Responses of Lowland Rice Genotypes under Terminal Water Stress and Identification of Drought Tolerance to Stabilize Rice Productivity in Southern Thailand
Abstract
:1. Introduction
2. Results
2.1. Effect of Water Stress on Yield Performance and Productivity
2.2. Association among Yield and Yield Attributes under Terminal Water Stress
2.3. Genotypic Classification Corresponding to Stress Indices
2.4. Association among Stress Tolerance Indices and Grain Yield
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Site Description and Crop Management
4.3. Crop Data Collection
4.4. Computation of Stress Tolerance Indices
4.5. Analysis of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. FAO (Online). 2020. Available online: http://www.fao.org/faostat/en/#home (accessed on 18 August 2021).
- Ichsan, C.N.; Basyah, B.; Zakaria, S.; Efendi, E. Differences of water status and relationship with roots growth and yield of rice under water stress. Syst. Rev. Pharm. 2020, 11, 611–618. [Google Scholar]
- Varinruk, B. Thailand rice production and rice research and development on climate change. In Proceedings of the Workshop on Strengthening APEC Cooperation on Food Security and Climate Change, Ha Noi, Vietnam, 19–21 April 2017. [Google Scholar]
- USDA Thailand: Irrigation Shortage Reduces 2015/16 Rice Production. 2015. Available online: https://ipad.fas.usda.gov/highlights/2015/10/th/index.htm (accessed on 25 June 2020).
- GRiSP. Rice Almanac, 4th ed.; International Rice Research Institute: Los Baños, Philippines, 2013. [Google Scholar]
- Mansour, E.; Desoky, E.S.M.; Ali, M.M.A.; Abdul-Hamid, M.I.; Ullah, H.; Attia, A.; Datta, A. Identifying drought-tolerant genotypes of faba bean and their agro-physiological responses to different water regimes in an arid Mediterranean environment. Agric. Water Manag. 2021, 247, 106754. [Google Scholar] [CrossRef]
- Ullah, H.; Santiago-Arenas, R.; Ferdous, Z.; Attia, A.; Datta, A. Improving Water Use Efficiency, Nitrogen Use Efficiency, and Radiation Use Efficiency in Field Crops under Drought Stress: A Review, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 156, ISBN 9780128175989. [Google Scholar]
