Sophocarpine Alleviates Injury-Induced Intima Hyperplasia of Carotid Arteries by Suppressing Inflammation in a Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Histological Analysis
2.3. Western Blot Analysis
2.4. Statistical Analysis
3. Results
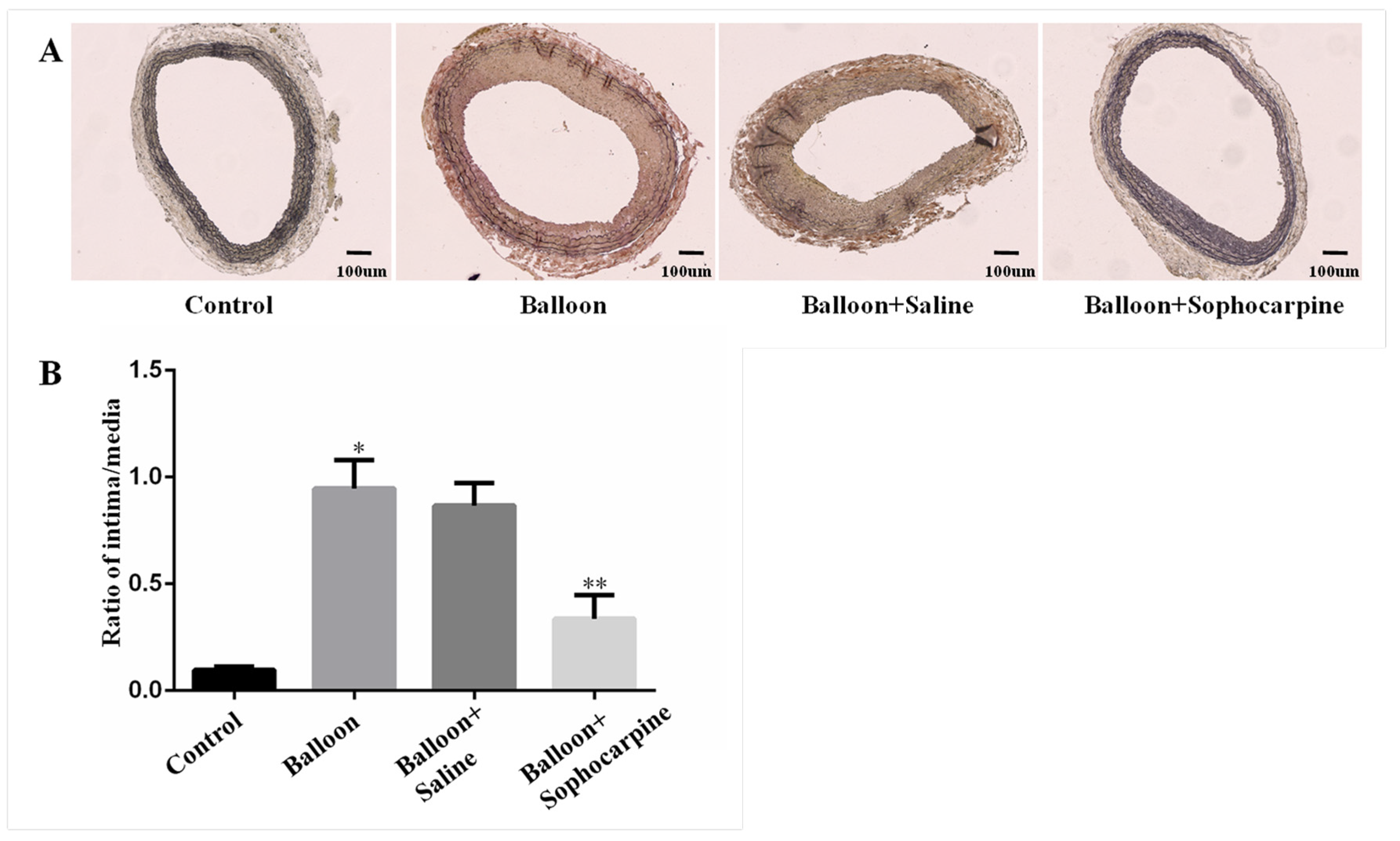
3.1. Balloon Injury Induces Stenosis in Rat Carotid Artery, and this Process Can Be Prevented by SPC
3.2. Balloon Injury Increases the Protein Levels of IL-6, IL-1β, MCP-1, NF-κB, TNF-α, ICAM-1 and VCAM-1, and These Effects Can Be Alleviated by SPC
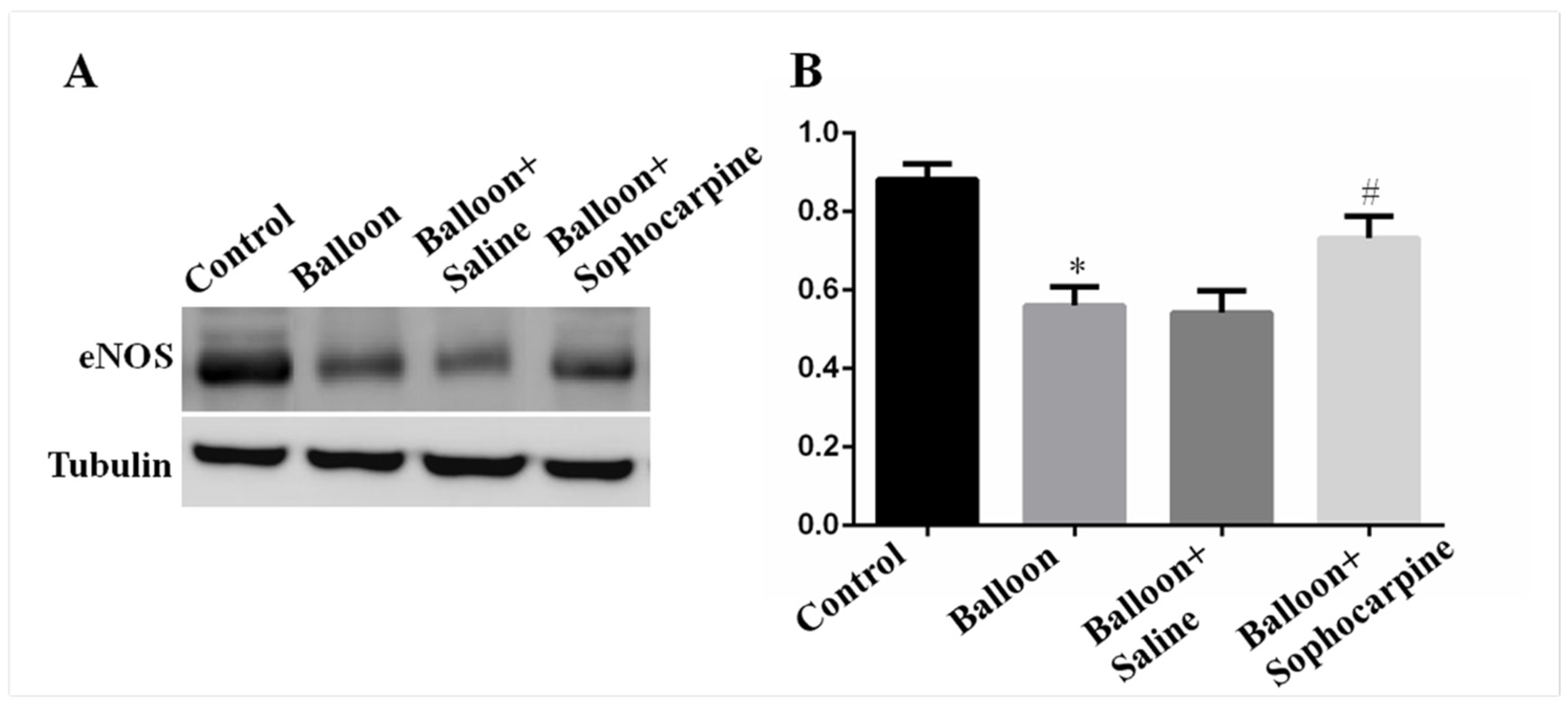
3.3. Balloon Injury Decreases the Expression of eNOS, and Its Level Can Be Elevated by SPC
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabani, V.; Davani, S. Translational Approaches in Cardiovascular Diseases by Omics. Curr. Issues Mol. Bio. 2018, 28, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobiyama, K.; Ley, K. Atherosclerosis. Cir. Res. 2018, 123, 1118–1120. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S. Inflammation in atherosclerosis. Arch. Cardiovasc. Dis. 2016, 109, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Stone, G.W.; Ormiston, J.; Kastrati, A. Coronary balloon angioplasty, stents, and scaffolds. Lancet 2017, 390, 781–792. [Google Scholar] [CrossRef]
- Hall, S.; Agrawal, D.K. Delivery of viral vectors for gene therapy in intimal hyperplasia and restenosis in atherosclerotic swine. Drug Deliv. Trans. Res. 2018, 8, 918–927. [Google Scholar] [CrossRef]
- Demirtas, K. Inflammation and In-Stent Restenosis. Angiology 2018, 69, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorriento, D.; Iaccarino, G. Inflammation and Cardiovascular Diseases: From pathogenesis to therapeutic target. Curr. Atheroscler Rep. 2014, 19, 435. [Google Scholar]
- Cai, X.H.; Guo, H.; Xie, B. Structural Modifications of Matrine-Type Alkaloids. Mini Rev. Med. Chem. 2018, 18, 730–744. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Tang, Z.; Xu, J.; Ma, M.; Pan, S.; Qiu, C.; Guan, G.; Wang, J. Matrine attenuates cardiac fibrosis by affecting ATF6 signaling pathway in diabetic cardiomyopathy. Eur. J. Pharmacol. 2017, 804, 21–30. