The Effect of Foliar Application of K2SO4 or KH2PO4 on Skin Color of the ‘Kyoho’ Grape
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Experimental Design
2.2. Determination of Physicochemical Indices of Berries
2.3. Chromaticity Values of Fruits
2.4. Pigment Content in Fruits
2.5. Transcriptome Sequencing and Analysis
2.6. Statistical Analysis
3. Results
3.1. Effect of Different Potassium Fertilizers on ‘Kyoho’ Grape Berries Quality
3.2. Effect of Different Potassium Fertilizers on Colorimetric Characteristics of ‘Kyoho’ Grape Berry Color
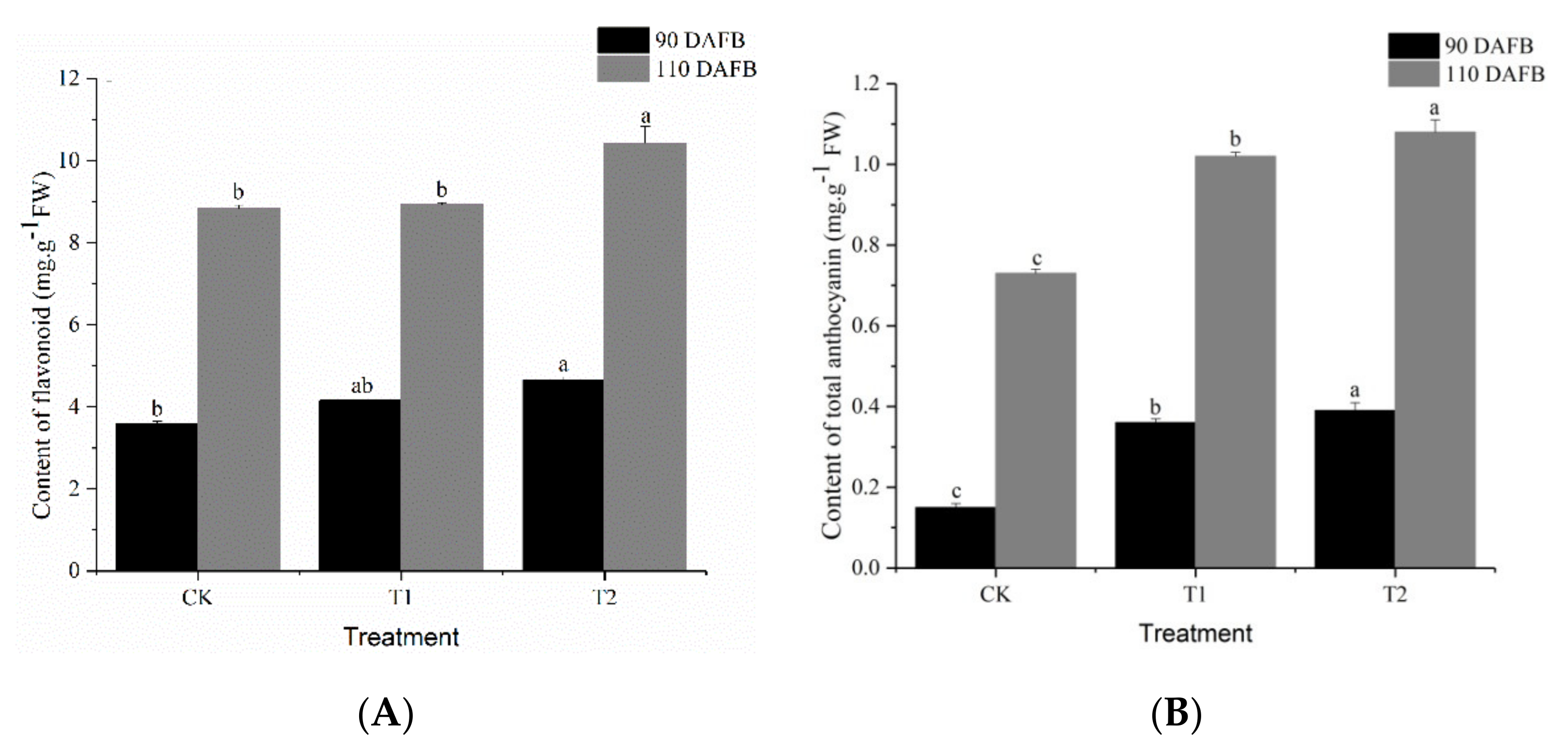
3.3. Effect of Different Potassium Fertilizers on Pigment Content in ‘Kyoho’ Grape Skin
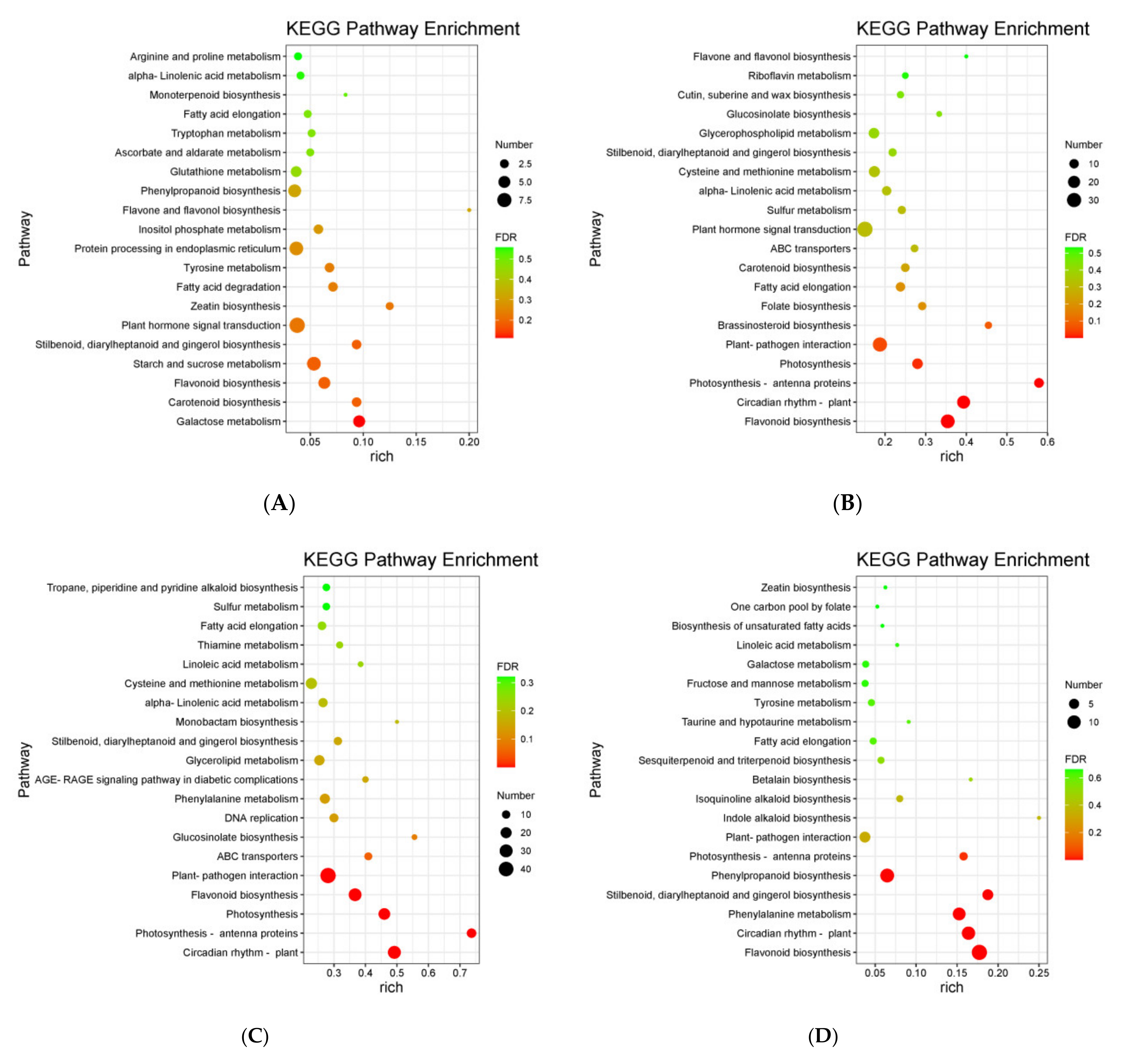
3.4. KEGG Enrichment Analysis of DEGs
3.5. Candidate Genes Associated with the Flavonoid Biosynthesis Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yang, B.; He, S.; Liu, Y.; Liu, B.; Ju, Y.; Kang, D.; Sun, X.; Fang, Y. Transcriptomics integrated with metabolomics reveals the effect of regulated deficit irrigation on anthocyanin biosynthesis in Cabernet Sauvignon grape berries. Food Chem. 2020, 314, 126170. [Google Scholar] [CrossRef]
- Conesa, M.R.; Falagán, N.; Rosa, J.M.D.L.; Aguayo, E.; Domingo, R.; Pastor, A.P. Post-veraison deficit irrigation regimes enhance berry coloration and health-promoting bioactive compounds in ‘Crimson Seedless’ table grapes. Agric. Water Manag. 2016, 163, 9–18. [Google Scholar] [CrossRef]
- Ding, S.; Che, C.; Xu, Z.; Du, X.; Li, J.; Li, J.-Q.; Xiao, Y.; Qin, Z. The effect of a novel powerful ABA mimic on the improvement of color in grapes and its mechanism. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Soubeyrand, E.; Basteau, C.; Hilbert, G.; van Leeuwen, C.; Delrot, S.; Gomes, E. Nitrogen supply affects anthocyanin biosynthetic and regulatory genes in grapevine cv. Cabernet-Sauvignon berries. Phytochemistry 2014, 103, 38–49. [Google Scholar] [CrossRef]
- Ren, Z.; Qiu, F.; Wang, Y.; Yu, W.; Liu, C.; Sun, Y.; Wang, Y.; Zhang, X.; Xing, S.; Tao, S.; et al. Network Analysis of Transcriptome and LC-MS Reveals a Possible Biosynthesis Pathway of Anthocyanins in Dendrobium officinale. Biomed. Res. Int. 2020, 2020, 6512895. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Flavonoid intake and mortality from cardiovascular disease and all causes: A meta-analysis of prospective cohort studies. Clin. Nutr. ESPEN 2017, 20, 68–77. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, H.; Gao, S.; Li, H.; Tao, J. Transcriptomic analysis of berry development and a corresponding analysis of anthocyanin biosynthesis in teinturier grape. J. Plant Interact. 2019, 14, 617–629. [Google Scholar] [CrossRef]
