A Continental-Scale Connectivity Analysis to Predict Current and Future Colonization Trends of Biofuel Plant’s Pests for Sub-Saharan African Countries
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Species and Study Area
2.2. Connectivity Modelling
2.3. Connectivity Changes and Geostatistical Analyses
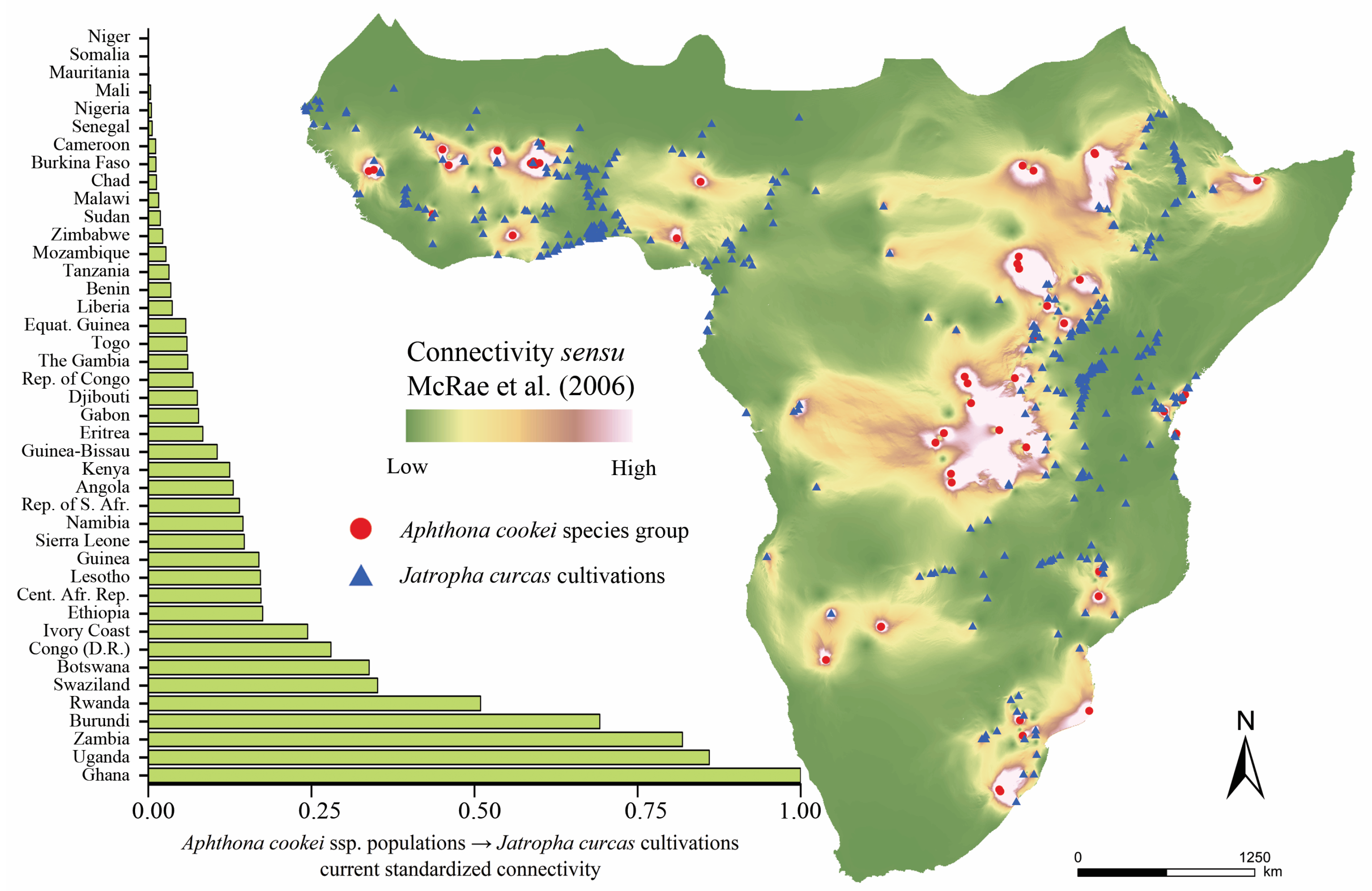
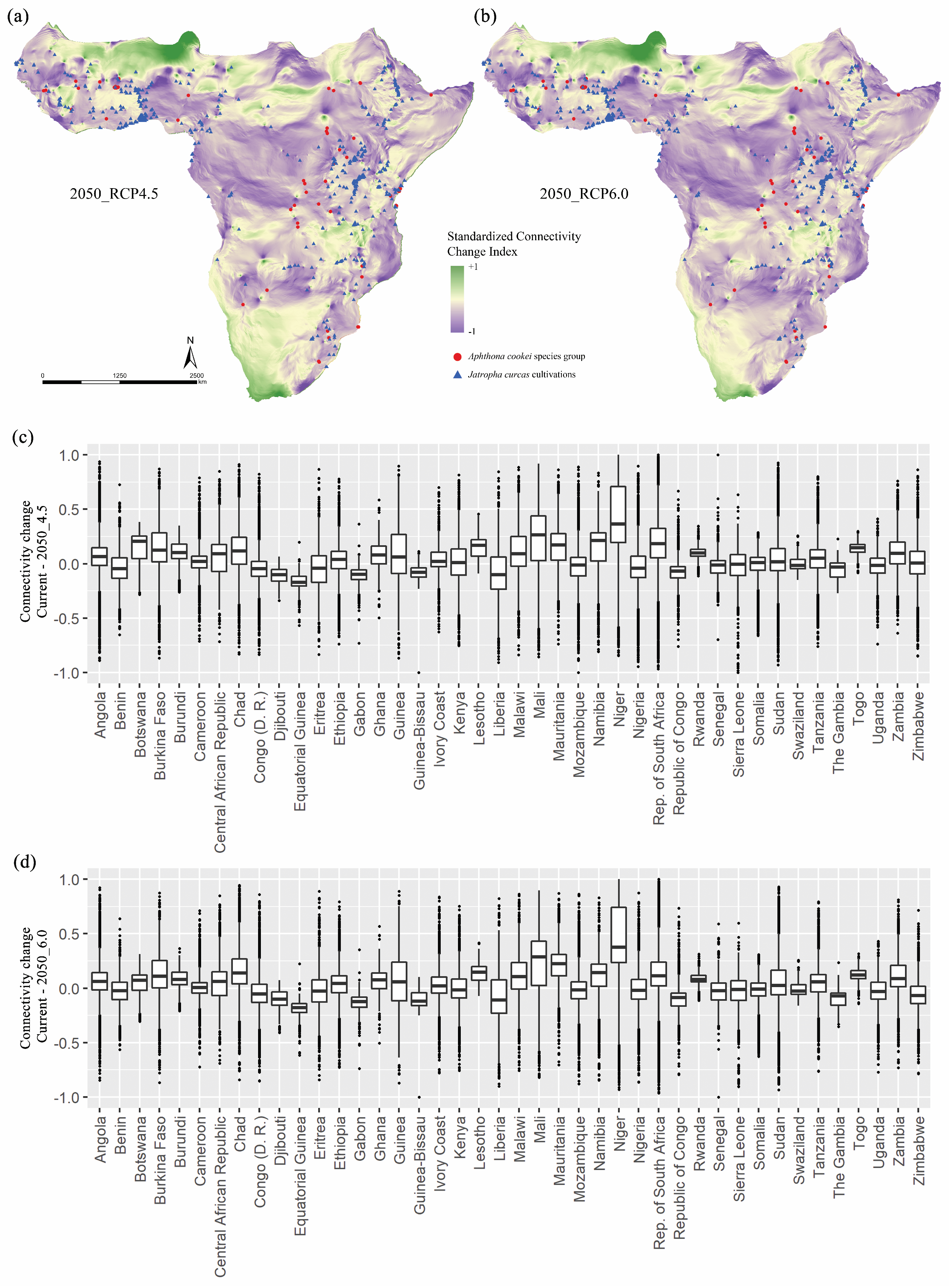
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koh, L.P.; Dunn, R.R.; Sodhi, N.S.; Colwell, R.K.; Proctor, H.C.; Smith, V.S. Species Coextinctions and the Biodiversity Crisis. Science 2004, 305, 1632–1634. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, F.; Loupa-Ramos, I.; Carvalheiro, J. Are Biodiversity Perception and Attitudes Context Dependent? A Comparative Study Using a Mixed-Method Approach. Land Use Policy 2021, 109, 105703. [Google Scholar]