- Ray, D.K.; West, P.C.; Clark, M.; Gerber, J.S.; Prishchepov, A.V.; Chatterjee, S. Climate change has likely already affected global food production. PLoS ONE 2019, 14, e0217148. [Google Scholar] [CrossRef] [PubMed]
- Watcharin, S.; Kanrob, P.; Charassri, N.; Nattapon, J. Improving photoperiod insensitivity of a Thai upland rice variety by marker-assisted foreground selection. Int. J. Agric. Technol. 2020, 16, 199–206. [Google Scholar]
- Chutia, J.; Borah, S.P. Water stress effects on leaf growth and chlorophyll content but not the grain yield in traditional rice (Oryza sativa Linn.) genotypes of Assam, India II. Protein and proline status in seedlings under PEG induced water stress. Am. J. Plant Sci. 2012, 3, 971–980. [Google Scholar] [CrossRef] [Green Version]
- Ahmadikhah, A.; Marufinia, A. Effect of reduced plant height on drought tolerance in rice. Biotech 2016, 6, 221. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wang, X.; Jiao, Y.; Qin, Y.; Liu, X.; Zhang, Q.; Fan, L.; Wang, X. Global genome expression analysis of rice in response to drought and high-salinity stresses in shoot, flag leaf, and panicle. Plant Mol. Biol. 2007, 63, 591–608. [Google Scholar] [CrossRef] [Green Version]
- Fischer, K.; Lafitte, R.; Fukai, S.; Atlin, G.; Hardy, B. Breeding Rice for Drought-Prone Environments; International Rice Research Institute: Los Baños, Philippines, 2003; ISBN 9712201899. [Google Scholar]
- Singh, B.; Reddy, K.R.; Redoña, E.D.; Walker, T. Screening of rice cultivars for morpho-physiological responses to early-season soil moisture stress. Rice Sci. 2017, 24, 322–335. [Google Scholar] [CrossRef]
- Swain, P.; Raman, A.; Singh, S.P.; Kumar, A. Breeding drought tolerant rice for shallow rainfed ecosystem of eastern India. Field Crops Res. 2017, 209, 168–178. [Google Scholar] [CrossRef]
- Agarwal, P.; Parida, S.K.; Raghuvanshi, S.; Kapoor, S.; Khurana, P.; Khurana, J.P.; Tyagi, A.K. Rice improvement through genome-based functional analysis and molecular breeding in India. Rice 2016, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.T.; Islam, M.T.; Islam, M.O. Effect of water stress at different growth stages on yield and yield contributing characters of transplanted Aman rice. Pak. J. Biol. Sci. 2002, 5, 169–172. [Google Scholar] [CrossRef]