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Chu, H.; Sun, X.; Ge, Z. Oral sophocarpine protects rat heart against pressure overload-induced cardiac fibrosis. Pharm. Bio. 2014, 52, 1045–1051. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Li, Y.; Xiao, Z.; Hu, Z.; Zhang, J. Sophocarpine and matrine inhibit the production of TNF-alpha and IL-6 in murine macrophages and prevent cachexia-related symptoms induced by colon26 adenocarcinoma in mice. Int. Immunopharmacol. 2008, 8, 1767–1772. [Google Scholar] [CrossRef]
- Li, C.; Gao, Y.; Tian, J.; Shen, J.; Xing, Y.; Liu, Z. Sophocarpine administration preserves myocardial function from ischemia-reperfusion in rats via NF-kappaB inactivation. J. Ethnopharmacol. 2011, 135, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Tulis, D.A.; Durante, W.; Liu, X.; Evans, A.J.; Peyton, K.J.; Schafer, A.I. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation 2001, 104, 2710–2715. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Lv, Y.; Zhang, Y.; Liu, F.; Zhu, L.; Pan, S.; Qiu, C.; Guo, Y.; Yang, T.; Wang, J. Matrine-Type Alkaloids Inhibit Advanced Glycation End Products Induced Reactive Oxygen Species-Mediated Apoptosis of Aortic Endothelial Cells In Vivo and In Vitro by Targeting MKK3 and p38MAPK Signaling. J. Am. Heart Assoc. 2017, 6, e007441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, N.; Guevara-Cruz, M.; Velázquez-Villegas, L.A.; Tovar, A.R. Nutrition and Atherosclerosis. Arch. Med. Res. 2015, 46, 408–426. [Google Scholar] [CrossRef]
- McGill, H.C., Jr. Smoking and the pathogenesis of atherosclerosis. Adv. Exp. Med. Biol. 1990, 273, 9–16. [Google Scholar]
- David, Y. Intima hyperplasia in murine models. Curr. Drug Targets. 2008, 9, 251–260. [Google Scholar]
- Yang, G.H.; Li, Y.C.; Wang, Z.Q.; Liu, B.; Ye, W.; Ni, L.; Zeng, R.; Miao, S.Y.; Wang, L.F.; Liu, C.W. Protective effect of melatonin on cigarette smoke-induced restenosis in rat carotid arteries after balloon injury. J. Pineal Res. 2014, 57, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Wang, Z.; Yang, G.; Li, T.; Liu, X.; Liu, C. Heme oxygenase-1 alleviates cigarette smoke-induced restenosis after vascular angioplasty by attenuating inflammation in rat model. Toxicol. Lett. 2016, 245, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Fiordelisi, A.; Iaccarino, G. NFkappaB is a Key Player in the Crosstalk between Inflammation and Cardiovascular Diseases. Int. J. Mol. Sci. 2019, 20, 1599. [Google Scholar] [CrossRef] [Green Version]
