The Subsequent Effects of Soil Pollution by Petroleum Products and Its Bioremediation on the Antioxidant Response and Content of Elements in Vicia faba Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental
2.2. Heavy Metal Concentrations in the PDS Samples
2.3. Petroleum-Derived Hydrocarbons and Element Concentrations in the Soil Samples
2.4. Plants
2.5. Biochemical Parameters of the Plants
2.6. Element Concentration in the Plant Samples
2.7. Statistical Analysis
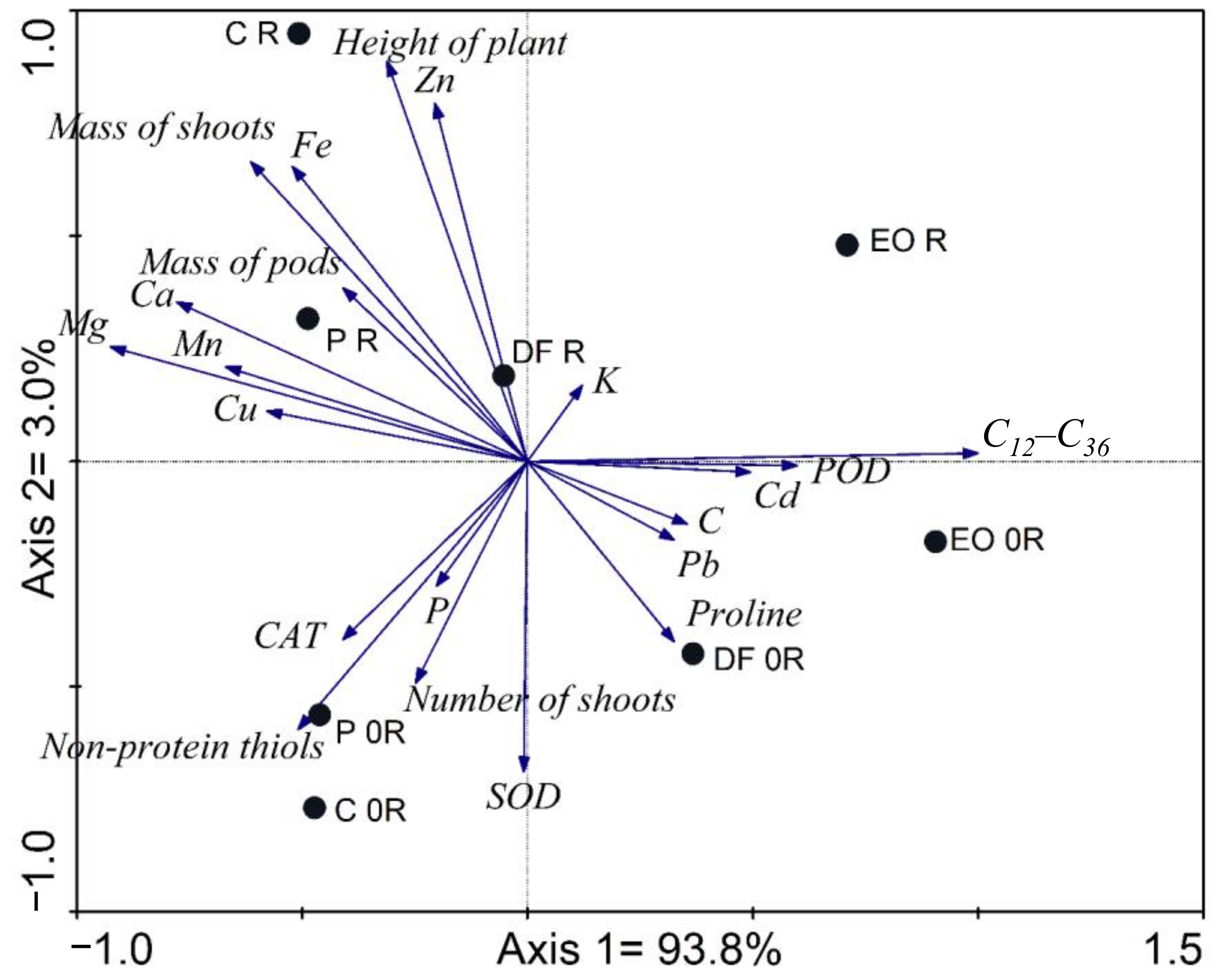
3. Results
3.1. Heavy Metal Concentrations in the PDS Samples
3.2. Petroleum-Derived Hydrocarbons and Element Concentrations in the Soil Samples
3.3. Plant Growth
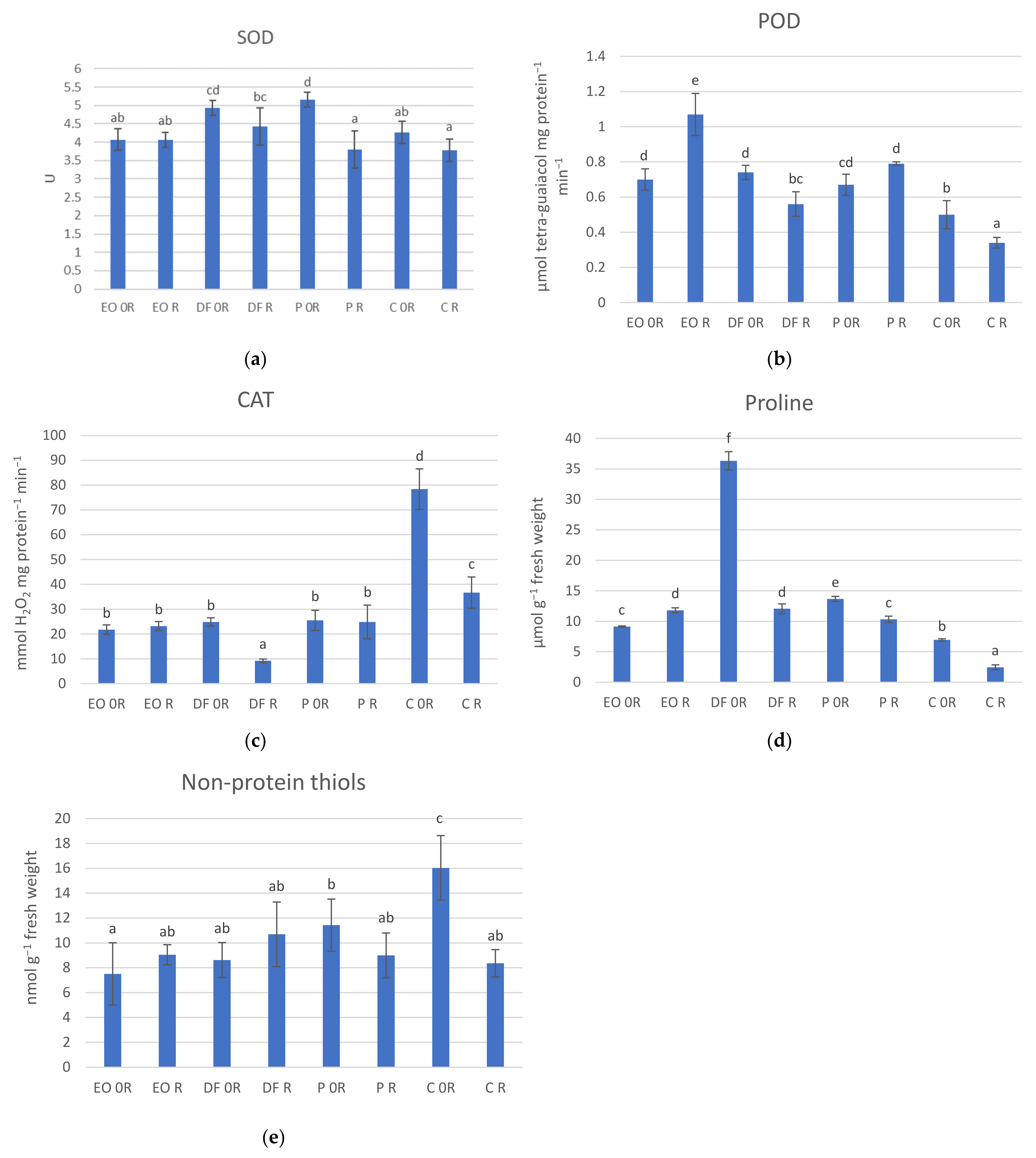
3.4. Biochemical Parameters of the Plants
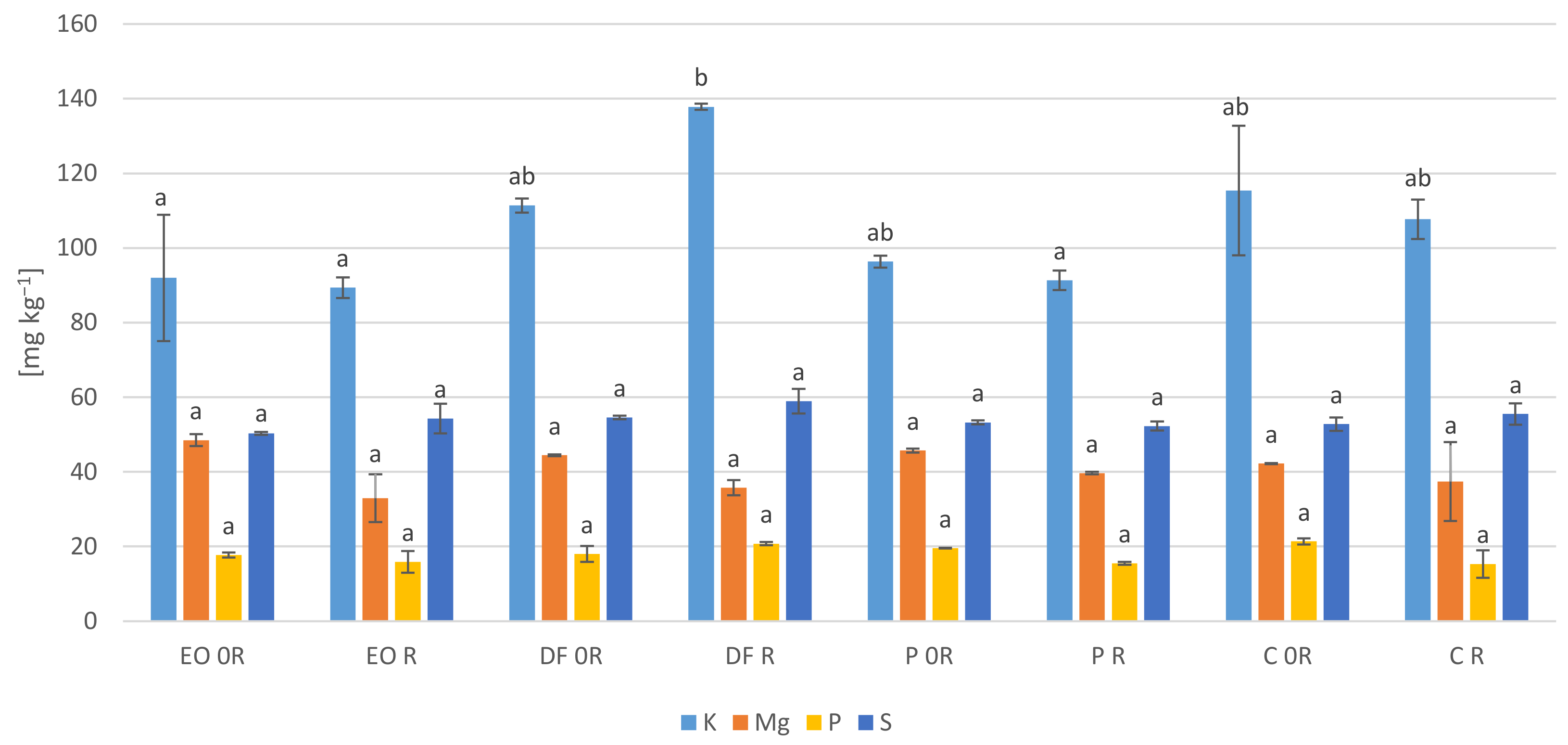
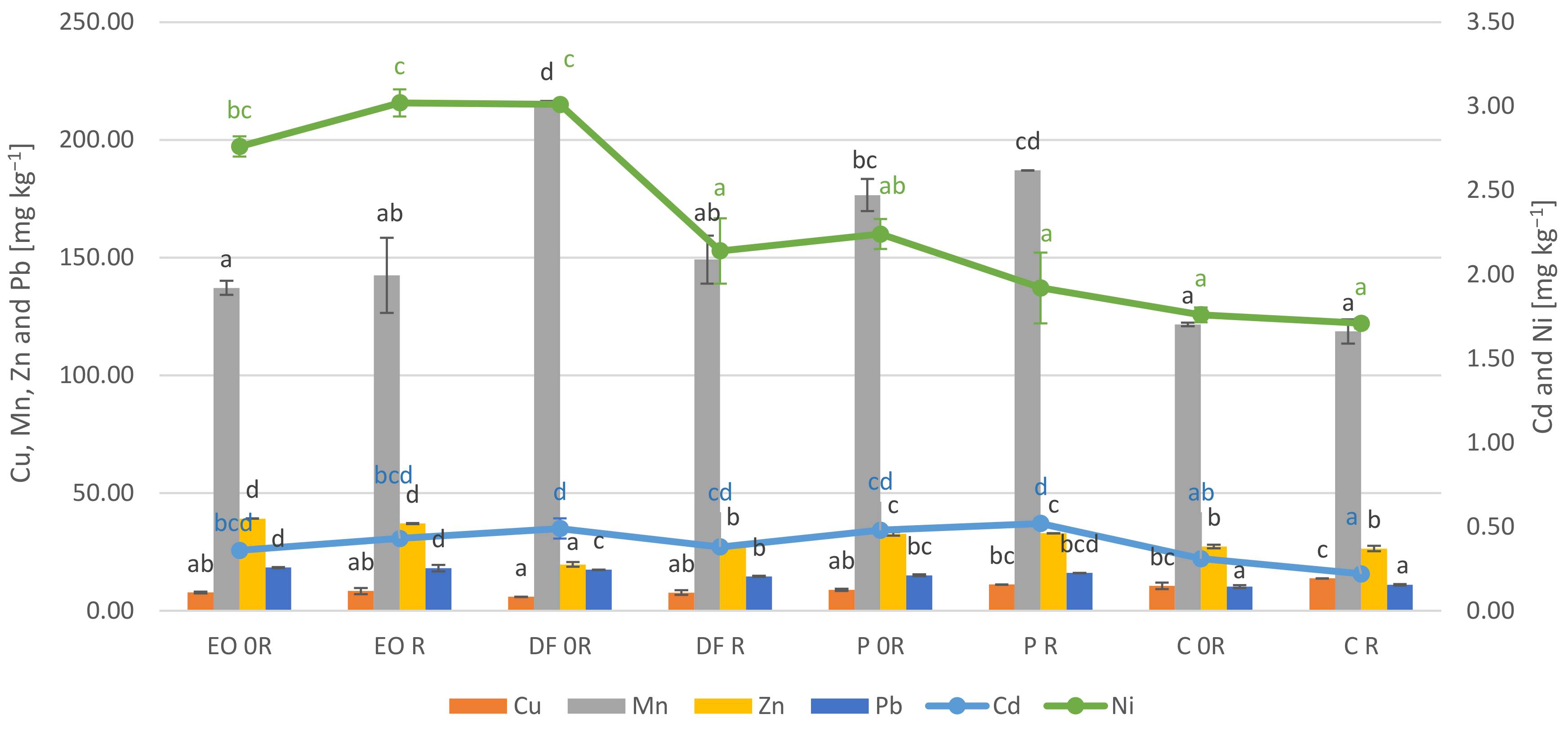
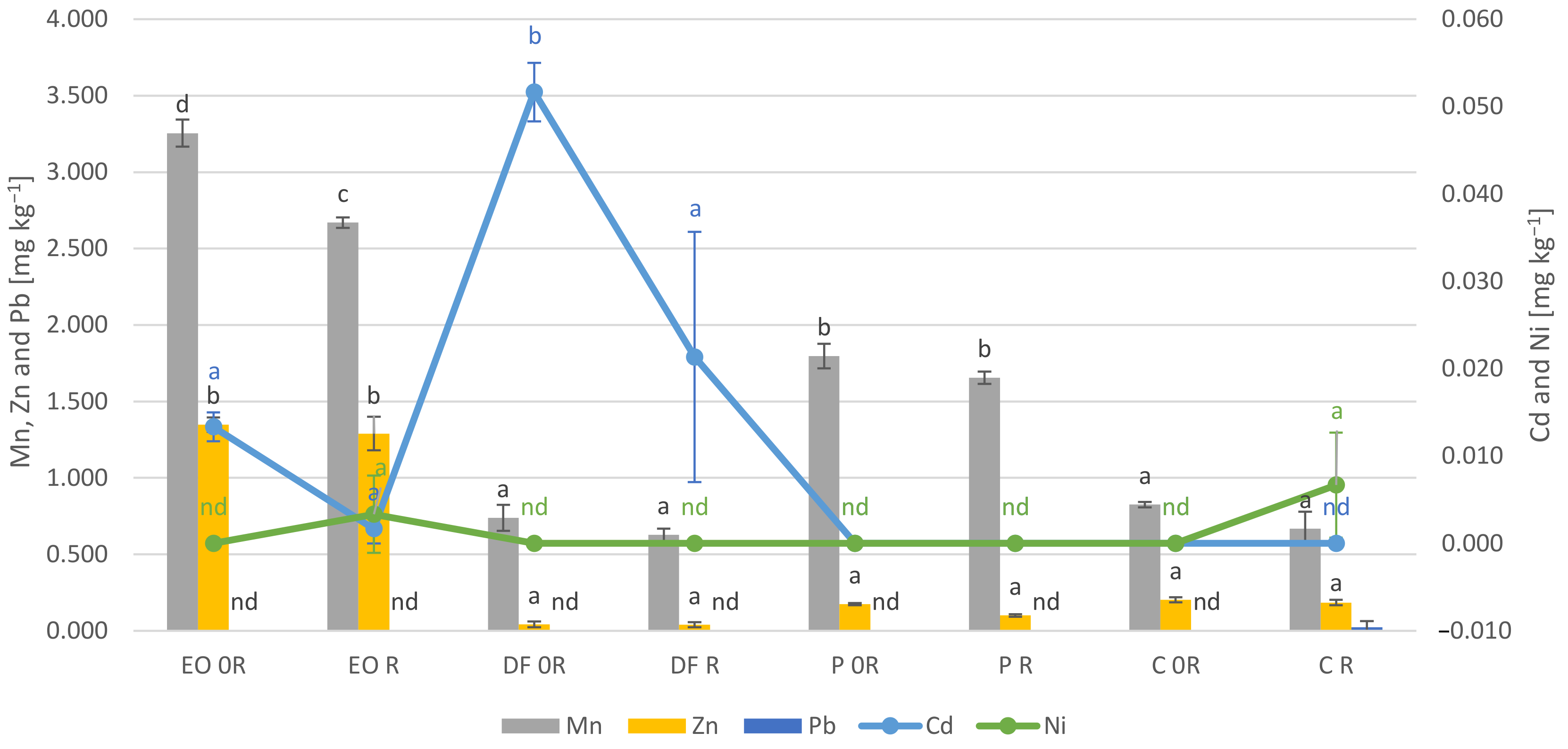
3.5. Element Concentration in the Plant Samples
4. Discussion
4.1. Petroleum-Derived Hydrocarbons and Heavy Metal Concentrations in Soil Samples
4.2. Plant Growth
4.3. Biochemical Parameters of the Plants
4.4. Element Concentration in the Plant Samples
5. Conclusions