- Yue, B.; Xue, W.; Xiong, L.; Yu, X.; Luo, L.; Cui, K.; Jin, D.; Xing, Y.; Zhang, Q. Genetic basis of drought resistance at reproductive stage in rice: Separation of drought tolerance from drought avoidance. Genetics 2006, 172, 1213–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantuwan, G.; Fukai, S.; Cooper, M.; Rajatasereekul, S. Yield response of rice (Oryza sativa L.) genotypes to drought under rainfed lowlands 4. Vegetative stage screening in the dry season. Field Crops Res. 2004, 89, 281–297. [Google Scholar] [CrossRef]
- Ovenden, B.; Milgate, A.; Lisle, C.; Wade, L.J.; Rebetzke, G.J.; Holland, J.B. Selection for water—Soluble carbohydrate accumulation and investigation of genetic × environment interactions in an elite wheat breeding population. Theor. Appl. Genet. 2017, 130, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Kobayashi, N.; Ito, O.; Wahid, A.; Serraj, R. Broader leaves result in better performance of indica rice under drought stress. J. Plant Physiol. 2010, 167, 1066–1075. [Google Scholar] [CrossRef]
- Wadhwa, R.; Kumari, N.; Sharma, V. Varying light regimes in naturally growing Jatropha curcus: Pigment, proline and photosynthetic performance. J. Stress Physiol. Biochem. 2010, 6, 67–80. [Google Scholar]
- Blum, A. Drought adaptation in cereal crops. In Drought Adaptation in Cereals; Haworth Food Products Press: New York, NY, USA, 2006; pp. 301–333. [Google Scholar]
- Cha-um, S.; Yooyongwech, S.; Supaibulwatana, K. Water deficit stress in the reproductive stage of four indica rice (Oryza sativa L.) genotypes. Pak. J. Bot. 2010, 42, 3387–3398. [Google Scholar]
- Majidi, M.M.; Tavakoli, V.; Mirlohi, A.; Sabzalian, M.R. Wild safflower species (Carthamus oxyacanthus Bieb.): A possible source of drought tolerance for arid environments. Aust. J. Crop Sci. 2011, 5, 1055–1063. [Google Scholar]
- Todaka, D.; Shinozaki, K.; Yamaguchi-shinozaki, K. Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front. Plant Sci. 2015, 6, 84. [Google Scholar] [CrossRef] [Green Version]
- Hussain, N.; Ahmed, M.; Duangpan, S.; Hussain, T.; Taweekun, J. Potential impacts of water stress on rice biomass composition and feedstock availability for bioenergy production. Sustainability 2021, 13, 10449. [Google Scholar] [CrossRef]