- Zhao, J.; Dixon, R.A. The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci. 2010, 15, 72–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, S.T.; Goto-Yamamoto, N.; Hashizume, K.; Esaka, M. Expression of the flavonoid 3′-hydroxylase and flavonoid 3′, 5′-hydroxylase genes and flavonoid composition in grape (Vitis vinifera). Plant Sci. 2006, 170, 61–69. [Google Scholar] [CrossRef]
- Premathilake, A.T.; Ni, J.; Shen, J.; Bai, S.; Teng, Y. Transcriptome analysis provides new insights into the transcriptional regulation of methyl jasmonate-induced flavonoid biosynthesis in pear calli. BMC Plant Biol. 2020, 20, 388. [Google Scholar] [CrossRef]
- Dong, W.; Li, M.; Li, Z.; Li, S.; Zhu, Y.; Xu, H.; Wang, Z. Transcriptome analysis of the molecular mechanism of Chrysanthemum flower color change under short-day photoperiods. Plant Physiol. Biochem. 2020, 146, 315–328. [Google Scholar] [CrossRef]
- Yue, J.; Zhu, C.; Zhou, Y.; Niu, X.; Miao, M.; Tang, X.; Chen, F.; Zhao, W.; Liu, Y. Transcriptome analysis of differentially expressed unigenes involved in flavonoid biosynthesis during flower development of Chrysanthemum morifolium ‘Chuju’. Sci. Rep. 2018, 8, 13414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.B.; Howard, S.; Chen, S.; Wang, Y.; Yu, O.; Kovacs, L.G.; Qiu, W. Berry skin development in Norton grape: Distinct patterns of transcriptional regulation and flavonoid biosynthesis. BMC Plant Biol. 2011, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Topalovic, A.; Slatnar, A.; Stampar, F.; Knezevic, M.; Veberic, R. Influence of foliar fertilization with P and K on chemical constituents of grape cv. ‘Cardinal’. J. Agric. Food Chem. 2011, 59, 10303–10310. [Google Scholar] [CrossRef]
- Souri, M.K.; Dehnavard, S. Tomato plant growth, leaf nutrient concentrations and fruit quality under nitrogen foliar applications. Adv. Hort. Sci. 2018, 32, 41–47. [Google Scholar]
- Nong, M.; Chen, J.; Gan, W.; Pan, L.; Xing, Y.; Liu, Y. Effects of Amino Acid Selenium Foliar Fertilizer on Selenium Content and Quality of Mango. Agric. Biotech. 2020, 9, 92–94. [Google Scholar]
- Shi, P.; Song, C.; Chen, H.; Duan, B.; Zhang, Z.; Meng, J. Foliar applications of iron promote flavonoids accumulation in grape berry of Vitis vinifera cv. Merlot grown in the iron deficiency soil. Food Chem. 2018, 253, 164–170. [Google Scholar] [CrossRef]