- Zou, F.; Wang, L.; Liu, H.; Wang, W.; Hu, L.; Xiong, X.; Wu, L.; Shen, Y.; Yang, R. Sophocarpine Suppresses NF-κB-Mediated Inflammation Both In Vitro and In Vivo and Inhibits Diabetic Cardiomyopathy. Front Pharmacol. 2019, 10, 1219. [Google Scholar] [CrossRef]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Torres, I.; Manzano-Pech, L.; Rubio-Ruíz, M.E.; Soto, M.E.; Guarner-Lans, V. Nitrosative Stress and Its Association with Cardiometabolic Disorders. Molecules 2020, 25, 2555. [Google Scholar] [CrossRef] [PubMed]
- Indolfi, C.; Torella, D.; Coppola, C.; Curcio, A.; Rodriguez, F.; Bilancio, A.; Leccia, A.; Arcucci, O.; Falco, M.; Leosco, D. Physical training increases eNOS vascular expression and activity and reduces restenosis after balloon angioplasty or arterial stenting in rats. Circ. Res. 2002, 91, 1190–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Zeng, R.; Song, X.; Liu, C.; Ni, L. Sophocarpine Alleviates Injury-Induced Intima Hyperplasia of Carotid Arteries by Suppressing Inflammation in a Rat Model. J. Clin. Med. 2021, 10, 5449. https://doi.org/10.3390/jcm10225449
Yang G, Zeng R, Song X, Liu C, Ni L. Sophocarpine Alleviates Injury-Induced Intima Hyperplasia of Carotid Arteries by Suppressing Inflammation in a Rat Model. Journal of Clinical Medicine. 2021; 10(22):5449. https://doi.org/10.3390/jcm10225449
Chicago/Turabian StyleYang, Genhuan, Rong Zeng, Xitao Song, Changwei Liu, and Leng Ni. 2021. "Sophocarpine Alleviates Injury-Induced Intima Hyperplasia of Carotid Arteries by Suppressing Inflammation in a Rat Model" Journal of Clinical Medicine 10, no. 22: 5449. https://doi.org/10.3390/jcm10225449
APA StyleYang, G., Zeng, R., Song, X., Liu, C., & Ni, L. (2021). Sophocarpine Alleviates Injury-Induced Intima Hyperplasia of Carotid Arteries by Suppressing Inflammation in a Rat Model. Journal of Clinical Medicine, 10(22), 5449. https://doi.org/10.3390/jcm10225449