- IRRI. Standard Evaluation System for Rice, 5th ed.; The Philippines International Rice Research Institute: Los Banos, CA, USA, 2014. [Google Scholar]
- Anwar, J.; Subhani, G.M.; Hussain, M.; Ahmad, J.; Hussain, M.; Munir, M. Drought tolerance indices and their correlation with yield in exotic wheat genotypes. Pak. J. Bot. 2011, 43, 1527–1530. [Google Scholar]
- Rashid, A.; Saleem, Q.; Nazir, A.; Kazım, H.S. Yield potential and stability of nine wheat varieties under water stress conditions. Int. J. Agric. Biol. 2003, 5, 7–9. [Google Scholar]
- Wasae, A. Evaluation of drought stress tolerance based on selection indices in Haricot Bean varieties exposed to stress at different growth stages. Int. J. Agron. 2021, 2021, 6617874. [Google Scholar] [CrossRef]
- Fernandez, G.C. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and other Food Crops in Temperature and Water Stress, Shanhua, Taiwan, 13–16 August 1992; pp. 257–270. [Google Scholar]
- Hossain, A.B.S.; Sears, R.G.; Cox, T.S.; Paulsen, G.M. Desiccation tolerance and its relationship to assimilate partitioning in winter wheat. Crop Sci. 1990, 30, 622–627. [Google Scholar] [CrossRef]
- Schneider, K.A.; Rosales-serna, R.; Ibarra-perez, F.; Cazares-enriquez, B.; Acosta-gallegos, J.A.; Ramirez-vallejo, P.; Wassimi, N.; Kelly, J.D. Improving common bean performance under drought stress. Crop Sci. 1997, 37, 43–50. [Google Scholar] [CrossRef]
- Rosielle, A.A.; Hamblin, J. Theoretical aspects of selection for yield in stress and non-stress environments. Crop Sci. 1981, 21, 943–946. [Google Scholar] [CrossRef]
- Bouslama, M.; Schapaugh, W.T. Stress tolerance in soybeans. I. Evaluation of three screening techniques for heat and drought tolerance. Crop Sci. 1984, 24, 933–937. [Google Scholar] [CrossRef]
- Fischer, R.A.; Maurer, R. Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Clarke, J.M.; Townley-Smith, F.; McCaig, T.N.; Green, D.G. Growth analysis of spring wheat cultivars of varying drought resistance. Crop Sci. 1984, 24, 537–541. [Google Scholar] [CrossRef]
- Mitra, J. Genetics and genetic improvement of drought resistance in crop plants. Curr. Sci. 2001, 80, 758–762. [Google Scholar]