- Román, S.M.-S.; Garde-Cerdán, T.; Baroja, E.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Foliar application of phenylalanine plus methyl jasmonate as a tool to improve Grenache grape aromatic composition. Sci. Hortic. 2020, 272, 109515. [Google Scholar] [CrossRef]
- Yamamoto, L.Y.; Marinho de Assis, A.; Roberto, S.R.; Bovolenta, Y.R.; Nixdorf, S.L.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Application of abscisic acid (S-ABA) to cv. Isabel grapes (Vitis vinifera × Vitis labrusca) for color improvement: Effects on color, phenolic composition and antioxidant capacity of their grape juice. Food Res. Int. 2015, 77, 572–583. [Google Scholar] [CrossRef]
- Olivares, D.; Contreras, C.; Muñoz, V.; Rivera, S.; González-Agüero, M.; Retamales, J.; Defilippi, B.G. Relationship among color development, anthocyanin and pigment-related gene expression in ‘Crimson Seedless’ grapes treated with abscisic acid and sucrose. Plant Physiol. Bioch. 2017, 115, 286–297. [Google Scholar] [CrossRef]
- Aslani, M.; Souri, M.K. Beneficial effects of foliar application of organic chelate fertilizers on French bean production under field conditions in a calcareous soil. AHS 2018, 32, 265–272. [Google Scholar]
- Sekhon, N.K.; Singh, C.B. Plant Nutrient Status during Boll Development and Seed Cotton Yield as Affected by Foliar Application of Different Sources of Potassium. AJPS 2013, 4, 1409–1417. [Google Scholar] [CrossRef] [Green Version]
- Zareei, E.; Javadi, T.; Aryal, R. Biochemical composition and antioxidant activity affected by spraying potassium sulfate in black grape (Vitis vinifera L. cv. Rasha). J. Sci. Food. Agri. 2018, 98, 5632–5638. [Google Scholar] [CrossRef]
- Haldankar, P.M.; Thorat, V.; Mayekar, A.J.; Khirsagar, P.J.; Korake, G.N.; Khandekar, R.G. Effect of paclobutrazol and post flowering foliar sprays of nutrients for accelerating harvesting of jackfruit. Indian. J. Hortic. 2014, 71, 476–480. [Google Scholar]
- Li, A.; Gui, M.; Wang, M.; Liu, Z. Effects of foliar application of NaH2PO4, K2SO4 and KH2PO4 on Expression of Genes Related to Phosphorus and Potassium Assimilate in Grape. MPB 2018, 16, 5415–5426. [Google Scholar]