- Even five years after contamination with PDSs, engine oil and diesel fuel adversely affected the morphological features of broad bean plants. This confirms the susceptibility of the analysed plant to this kind of contamination, and also indicates the durability of the xenobiotics in the soil environment, which was confirmed by chemical analysis of the soil;
- The applied contaminants significantly affected the activity of antioxidative enzymes and the content of antioxidants, which resulted in a significant increase in the activity of POD and a higher content of proline content in the plant leaves;
- The contamination of the soil with spent engine oil and diesel fuel caused a decrease in the content of calcium, magnesium and phosphorus in the plants. Diesel fuel also decreased the content of potassium and nitrogen while engine oil decreased the content of iron. Petrol had no significant effect on the content of the majority of the analysed nutrient components in the leaves of the broad bean. The introduction of the biopreparation into non-contaminated soil decreased the content of potassium and phosphorus as much as contamination with petroleum products;
- PDSs significantly modified the content of heavy metals in the plants. In particular they increased the content of lead and cadmium, while some of them caused a decrease in the content of zinc (diesel fuel), manganese (engine oil) and copper (engine oil and diesel fuel);
- The applied ZB-01 biopreparation had a generally beneficial effect on the analysed morphological features of the plants and contributed to lowering the activity of the analysed antioxidative enzymes, as well as to a decrease in the antioxidants in the plants from the soil that had been contaminated with the diesel fuel. Furthermore, ZB-01 most often caused an increase in the nutrients in the leaves of the plants. However, its effect on the content of heavy metals varied depending on the contaminant and the analysed metal.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef]
- Iturbe, R.; Flores, C.; Castro, A.; Torres, L.G. Sub-soil contamination due to oil spills in zones surrounding oil pipeline-pump stations and oil pipeline right-of-ways in Southwest-Mexico. Environ. Monit. Assess. 2007, 133, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Shirdam, R.; Zand, A.D.; Bidhendi, G.N.; Mehrdadi, N. Phytoremediation of hydrocarbon-contaminated soils with emphasis on the effect of petroleum hydrocarbons on the growth of plant species. Phytoprotection 2008, 89, 21–29. [Google Scholar] [CrossRef]
