin inhibitor of Bombyx mori. Eur. J. Biochem. 1999, 259, 120–126. [Google Scholar] [CrossRef]
- Zhao, P.; Dong, Z.; Duan, J.; Wang, G.; Wang, L.; Li, Y.; Xiang, Z.; Xia, Q. Genome-wide identification and immune response analysis of serine protease inhibitor genes in the silkworm, Bombyx mori. PLoS ONE 2012, 7, e31168. [Google Scholar] [CrossRef] [PubMed]
- Abadines, I.B.; Le, K.; Newman, D.J.; Glaser, K.B.; Mayer, A.M. The marine pharmacology and pharmaceuticals pipeline in 2018. FASEB J. 2019, 33, 501–504. [Google Scholar] [CrossRef]
- Benkendor, K. Chemical diversity in molluscan communities: From natural products to chemical ecology. In Neuroecology and Neuroethology in Molluscs: The Interface between Behaviour and Environment; Cosmo, A.D., Winlow, W., Eds.; Nova Science Publishers: New York, NY, USA, 2014; pp. 13–41. [Google Scholar]
- Abbenante, G.; Fairlie, D.P. Protease Inhibitors in the Clinic. Med. Chem. (Los. Angeles) 2005, 1, 71–104. [Google Scholar] [CrossRef] [PubMed]
- Randolph, J.T.; DeGoey, D.A. Peptidomimetic inhibitors of HIV protease. Curr. Top. Med. Chem. 2004, 4, 1079–1095. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A.; De Bernardis, F.; Torosantucci, A.; Tacconelli, E.; Tumbarello, M.; Cauda, R. In Vitro and In Vivo Anticandidal Activity of Human Immunodeficiency Virus Protease Inhibitors. J. Infect. Dis. 1999, 180, 448–453. [Google Scholar] [CrossRef]
- Blasi, E.; Colombari, B.; Francesca, C.; Pinti, M.; Troiano, L.; Cossarizza, A.; Esposito, R.; Peppoloni, S.; Mussini, C.; Neglia, R. The human immunodeficiency virus (HIV) protease inhibitor indinavir directly affects the opportunistic fungal pathogen Cryptococcus neoformans. FEMS Immunol. Med. Microbiol. 2004, 42, 187–195. [Google Scholar] [CrossRef]
- Manfredi, R.; Calza, L.; Chiodo, F. AIDS-associated Cryptococcus infection before and after the highly active antiretroviral therapy era: Emerging management problems. Int. J. Antimicrob. Agents 2003, 22, 449–452. [Google Scholar] [CrossRef]
- Quintero, D.; Bermudes, D. A culture-based method for determining the production of secreted protease inhibitors. J. Microbiol. Methods 2014, 100, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Supuran, C.T.; Scozzafava, A.; Clare, B.W. Bacterial Protease Inhibitors. Med. Res. Rev. 2002, 22, 329–372. [Google Scholar] [CrossRef] [PubMed]
- Vathipadiekal, V.; Umasankar, P.K.; Patole, M.S.; Rao, M. Molecular cloning, over expression, and activity studies of a peptidic HIV-1 protease inhibitor: Designed synthetic gene to functional recombinant peptide. Peptides 2010, 31, 16–21. [Google Scholar] [CrossRef]
- Wolber, G.; Hell, M.; Heinrich, I.E.; Cadicamo, C.D.; Michel, D.; Semlin, L.; Berger, U.; Korting, H.C.; Ho, H.; Koksch, B.; et al. Design, synthesis, inhibition studies, and molecular modeling of pepstatin analogues addressing different secreted aspartic proteinases of Candida albicans. Biochem. Pharmacol. 2013, 85, 881–887. [Google Scholar] [CrossRef]
- Bondaryk, M.; Staniszewska, M.; Zielinska, P.; Urbanczyk-Lipkowska, Z.; Zieli, P. Natural Antimicrobial Peptides as Inspiration for Design of a New Generation Antifungal Compounds. J. Fungi 2017, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Ruchel, R.; Ritter, B. Modulation of experimental systemic murine candidosis by intravenous pepstatin. Zentralbl. Bakterio 1996, 273, 391–403. [Google Scholar] [CrossRef]
- Sidrim, J.J.C.; Perdigão-Neto, L.V.; Cordeiro, R.A.; Brilhante, R.S.N.; Leite, J.J.G.; Teixeira, C.E.C.; Monteiro, A.J.; Freitas, R.M.F.; Ribeiro, J.F.; Mesquita, J.R.L.; et al. Viral protease inhibitors affect the production of virulence factors in Cryptococcus neoformans. Can. J. Microbiol. 2012, 58, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Monari, C.; Pericolini, E.; Bistoni, G.; Cenci, E.; Bistoni, F.; Vecchiarelli, A. Influence of Indinavir on Virulence and Growth of Cryptococcus neoformans. J. Infect. Dis. 2005, 191, 307–311. [Google Scholar] [CrossRef]