- Hall, A.E. Is dehydration tolerance relevant to genotypic differences in leaf senescence and crop adaptation to dry environments. In Plant Responses to Cellular Dehydration during Environmental Stress; Close, T.J., Bray, E.A., Eds.; Current Topics in Plant Physiology: Washington, DC, USA, 1993; pp. 1–10. [Google Scholar]
- Blum, A. Plant Breeding for Stress Environments; CRC Press: Boca Raton, FL, USA, 2018; p. 212. [Google Scholar]
- Golabadi, M.; Arzani, A.; Maibody, M. Assessment of drought tolerance in segregating populations in Durum Wheat. African J. Agric. Res. 2006, 1, 162–171. [Google Scholar]
- Saba, J.; Moghadam, M.; Ghasemi, K.; Nishabouri, M. Genetic properties of drought resistance indices. J. Agric. Sci. Technol. 2001, 3, 43–49. [Google Scholar]
- Arif, A.; Parveen, N.; Waheed, M.Q.; Atif, R.M.; Waqar, I.; Shah, T.M. A comparative study for assessing the drought-tolerance of Chickpea under varying natural growth environments. Front. Plant Sci. 2021, 11, 2228. [Google Scholar] [CrossRef]
- Campozano, L.; Ballari, D.; Montenegro, M.; Avilés, A. Future meteorological droughts in ecuador: Decreasing trends and associated spatio-temporal features derived from CMIP5 models. Front. Earth Sci. 2020, 8, 17. [Google Scholar] [CrossRef]
- Spinoni, J.; Vogt, J.V.; Naumann, G.; Barbosa, P.; Dosio, A. Will drought events become more frequent and severe in Europe? Int. J. Climatol. 2018, 1736, 1718–1736. [Google Scholar] [CrossRef] [Green Version]
- Davatgar, N.; Neishabouri, M.R.; Sepaskhah, A.R.; Soltani, A. Physiological and morphological responses of rice (Oryza sativa L.) to varying water stress management strategies. Int. J. Plant Prod. 2009, 3, 19–32. [Google Scholar] [CrossRef]
- Saikumar, S.; Varma, C.M.K.; Saiharini, A.; Kalmeshwer, G.P.; Nagendra, K.; Lavanya, K.; Ayyappa, D. Grain yield responses to varied level of moisture stress at reproductive stage in an interspecific population derived from Swarna/O. glaberrima introgression line. NJAS Wagening. J. Life Sci. 2016, 78, 111–122. [Google Scholar] [CrossRef]
- Hussain, T.; Anothai, J.; Nualsri, C.; Soonsuwon, W. Application of CSM-CERES-Rice in scheduling irrigation and simulating effect of drought stress on upland rice yield. Indian J. Agric. Res. 2018, 52, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Pantuwan, G.; Fukai, S.; Cooper, M.; Rajatasereekul, S.; O’Toole, J.C. Yield response of rice (Oryza sativa L.) genotypes to drought under rainfed lowland 3. Plant factors contributing to drought resistance. Field Crops Res. 2002, 73, 181–200. [Google Scholar] [CrossRef]