- Gbadebo, A.M.; Adenuga, M.D. Effect of crude oil on the emergence and growth of cowpea in two contrasting soil types from Abeokuta, Southwestern Nigeria. Asian J. Appl. Sci. 2012, 5, 232–239. [Google Scholar]
- Shukry, W.M.; Al-Hawas, G.H.S.; Al-Moaikal, R.; El-Bendary, M.A. Effect of petroleum crude oil on mineral nutrient elements and soil properties of jojoba plant (Simmondsia chinensis). Acta Bot. Hung. 2013, 55, 117–133. [Google Scholar] [CrossRef]
- Rusin, M.; Gospodarek, J.; Nadgorska-Socha, A. Antioxidant responses of Triticum aestivum plants to petroleum-derived sub-stances. Ecotoxicology 2018, 27, 1353–1367. [Google Scholar] [CrossRef]
- Ujowundu, C.O.; Kalu, F.N.; Nwaoguikpe, R.N.; Kalu, O.I.; Ihejirika, C.E.; Nwosunjoku, E.C.; Okechukwu, R.I. Biochemical and physical characterization of diesel petroleum contaminated soil in southeastern Nigeria. Res. J. Chem. Sci. Ences. 2011, 1, 57–62. [Google Scholar]
- Pawlak-Sprada, S.; Arasimowicz-Jelonek, M.; Podgórska, M.; Deckert, J. Activation of phenylpropanoid pathway in legume plants exposed to heavy metals. Part I. Effects of cadmium and lead on phenylalanine ammonia-lyase gene expression, enzyme activity and lignin content. Acta Biochim. Pol. 2011, 58, 211–216. [Google Scholar] [CrossRef]
- Molassiotis, A.; Sotiropoulos, T.; Tanou, G.; Diamantidis, G.; Therios, I. Boron-induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM 9 (Malus domestica Borkh). Environ. Exp. Bot. 2006, 56, 54–62. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Baek, K.-H.; Skinner, D.Z. Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant Sci. 2003, 165, 1221–1227. [Google Scholar] [CrossRef]
- Kafel, A.; Nadgórska-Socha, A.; Gospodarek, J.; Babczynska, A.; Skowronek, M.; Kandziora-Ciupa, M.; Rozpędek, K. The effects of Aphis fabae infestation on the antioxidant response and heavy metal content in field grown Philadelphus coronarius plants. Sci. Total Environ. 2010, 408, 1111–1119. [Google Scholar] [CrossRef]
- Boojar, M.; Tavakkoli, Z. Antioxidative Responses and Metal Accumulation in Invasive Plant Species Growing on Mine Tailings in Zanjan, Iran. Pedosphere 2011, 21, 802–812. [Google Scholar] [CrossRef]