- Ben Rejeb, I.; Dhen, N.; Kassebi, S.; Gargouri, M.; Roussis, I.G. Quality Evaluation and Functional Properties of Reduced Sugar Jellies Formulated from Citrus Fruits. J. Chem. 2020, 2020, 5476872. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Skvarc, A.; Rusjan, D. Biochemical composition of different table grape cultivars produced in Slovenia. J. Hortic. Sci. Biotech. 2019, 94, 368–377. [Google Scholar] [CrossRef]
- Carreño, J.; Martínez, A.; Almela, L.; Fernández-López, J.A. Measuring the color of table grapes. Res. Appl. 1996, 21, 50–54. [Google Scholar]
- Jiang, W.; Na, L.; Dapeng, Z.; Lyndel, M.; Bing, C.; Yuanjing, L.; Lihua, S. Elevated temperature and drought stress significantly affect fruit quality and activity of anthocyanin-related enzymes in jujube (Ziziphus jujuba Mill. cv. ‘Lingwuchangzao’). PLoS ONE 2020, 15, e0241491. [Google Scholar]
- Guo, Y.; Bai, J.; Duan, X.; Wang, J. Accumulation characteristics of carotenoids and adaptive fruit color variation in ornamental pepper. Sci. Hortic. 2021, 275, 109699. [Google Scholar] [CrossRef]
- Haider, M.S.; Kurjogi, M.M.; Khalil-Ur-Rehman, M.; Fiaz, M.; Pervaiz, T.; Jiu, S.; Haifeng, J.; Chen, W.; Fang, J. Grapevine immune signaling network in response to drought stress as revealed by transcriptomic analysis. Plant Physiol. Biochem. 2017, 121, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, M.; Wang, M.; Xu, Y.; Chen, W.; Yang, G. Transcriptome analysis of calcium-induced accumulation of anthocyanins in grape skin. Sci. Hortic. 2020, 260, 108871. [Google Scholar] [CrossRef]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Yao, Q.; Huang, H.; Tong, Y.; Tong, E.; Gao, L. Transcriptome Analysis Identifies Candidate Genes Related to Triacylglycerol and Pigment Biosynthesis and Photoperiodic Flowering in the Ornamental and Oil-Producing Plant, Camellia reticulata (Theaceae). Front. Plant. Sci. 2016, 7, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.-Y.; Dillies, M.-A. SARTools: A DESeq2- and EdgeR-Based R Pipeline for Comprehensive Differential Analysis of RNA-Seq Data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef] [Green Version]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, 161–476. [Google Scholar] [CrossRef] [Green Version]
- Minoru, K.; Michihiro, A.; Susumu, G.; Masahiro, H.; Mika, H.; Masumi, I.; Toshiaki, K.; Shuichi, K.; Shujiro, O.; Toshiaki, T.; et al. KEGG for linking genomes to life and the environment. Narnia 2008, 36 (Suppl. 1), D480–D484. [Google Scholar]
- Mao, X.; Cai, T.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 7. [Google Scholar] [CrossRef]