- Marzialetti, F.; Frate, L.; De Simone, W.; Frattaroli, A.R.; Acosta, A.T.R.; Carranza, M.L. Unmanned Aerial Vehicle (UAV)-Based Mapping of Acacia saligna Invasion in the Mediterranean Coast. Remote Sens. 2021, 13, 3361. [Google Scholar] [CrossRef]
- De Simone, W.; Allegrezza, M.; Frattaroli, A.R.; Montecchiari, S.; Tesei, G.; Zuccarello, V.; Di Musciano, M. From Remote Sensing to Species Distribution Modelling: An Integrated Workflow to Monitor Spreading Species in Key Grassland Habitats. Remote Sens. 2021, 13, 1904. [Google Scholar] [CrossRef]
- Iannella, M.; Console, G.; D’Alessandro, P.; Cerasoli, F.; Mantoni, C.; Ruggieri, F.; Di Donato, F.; Biondi, M. Preliminary Analysis of the Diet of Triturus carnifex and Pollution in Mountain Karst Ponds in Central Apennines. Water 2020, 12, 44. [Google Scholar] [CrossRef]
- IPBES, D.S. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES secretariat: Bonn, Germany, 2019; Available online: https://www.ipbes.net/global-assessment-report-biodiversity-ecosystem-services (accessed on 18 August 2021).