- Naglik, J.; Albrecht, A.; Bader, O.; Hube, B. Candida albicans proteinases and host/pathogen interactions. Cell. Microbiol. 2004, 6, 915–926. [Google Scholar] [CrossRef]
- Muselius, B.; Durand, S.; Geddes-McAlister, J. Proteomics of Cryptococcus neoformans: From the Lab to the Clinic. Int. J. Mol. Sci. 2021, 22, 12390. [Google Scholar] [CrossRef]
| Source | Protease Inhibitor Designation (Source) | Enzymatic Family | MW (kDa) | Activity (Mechanism of Action) | Reference |
|---|---|---|---|---|---|
| Fabaceae (Leguminosae) | IETI (Inga edulis) | Kunitz | 19.7 | Antifungal (Protease inhibition, membrane disruption and oxidative stress) | [31] |
| ILTI (Inga laurica) | 20 | [30] | |||
| ApTI (A, B, C) (Acacia plumosa) | Kunitz | 20 | Antifungal (Secreted protease inhibition and nutrition impairment) | [32] | |
| API (Albizia amara) | Unknown | 49 | Antifungal and Antibacterial | [55] | |
| Lupinine (Lupinus spp.) | Quinolizidine alkaloid | 0.17 | Anticryptococcal (secreted metallopeptidase inhibition) | [33] | |
| Diosgenin (Trigonella foenum-graecum) | Steroidal sapogenin | 0.41 | |||
| Solanaceae | Potide-G (S. tuberosum L. Cv. Golden Valley) | Kunitz | 5.57 | Antibacterial and Antifungal (Secreted protease inhibition and nutrition impairment) | [44] |
| PG-2 (S. tuberosum L. Cv. Gogu Valley) | Kunitz | 3.2 | Antibacterial and Antifungal | [56] | |
| AFP-J (S. tuberosum L. Cv. L. Jopung) | Kunitz | 13.5 | Antifungal | [64] | |
| Rhamnaceae | RflP-1 (Rhamnus frangula) | Kunitz | 22.5 | Antibacterial and Antifungal | [57,58] |
| Rutaceae | CLTI (Clausena lamsium) | Unknown | 54 | Anti-HIV-1 reverse transcriptase activity and Antifungal | [65] |
| Pinaceae | Abietic acid (Pinus spp.) | Abietane diterpenoid | 0.3 | Anticryptococcal (secreted metallopeptidase inhibition) | [33,66] |
| Source | Protease Inhibitor Designation (Source) | Family/Chemical Class | MW (kDa) | Activity (Mechanism of Action) | Reference |
|---|---|---|---|---|---|
| Arthropoda | MjSerp1 (Marsupenaeus japonicas) | Serpin | 46.3 | Antibacterial | [84] |
| SWDPm2 (Penaeus monodon) | Type III crustin | 7.38 | [85] | ||
| BmoSPI51 (Bombyx mori) | Kunitz-type | 14 | Antifungal | [86] | |
| Mollusk | Peptides (Crassostrea gigas) | Unknown | Unknown | HIV protease inhibitor (Competitive inhibition) | [87] |
| Source | Protease Inhibitor Designation (Source) | Family/Chemical Class | MW (kDa) | Activity (Mechanism of Action) | Ref. |
|---|---|---|---|---|---|
| Actinomycetaceae | Pepstatin A (Actinomycetes spp.) | Hexapeptide | 0.68 | Antifungal (Secreted protease inhibition) and HIV protease inhibitor | [42] |
| Bacillaceae | ATBI (Bacillus spp.) | Heptapeptide | 1.1 | HIV-1 protease inhibitor (Competitive inhibition) | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez-Gongora, D.; Geddes-McAlister, J. From Naturally-Sourced Protease Inhibitors to New Treatments for Fungal Infections. J. Fungi 2021, 7, 1016. https://doi.org/10.3390/jof7121016
Gutierrez-Gongora D, Geddes-McAlister J. From Naturally-Sourced Protease Inhibitors to New Treatments for Fungal Infections. Journal of Fungi. 2021; 7(12):1016. https://doi.org/10.3390/jof7121016
Chicago/Turabian StyleGutierrez-Gongora, Davier, and Jennifer Geddes-McAlister. 2021. "From Naturally-Sourced Protease Inhibitors to New Treatments for Fungal Infections" Journal of Fungi 7, no. 12: 1016. https://doi.org/10.3390/jof7121016