- Chapagain, B.P.; Wiesman, Z. Effect of Nutri-Vant-PeaK foliar spray on plant development, yield, and fruit quality in greenhouse tomatoes. Sci. Hortic. 2003, 102, 177–188. [Google Scholar] [CrossRef]
- Haldankar, P.M.; Somavanshi, A.V. Studies on the effect of foliar sprays of nutrients after fruit set on harvesting, yield and quality of kokum (Garcinia indica Choisy). Indian J. Hortic. 2015, 72, 38–42. [Google Scholar] [CrossRef]
- Ferrara, G.; Mazzeo, A.; Matarrese, A.M.S.; Pacucci, C.; Pacifico, A.; Gambacorta, G.; Faccia, M.; Trani, A.; Gallo, V.; Cafagna, I.; et al. Application of Abscisic Acid (S-ABA) to ‘Crimson Seedless’ Grape Berries in a Mediterranean Climate: Effects on Color, Chemical Characteristics, Metabolic Profile, and S-ABA Concentration. J. Plant. Growth Regul. 2013, 32, 491–505. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J.; Deng, X.; Lei, Y.; Xie, S.; Guo, S.; Ren, R.; Li, J.; Zhang, Z.; Xu, T. Foliar-sprayed manganese sulfate improves flavonoid content in grape berry skin of Cabernet Sauvignon (Vitis vinifera L.) growing on alkaline soil and wine chromatic characteristics. Food Chem. 2020, 314, 126182. [Google Scholar] [CrossRef]
- Shahab, M.; Roberto, S.R.; Ahmed, S.; Colombo, R.C.; Silvestre, J.P.; Koyama, R.; Souza, R.T.D. Relationship between anthocyanins and skin color of table grapes treated with abscisic acid at different stages of berry ripening. Sci. Hortic. 2020, 259, 108859. [Google Scholar] [CrossRef]
- Deng, Q.; Xia, H.; Lin, L.; Wang, J.; Yuan, L.; Li, K.; Zhang, J.; Lv, X.; Liang, D. SUNRED, a natural extract-based biostimulant, application stimulates anthocyanin production in the skins of grapes. Sci. Rep. 2019, 9, 2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornero-Méndez, D.; de Gómez-Ladrón Guevara, R.; Mínguez-Mosquera, M.I. Carotenoid biosynthesis changes in five red pepper (Capsicum annuum L.) cultivars during ripening. Cultivar selection for breeding. J. Agric. Food Chem. 2000, 48, 3857–3864. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Almela, L.; Muñoz, J.A.; Hidalgo, V.; Carreño, J. Dependence between colour and individual anthocyanin content in ripening grapes. Food Res. Int. 1998, 31, 667–672. [Google Scholar] [CrossRef]
- Ban, T.; Ishimaru, M.; Kobayashi, S.; Goto-Yamamoto, N.; Horiuchi, S. Abscisic acid and 2,4-dichlorophenoxyacetic acid affect the expression of anthocyanin biosynthetic pathway genes in ‘Kyoho’ grape berries. J. Hortic. Sci. Biotech. 2015, 78, 586–589. [Google Scholar] [CrossRef]