- Iannella, M.; De Simone, W.; D’Alessandro, P.; Console, G.; Biondi, M. Investigating the Current and Future Co-Occurrence of Ambrosia Artemisiifolia and Ophraella communa in Europe through Ecological Modelling and Remote Sensing Data Analysis. Int. J. Environ. Res. Public Health 2019, 16, 3416. [Google Scholar] [CrossRef] [PubMed]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P. Global Threats from Invasive Alien Species in the Twenty-First Century and National Response Capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.N.; Balmford, A.; Brook, B.W.; Buettel, J.C.; Galetti, M.; Guangchun, L.; Wilmshurst, J.M. Biodiversity Losses and Conservation Responses in the Anthropocene. Science 2017, 356, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Daily, G.C.; Kareiva, P.M.; Polasky, S.; Ricketts, T.H.; Tallis, H. Natural Capital: Theory & Practice of Mapping Ecosystem Services; Oxford University Press: Oxford, UK, 2011; ISBN 6613264938. [Google Scholar]
- Secretariat of the Convention on Biological Diversity. Global Biodiversity Outlook 5; Secretariat of the Convention on Biological Diversity, UN environment programme: Montreal, QC, Canada, 2020. [Google Scholar]
- Kamel, D.A.; Farag, H.A.; Amin, N.K.; Zatout, A.A.; Ali, R.M. Smart Utilization of Jatropha (Jatropha curcas Linnaeus) Seeds for Biodiesel Production: Optimization and Mechanism. Ind. Crop. Prod. 2018, 111, 407–413. [Google Scholar] [CrossRef]
- Gonzàles, N.F.C. International Experiences with the Cultivation of Jatropha curcas for Biodiesel Production. Energy 2016, 112, 1245–1258. [Google Scholar] [CrossRef]
- Trabucco, A.; Achten, W.M.; Bowe, C.; Aerts, R.; Van Orshoven, J.; Norgrove, L.; Muys, B. Global Mapping of Jatropha curcas Yield Based on Response of Fitness to Present and Future Climate. Gcb Bioenergy 2010, 2, 139–151. [Google Scholar] [CrossRef]
- Correa, D.F.; Beyer, H.L.; Possingham, H.P.; Thomas-Hall, S.R.; Schenk, P.M. Biodiversity Impacts of Bioenergy Production: Microalgae vs. First Generation Biofuels. Renew. Sustain. Energy Rev. 2017, 74, 1131–1146. [Google Scholar] [CrossRef]
- Dale, V.H.; Kline, K.L.; Wiens, J.; Fargione, J. Biofuels: Implications for Land Use and Biodiversity; Ecological Society of America: Washington, DC, USA, 2010; p. 13. [Google Scholar]
- Parawira, W. Biodiesel Production from Jatropha curcas: A Review. Sci. Res. Essays 2010, 5, 1796–1808. [Google Scholar]
- Iannella, M.; D’Alessandro, P.; Biondi, M. Forecasting the Spread Associated with Climate Change in Eastern Europe of the Invasive Asiatic Flea Beetle, Luperomorpha xanthodera (Coleoptera: Chrysomelidae). Eur. J. Entomol. 2020, 117, 130–138. [Google Scholar] [CrossRef]