- Haritash, A.; Kaushik, C. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Ilic, M.; Beskoski, V.; Ali, S.; Gojgic-Cvijovic, G.; Vrvic, M. Bioremediation of soil heavily contaminated with crude oil and its products: Composition of the microbial consortium. J. Serbian Chem. Soc. 2009, 74, 455–460. [Google Scholar] [CrossRef]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.-W. Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar] [CrossRef] [PubMed]
- Petryszak, P.; Kołoczek, H.; Kaszycki, P. Biological treatment of wastewaters generated by furniture industry. Part 1. Labor-atory-scale process for biodegradation of recalcitrant xenobiotics. Ecol. Chem. Eng. A 2008, 15, 1129–1141. [Google Scholar]
- Kaszycki, P.; Pawlik, M.; Petryszak, P.; Kołoczek, H. Aerobic process for in situ bioremediation of petroleum-derived con-tamination of soil: A field study based on laboratory microcosm tests. Ecol. Chem. Eng. A 2010, 17, 405–414. [Google Scholar]
- Kaszycki, P.; Petryszak, P.; Pawlik, M.; Koloczek, H. Ex situ bioremediation of soil polluted with oily waste: The use of spe-cialized microbial consortia for process bioaugmentation. Ecol. Chem. Eng. S-Chem. I Inz. Ekol. S 2011, 18, 83–92. [Google Scholar]