- Wei, L.; Cao, Y.; Cheng, J.; Xiang, J.; Shen, B.; Wu, J. Comparative transcriptome analyses of a table grape ‘Summer Black’ and its early-ripening mutant ‘Tiangong Moyu’ identify candidate genes potentially involved in berry development and ripening. J. Plant Interact. 2020, 15, 213–222. [Google Scholar] [CrossRef]




| Treatment | Total Soluble Solids (%) | Titratable Acid (%) | Solidity–Acid Ratio | pH | |
|---|---|---|---|---|---|
| CK | 14.20 ± 0.76 b | 0.66 ± 0.05 a | 21.43 ± 0.25 c | 3.34 ± 0.01 c | |
| 90 DAFB | T1 | 13.80 ± 0.15 b | 0.51 ± 0.02 b | 27.19 ± 0.19 b | 3.42 ± 0.02 b |
| T2 | 14.90 ± 0.39 a | 0.34 ± 0.01 c | 44.15 ± 0.22 a | 3.75 ± 0.01 a | |
| CK | 17.40 ± 0.43 b | 0.35 ± 0.01 a | 50.07 ± 0.43 c | 3.52 ± 0.01 c | |
| 110 DAFB | T1 | 17.80 ± 0.60 ab | 0.28 ± 0.06 b | 64.14 ± 0.16 b | 3.86 ± 0.02 b |
| T2 | 18.10 ± 0.08 a | 0.24 ± 0.01 c | 74.64 ± 0.28 a | 3.95 ± 0.03 a |
| 90 DAFB | 110 DAFB | |||||
|---|---|---|---|---|---|---|
| CK | T1 | T2 | CK | T1 | T2 | |
| L* | 33.39 ± 0.90 a | 30.65 ± 1.42 b | 29.02 ± 0.93 c | 27.18 ± 1.10 a | 26.73 ± 0.18 b | 26.54 ± 0.62 b |
| a* | 3.77 ± 1.16 b | 5.21 ± 0.64 a | 6.47 ± 0.82 a | 4.37 ± 0.76 a | 3.05 ± 1.63 b | 1.67 ± 0.51 c |
| b* | 3.12 ± 1.24 a | 2.19 ± 0.73 b | −0.71 ± 0.39 c | −0.45 ± 0.11 a | −0.50 ± 0.13 b | −0.91 ± 0.23 a |
| C* | 4.89 ± 0.42 b | 5.65 ± 0.56 a | 6.51 ± 0.80 a | 4.39 ± 0.77 a | 3.09 ± 1.58 b | 1.90 ± 0.48 c |
| h° | 39.61 ± 2.54 b | 22.79 ± 1.29 b | 353.74 ± 10.33 a | 354.12 ± 11.24 a | 350.69 ± 5.67 ab | 331.41 ± 7.86 b |
| CIRG | 3.67 ± 0.54 c | 4.33 ± 0.57 b | 5.24 ± 0.87 a | 5.88 ± 0.23 c | 6.34 ± 0.56 b | 7.60 ± 1.21 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Li, P.; Jia, H.; Phillip, F.O.; Bao, X.; Zhao, F.; Zhao, B.; Feng, J.; Yu, K. The Effect of Foliar Application of K2SO4 or KH2PO4 on Skin Color of the ‘Kyoho’ Grape. Agronomy 2021, 11, 2361. https://doi.org/10.3390/agronomy11112361
Wu L, Li P, Jia H, Phillip FO, Bao X, Zhao F, Zhao B, Feng J, Yu K. The Effect of Foliar Application of K2SO4 or KH2PO4 on Skin Color of the ‘Kyoho’ Grape. Agronomy. 2021; 11(11):2361. https://doi.org/10.3390/agronomy11112361
Chicago/Turabian StyleWu, Linnan, Pengcheng Li, Haifeng Jia, Fesobi Olumide Phillip, Xingcheng Bao, Fengyun Zhao, Benzhou Zhao, Jianrong Feng, and Kun Yu. 2021. "The Effect of Foliar Application of K2SO4 or KH2PO4 on Skin Color of the ‘Kyoho’ Grape" Agronomy 11, no. 11: 2361. https://doi.org/10.3390/agronomy11112361