- Iannella, M.; D’Alessandro, P.; Longo, S.; Biondi, M. New Records and Potential Distribution by Ecological Niche Modelling of the Adventive Leaf Beetle Monoxia obesula Blake in the Mediterranean Area (Coleoptera, Chrysomelidae, Galerucinae). Bull. Insectol. 2019, 72, 135–142. [Google Scholar]
- Iannella, M.; De Simone, W.; D’Alessandro, P.; Biondi, M. Climate Change Favours Connectivity between Virus-Bearing Pest and Rice Cultivations in Sub-Saharan Africa, Depressing Local Economies. PeerJ 2021. [Google Scholar] [CrossRef]
- De Simone, W.; Iannella, M.; D’Alessandro, P.; Biondi, M. Assessing Influence in Biofuel Production and Ecosystem Services When Environmental Changes Affect Plant–Pest Relationships. Gcb Bioenergy 2020, 12, 864–877. [Google Scholar] [CrossRef]
- Biondi, M.; Urbani, F.; D’Alessandro, P. Revision of the Aphthona cookei Species Group in Sub-Saharan Africa: Pests of Jatropha curcas L. in Biodiesel Plantations (Coleoptera, Chrysomelidae, Galerucinae, Alticini). Entomologia 2013, 1, e7. [Google Scholar] [CrossRef][Green Version]
- Sawadogo, A.; Nacro, S. The Effect of Aphthona whitfieldi (Coleoptera: Chrysomelidae) Populations’ Density on the Growth of Jatropha curcas in Burkina Faso. Adv. Entomol. 2017, 5, 127–137. [Google Scholar] [CrossRef]
- Sawadogo, A.; Nagalo, E.; Nacro, S.; Rouamba, M.; Kenis, M. Population Dynamics of Aphthona whitfieldi (Coleoptera: Chrysomelidae), Pest of Jatropha curcas, and Environmental Factors Favoring Its Abundance in Burkina Faso. J. Insect Sci. 2015, 15, 108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mačić, V.; Albano, P.G.; Almpanidou, V.; Claudet, J.; Corrales, X.; Essl, F.; Evagelopoulos, A.; Giovos, I.; Jimenez, C.; Kark, S. Biological Invasions in Conservation Planning: A Global Systematic Review. Front. Mar. Sci. 2018, 5, 178. [Google Scholar] [CrossRef]
- Iannella, M.; Console, G.; Cerasoli, F.; De Simone, W.; D’Alessandro, P.; Biondi, M. A Step towards SDMs: A “Couple-and-Weigh” Framework Based on Accessible Data for Biodiversity Conservation and Landscape Planning. Divers. Distrib. 2021, 00, 1–16. [Google Scholar] [CrossRef]
- Heller, J. Physic Nut, Jatropha Curcas L.; International Plant Genetic Resources Institute: Rome, Italiy, 1996; Volume 1, ISBN 92-9043-278-0. [Google Scholar]
- Lama, A.D.; Vuorisalo, T.; Niemelä, P. Global Patterns of Arthropod Herbivory on an Invasive Plant, the Physic Nut (Jatropha curcas L.). J. Appl. Entomol. 2015, 139, 1–10. [Google Scholar] [CrossRef]
- USDA. Germplasm Resources Information Network (GRIN-Taxonomy); Von National Germplasm Resources Laboratory: Beltsville, Maryland, 2020; p. 997. Available online: https://npgsweb.ars-grin.gov/gringlobal/taxonomyfamily.aspx (accessed on 20 August 2021).