- Zhao, D.L.; Atlin, G.N.; Amante, M.; Cruz, M.T.S.; Kumar, A. Developing aerobic rice cultivars for water-short irrigated and drought-prone rainfed areas in the tropics. Crop Sci. 2010, 50, 2268–2276. [Google Scholar] [CrossRef]
- Anantha, M.S.; Patel, D.; Quintana, M.; Swain, P.; Dwivedi, J.L.; Torres, R.O.; Verulkar, S.B.; Variar, M.; Mandal, N.P.; Kumar, A.; et al. Trait combinations that improve rice yield under drought: Sahbhagi Dhan and new drought-tolerant varieties in South Asia. Crop Sci. 2016, 56, 408–421. [Google Scholar] [CrossRef] [Green Version]
- Torres, R.O.; Henry, A. Yield stability of selected rice breeding lines and donors across conditions of mild to moderately severe drought stress. Field Crops Res. 2018, 220, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zain, N.A.M.; Ismail, M.R.; Puteh, A.; Mahmood, M.; Islam, M.R. Impact of cyclic water stress on growth, physiological responses and yield of rice (Oryza sativa L.) grown in tropical environment. Ciência Rural 2014, 44, 2136–2141. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Verulkar, S.; Dixit, S.; Chauhan, B.; Bernier, J.; Venuprasad, R.; Zhao, D.; Shrivastava, M.N. Yield and yield-attributing traits of rice (Oryza sativa L.) under lowland drought and suitability of early vigor as a selection criterion. Field Crops Res. 2009, 114, 99–107. [Google Scholar] [CrossRef]
- Torres, R.O.; McNally, K.L.; Cruz, C.V.; Serraj, R.; Henry, A. Screening of rice genebank germplasm for yield and selection of new drought tolerance donors. Field Crops Res. 2013, 147, 12–22. [Google Scholar] [CrossRef]
- Raman, A.; Verulkar, S.B.; Mandal, N.P.; Variar, M.; Shukla, V.D.; Dwivedi, J.L.; Singh, B.N.; Singh, O.N.; Swain, P.; Mall, A.K.; et al. Drought yield index to select high yielding rice lines under different drought stress severities. Rice 2012, 5, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duangpan, S.; Buapet, P.; Sujitto, S.; Eksomtramage, T. Early assessment of drought tolerance in oil palm D × P progenies using growth and physiological characters in seedling stage. Plant Genet. Resour. 2018, 16, 544–554. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acid Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]