- De Jongh, J.; van der Putten, E. The Jatropha Handbook: From Cultivation to Application; FACT Foundation: Eindhoven, The Netherlands, 2010; pp. 1–118. ISBN 978-90-815219-1-8. [Google Scholar]
- Biondi, M.; D’Alessandro, P. Afrotropical Flea Beetle Genera: A Key to Their Identification, Updated Catalogue and Biogeographical Analysis (Coleoptera, Chrysomelidae, Galerucinae, Alticini). ZooKeys 2012, 1–158. [Google Scholar] [CrossRef] [PubMed]
- Anitha, K.; Varaprasad, K.S. Jatropha pests and diseases: An overview. In Jatropha, Challenges for a New Energy Crop; Springer: New York, NY, USA, 2012; pp. 175–218. [Google Scholar]
- Nielsen, F.; De Jongh, J. Jatropha curcas Oil Production for Local Development in Mozambique. Afr. Crop. Sci. Conf. Proc. 2009, 9, 71–75. [Google Scholar]
- Sawadogo, A.; Nacro, S. Some Biological Features of Aphtona whitfieldi Bryant (Coleoptera: Chrysomelidae), an Insect Pest of Jatropha curcas L. in Burkina Faso. Adv. Entomol. 2016, 4, 231–239. [Google Scholar] [CrossRef]
- Dickson, B.G.; Albano, C.M.; Anantharaman, R.; Beier, P.; Fargione, J.; Graves, T.A.; Gray, M.E.; Hall, K.R.; Lawler, J.J.; Leonard, P.B. Circuit-theory Applications to Connectivity Science and Conservation. Conserv. Biol. 2019, 33, 239–249. [Google Scholar] [CrossRef] [PubMed]
- McRae, B.H.; Dickson, B.G.; Keitt, T.H.; Shah, V.B. Using Circuit Theory to Model Connectivity in Ecology, Evolution, and Conservation. Ecology 2008, 89, 2712–2724. [Google Scholar] [CrossRef] [PubMed]
- McRae, B.H.; Shah, V.; Edelman, A. Circuitscape: Modeling Landscape Connectivity to Promote Conservation and Human Health. Nat. Conserv. 2016, 14. [Google Scholar] [CrossRef]
- Anantharaman, R.; Hall, K.; Shah, V.B.; Edelman, A. Circuitscape in Julia: High Performance Connectivity Modelling to Support Conservation Decisions. Proc. JuliaCon Conf. 2020, 1, 58. [Google Scholar] [CrossRef]
- Bezanson, J.; Edelman, A.; Karpinski, S.; Shah, V.B. Julia: A Fresh Approach to Numerical Computing. SIAM Rev. 2017, 59, 65–98. [Google Scholar] [CrossRef]
- McClure, M.L.; Hansen, A.J.; Inman, R.M. Connecting Models to Movements: Testing Connectivity Model Predictions against Empirical Migration and Dispersal Data. Landsc. Ecol. 2016, 31, 1419–1432. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017; ISBN 0-521-76513-7. [Google Scholar]
- Gray, L.N.; Barley, A.J.; Poe, S.; Thomson, R.C.; Nieto-Montes de Oca, A.; Wang, I.J. Phylogeography of a Widespread Lizard Complex Reflects Patterns of Both Geographic and Ecological Isolation. Mol. Ecol. 2019, 28, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Meinshausen, M.; Smith, S.J.; Calvin, K.; Daniel, J.S.; Kainuma, M.L.T.; Lamarque, J.-F.; Matsumoto, K.; Montzka, S.A.; Raper, S.C.B.; Riahi, K. The RCP Greenhouse Gas Concentrations and Their Extensions from 1765 to 2300. Clim. Chang. 2011, 109, 213. [Google Scholar] [CrossRef]
- ESRI ArcMap 10.0. ESRI, Redlands, California 2010. Available online: https://www.esri.com/en-us/home (accessed on 17 August 2021).
- De Mendiburu, F.; Yaseen, M. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.4.0. 2020. Available online: https://myaseen208.github.io/agricolae/ (accessed on 20 August 2021).
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2016. Available online: http://www.R-project.org/ (accessed on 20 August 2021).
- Gexsi, L.L.P. Global Market Study on Jatropha; Final Report Prepared for the World Wide Fund for Nature (WWF); GEXSI: London, UK; Berlin, Germany, 2008. [Google Scholar]
- Terren, M.; Mignon, J.; De Clerck, C.; Jijakli, H.; Savery, S.; Jacquet de Haveskercke, P.; Winandy, S.; Mergeai, G. Principal Disease and Insect Pests of Jatropha curcas L. in the Lower Valley of the Senegal River. Tropicultura 2012, 30, 222–229. [Google Scholar]
- Prasad, D.R.; Izam, A.; Khan, M.M.R. Jatropha curcas: Plant of Medical Benefits. J. Med. Plants Res. 2012, 6, 2691–2699. [Google Scholar]
- Habou, Z.A.; Adam, T.; Haubruge, E.; Mergeai, G.; Verheggen, F.J. Insects Associated with Jatropha curcas Linn. (Euphorbiaceae) in West Niger. J. Insect Sci. 2014, 14. [Google Scholar] [CrossRef][Green Version]
- Chowdhury, J.; Al Basir, F.; Pal, J.; Roy, P.K. Pest Control for Jatropha curcas Plant through Viral Disease: A Mathematical Approach. Nonlinear Stud. 2016, 23, 515–530. [Google Scholar]
- Thunes, K.H.; Ratnadass, A.; Nikiema, A.; Claude, Z. Pest Damage on Jatropha curcas (Euphorbiaceae): The Effect of Seedling Irrigation in Sahelian Niger. Int. J. Trop. Insect Sci. 2016, 36, 140–145. [Google Scholar] [CrossRef]
- Lashari, M.A.; Sheikh, J.; Ali, A.; Memon, S.A.; un Nisa, M.; Rashid, M. Monitoring of Insect Pests and Their Natural Enemies on Biodiesel Plant, Jatropha curcas L. Sci. Int. (Lahore) 2017, 29, 985–991. [Google Scholar]
- Ewunie, G.A.; Morken, J.; Lekang, O.I.; Yigezu, Z.D. Factors Affecting the Potential of Jatropha curcas for Sustainable Biodiesel Production: A Critical Review. Renew. Sustain. Energy Rev. 2020, 137, 110500. [Google Scholar] [CrossRef]
- Von Maltitz, G.; Gasparatos, A.; Fabricius, C. The Rise, Fall and Potential Resilience Benefits of Jatropha in Southern Africa. Sustainability 2014, 6, 3615–3643. [Google Scholar] [CrossRef]
| Mann–Whitney U Test | High Number of Cultivations | High Current Connectivity | SCCI 2050 Trend | |||||
|---|---|---|---|---|---|---|---|---|
| Significance | U | p | ∆ (percentage) | 2050_4.5 | 2050_6.0 | |||
| Benin | Yes | 32592 | 0.004 | 1.5% | X | ↓ | ↓ | |
| Burundi | Yes | 34001 | 0.000 | 2.1% | X | ↑ | ↑↑ | |
| Centr. Afr. Rep. | No | 27316 | 0.503 | 0.7% | X | ↑↑ | ↑↑ | |
| Ivory Coast | No | 30750 | 0.106 | 1.4% | X | X | ↑ | ↑ |
| Ethiopia | No | 25691 | 0.080 | 1.9% | X | X | ↑↑ | ↑ |
| Ghana | Yes | 34962 | 0.000 | 2.1% | X | X | ↑ | ↑ |
| Guinea | Yes | 38722 | 0.000 | 5.5% | X | ↓↓ | ↓ | |
| Rwanda | Yes | 32635 | 0.004 | 2.7% | X | ↑ | ↑ | |
| Rep. of South Africa | Yes | 34351 | 0.000 | 5.8% | X | ↓↓ | ↓ | |
| Tanzania | No | 29307 | 0.512 | 1.1% | X | — | — | |
| Uganda | No | 28912 | 0.694 | 0.6% | X | X | ↓ | ↓ |
| Zambia | No | 27117 | 0.422 | 0.1% | X | X | ↑ | ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iannella, M.; De Simone, W.; Cerasoli, F.; D’Alessandro, P.; Biondi, M. A Continental-Scale Connectivity Analysis to Predict Current and Future Colonization Trends of Biofuel Plant’s Pests for Sub-Saharan African Countries. Land 2021, 10, 1276. https://doi.org/10.3390/land10111276
Iannella M, De Simone W, Cerasoli F, D’Alessandro P, Biondi M. A Continental-Scale Connectivity Analysis to Predict Current and Future Colonization Trends of Biofuel Plant’s Pests for Sub-Saharan African Countries. Land. 2021; 10(11):1276. https://doi.org/10.3390/land10111276
Chicago/Turabian StyleIannella, Mattia, Walter De Simone, Francesco Cerasoli, Paola D’Alessandro, and Maurizio Biondi. 2021. "A Continental-Scale Connectivity Analysis to Predict Current and Future Colonization Trends of Biofuel Plant’s Pests for Sub-Saharan African Countries" Land 10, no. 11: 1276. https://doi.org/10.3390/land10111276
APA StyleIannella, M., De Simone, W., Cerasoli, F., D’Alessandro, P., & Biondi, M. (2021). A Continental-Scale Connectivity Analysis to Predict Current and Future Colonization Trends of Biofuel Plant’s Pests for Sub-Saharan African Countries. Land, 10(11), 1276. https://doi.org/10.3390/land10111276