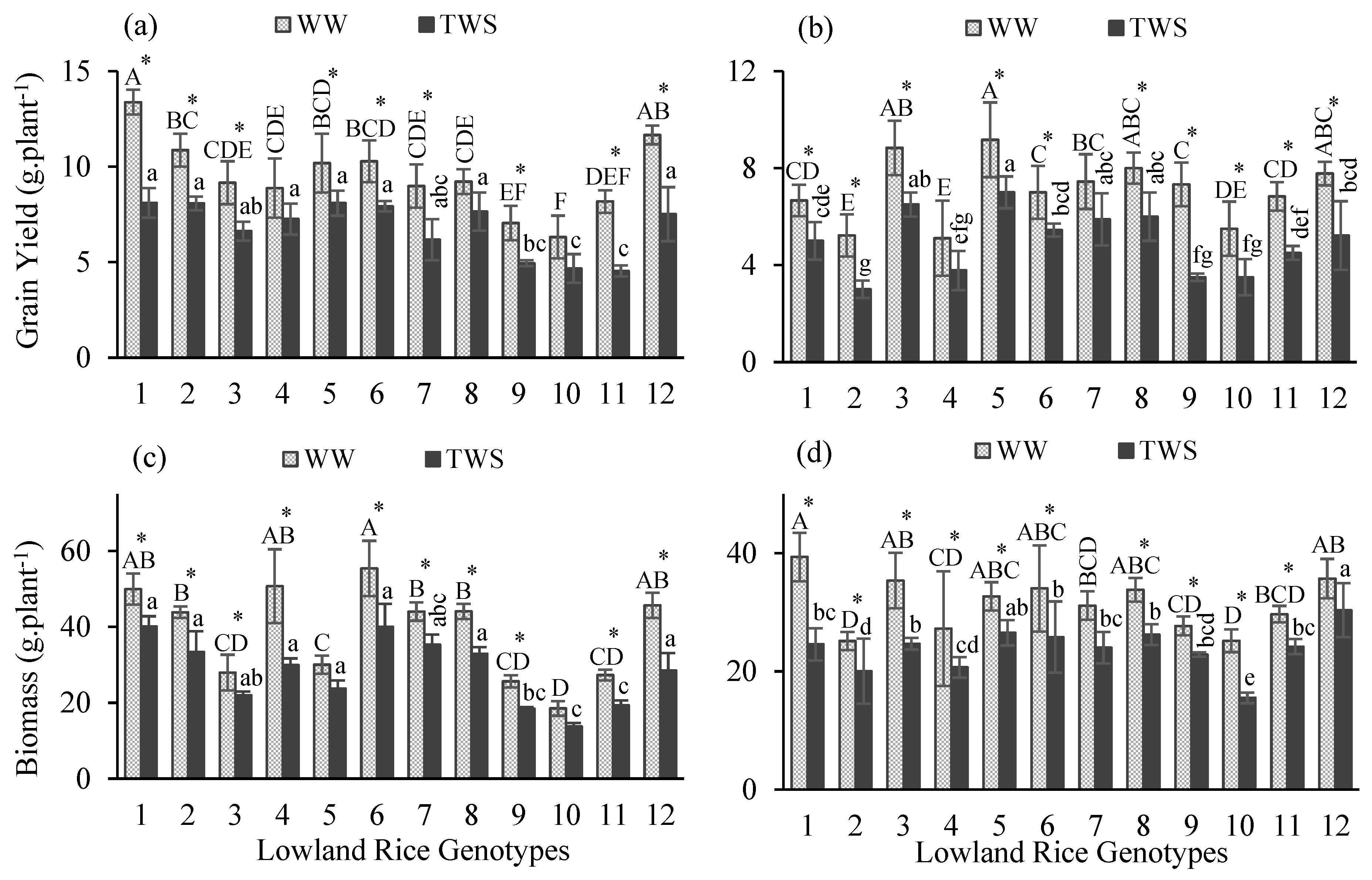
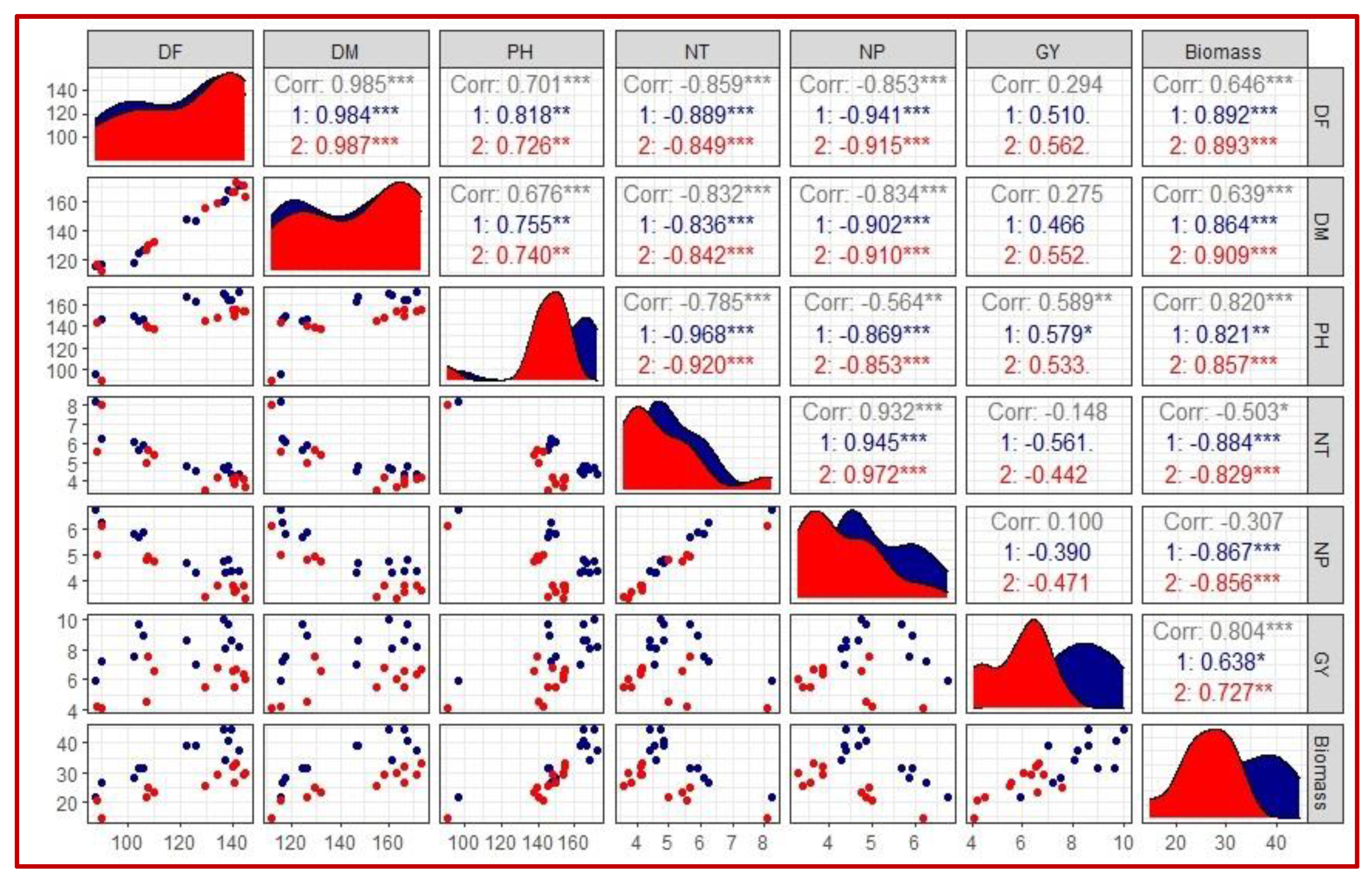
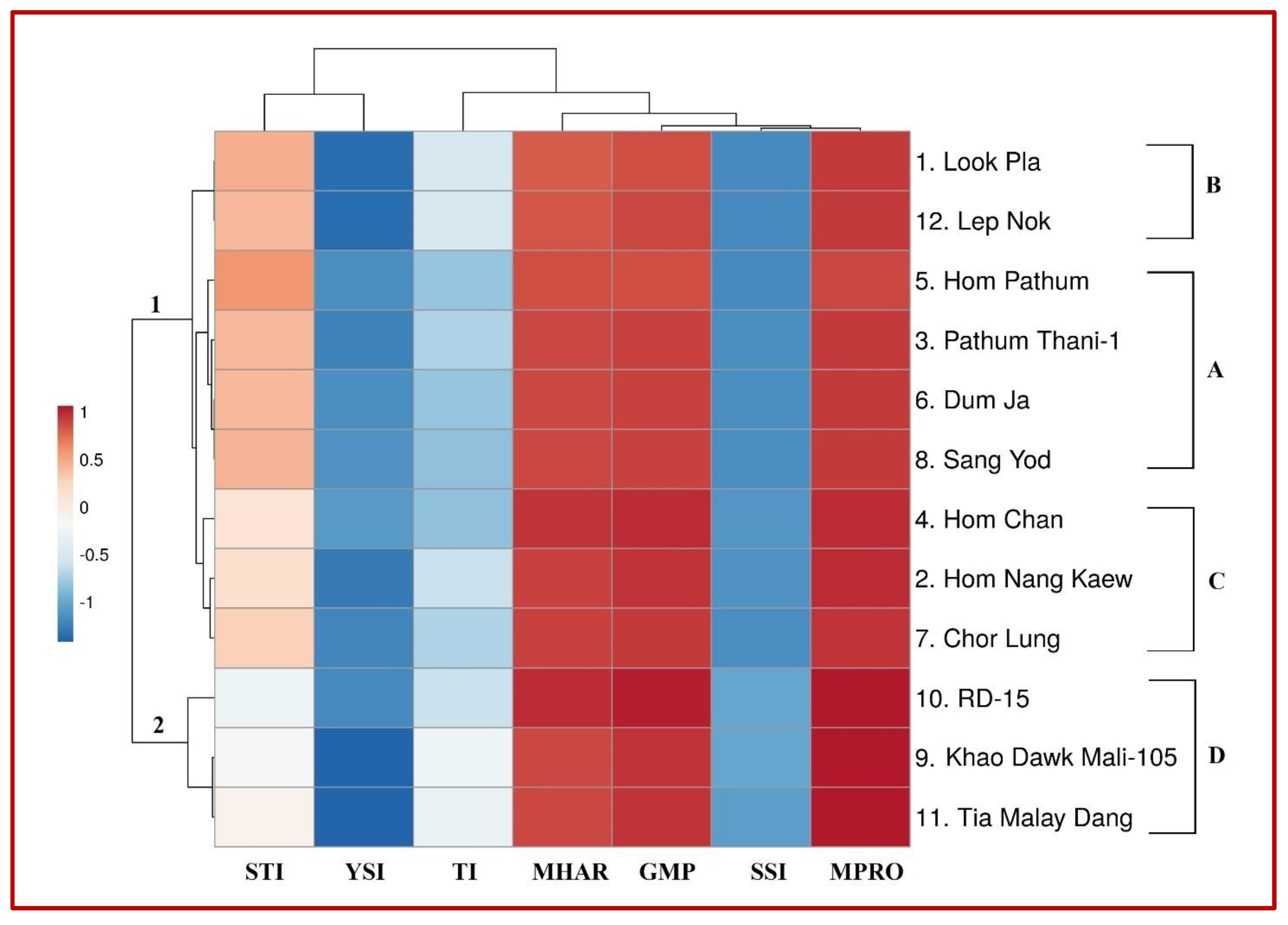

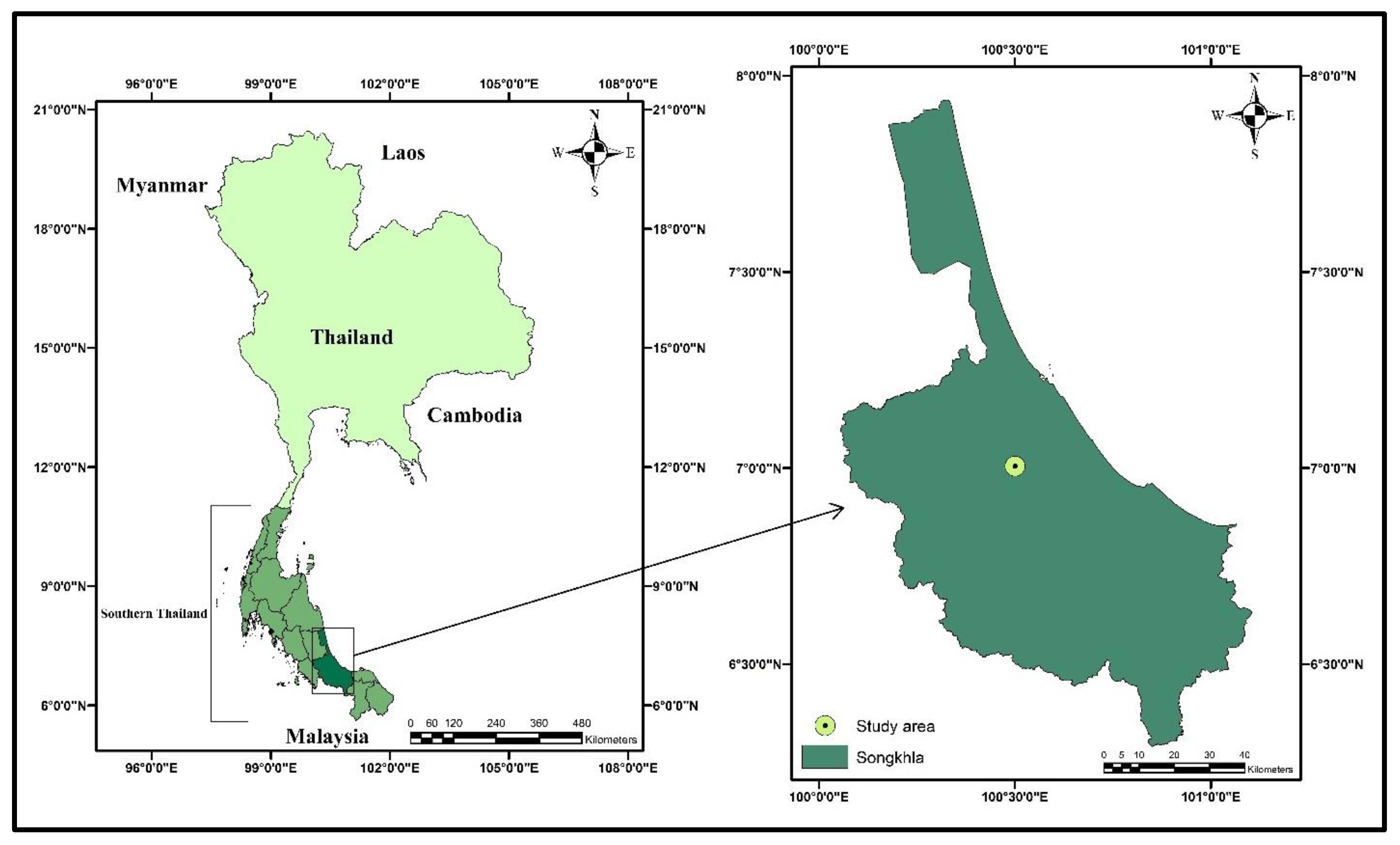
| Year | Traits | Treatment (T) Effect | Genotype (G) Effect | Interaction (T × G) | Year Effect |
|---|---|---|---|---|---|
| 2018–2019 | DF | *** | *** | * | *** |
| DM | ns | *** | *** | ** | |
| PH | *** | *** | ns | ns | |
| NT | *** | *** | ns | *** | |
| NP | *** | *** | ns | *** | |
| GY | *** | *** | ns | *** | |
| BM | *** | *** | ns | *** | |
| 2019–2020 | DF | *** | *** | ns | |
| DM | *** | *** | ns | ||
| PH | *** | *** | ns | ||
| NT | ** | *** | ns | ||
| NP | *** | *** | ns | ||
| GY | *** | *** | ns | ||
| BM | *** | *** | ns |
| Genotypes | 2018–2019 | 2019–2020 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | DM | PH | NT | NP | GY | BM | DF | DM | PH | NT | NP | GY | BM | |
| Days | Days | % | no. | no. | % | % | Days | Days | % | no. | no. | % | % | |
| 1 | 3 | 5 | −9 | 0 | 0 | −39 | −20 | 3 | 7 | −10 | −1 | −1 | −25 | −38 |
| 2 | 4 | 7 | −10 | −1 | −1 | −26 | −24 | 3 | 4 | −12 | 0 | −1 | −43 | −20 |
| 3 | 5 | 8 | −4 | 1 | −0 | −28 | −21 | 4 | 4 | −8 | −2 | −2 | −26 | −30 |
| 4 | 3 | 10 | −13 | −1 | −1 | −18 | −41 | 4 | 6 | −8 | −1 | −1 | −26 | −24 |
| 5 | 2 | 5 | −3 | 0 | −1 | −21 | −21 | 4 | 5 | −5 | 0 | −1 | −24 | −19 |
| 6 | 6 | 5 | −4 | 0 | −1 | −23 | −28 | −2 | 8 | −8 | 0 | −1 | −22 | −24 |
| 7 | 4 | −19 | −11 | −1 | −1 | −31 | −20 | 1 | 4 | −10 | −1 | −1 | −21 | −23 |
| 8 | 19 | 14 | −8 | −1 | −1 | −17 | −25 | 4 | 7 | −14 | 0 | −1 | −25 | −22 |
| 9 | −4 | −5 | −4 | −1 | −2 | −30 | −28 | 1 | 4 | −2 | 0 | −1 | −52 | −17 |
| 10 | 2 | −11 | −8 | −1 | −1 | −26 | −26 | 2 | 3 | −6 | 0 | −1 | −36 | −38 |
| 11 | 3 | 11 | −11 | −1 | −1 | −45 | −29 | 7 | 6 | −3 | −1 | −1 | −34 | −19 |
| 12 | 8 | 4 | −5 | −1 | −1 | −36 | −38 | 3 | 3 | −7 | −1 | −1 | −33 | −15 |
| Lowland Rice Genotypes | YWW | YTWS | RYTWS | SSI | GMP | STI | MPRO | MHAR | TI | YSI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Look Pla | 10.02 | 6.55 | 0.87 | 1.19 | 8.10 | 6.75 | 8.29 | 7.92 | 3.47 | 0.65 |
| 2 | Hom Nang Kaew | 8.04 | 5.54 | 0.73 | 1.07 | 6.67 | 4.58 | 6.79 | 6.56 | 2.50 | 0.69 |
| 3 | Pathum Thani-1 | 9.00 | 6.56 | 0.87 | 0.93 | 7.68 | 6.07 | 7.78 | 7.59 | 2.43 | 0.73 |
| 4 | Hom Chan | 7.00 | 5.52 | 0.73 | 0.72 | 6.21 | 3.97 | 6.26 | 6.17 | 1.48 | 0.79 |
| 5 | Hom Pathum | 9.68 | 7.55 | 1.00 | 0.75 | 8.54 | 7.51 | 8.61 | 8.48 | 2.13 | 0.78 |
| 6 | Dum Ja | 8.64 | 6.68 | 0.89 | 0.78 | 7.60 | 5.94 | 7.66 | 7.54 | 1.96 | 0.77 |
| 7 | Chor Lung | 8.22 | 6.03 | 0.80 | 0.91 | 7.04 | 5.10 | 7.12 | 6.96 | 2.18 | 0.73 |
| 8 | Sang Yod | 8.61 | 6.83 | 0.90 | 0.71 | 7.66 | 6.04 | 7.72 | 7.61 | 1.78 | 0.79 |
| 9 | Khao Dawk Mali-105 | 7.19 | 4.22 | 0.56 | 1.41 | 5.51 | 3.12 | 5.71 | 5.32 | 2.97 | 0.59 |
| 10 | RD-15 | 5.91 | 4.08 | 0.54 | 1.06 | 4.91 | 2.48 | 5.00 | 4.83 | 1.82 | 0.69 |
| 11 | Tia Malay Dang | 7.51 | 4.52 | 0.60 | 1.36 | 5.82 | 3.49 | 6.01 | 5.64 | 2.99 | 0.60 |
| 12 | Lep Nok | 9.72 | 6.37 | 0.84 | 1.18 | 7.87 | 6.37 | 8.04 | 7.69 | 3.35 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, T.; Hussain, N.; Ahmed, M.; Nualsri, C.; Duangpan, S. Responses of Lowland Rice Genotypes under Terminal Water Stress and Identification of Drought Tolerance to Stabilize Rice Productivity in Southern Thailand. Plants 2021, 10, 2565. https://doi.org/10.3390/plants10122565
Hussain T, Hussain N, Ahmed M, Nualsri C, Duangpan S. Responses of Lowland Rice Genotypes under Terminal Water Stress and Identification of Drought Tolerance to Stabilize Rice Productivity in Southern Thailand. Plants. 2021; 10(12):2565. https://doi.org/10.3390/plants10122565
Chicago/Turabian StyleHussain, Tajamul, Nurda Hussain, Mukhtar Ahmed, Charassri Nualsri, and Saowapa Duangpan. 2021. "Responses of Lowland Rice Genotypes under Terminal Water Stress and Identification of Drought Tolerance to Stabilize Rice Productivity in Southern Thailand" Plants 10, no. 12: 2565. https://doi.org/10.3390/plants10122565
APA StyleHussain, T., Hussain, N., Ahmed, M., Nualsri, C., & Duangpan, S. (2021). Responses of Lowland Rice Genotypes under Terminal Water Stress and Identification of Drought Tolerance to Stabilize Rice Productivity in Southern Thailand. Plants, 10(12), 2565. https://doi.org/10.3390/plants10122565