- Kaszycki, P.; Supel, P.; Petryszak, P. Bacterial population dynamics of biostimulated auto- and allochthonous microflora in waste oily emulsions from the metal-processing industry. J. Ecol. Eng. 2014, 15, 14–22. [Google Scholar]
- Kaszycki, P.; Petryszak, P.; Supel, P. Bioremediation Of A Spent Metalworking Fluid With Auto—And Allochthonous Bacterial Consortia. Ecol. Chem. Eng. S 2015, 22, 285–299. [Google Scholar] [CrossRef][Green Version]
- Pilipović, A.; Orlović, S.; Nikolić, N.; Borišev, M.; Krstić, B.; Rončević, S. Growth and Plant Physiological parameters as markers for selection of poplar clones for crude oil phytoremediation. Šumarski List 2012, 136, 273–281. [Google Scholar]
- Achuba, F.I. Petroleum Products in Soil Mediated Oxidative Stress in Cowpea (Vigna unguiculata) and Maize (Zea mays) Seedlings. Open J. Soil Sci. 2014, 04, 417–435. [Google Scholar] [CrossRef]
- Tabassum, S.; Shahid, N.; Wang, J.; Shafiq, M.; Mumtaz, M.; Arslan, M. The Oxidative Stress Response of Mirabilis jalapa to Exhausted Engine Oil (EEO) during Phytoremediation. Pol. J. Environ. Stud. 2016, 25, 2581–2587. [Google Scholar] [CrossRef]
- Malallah, G.; Afzal, M.; Gulshan, S.; Abraham, D.; Kurian, M.; Dhami, M. Vicia faba as a bioindicator of oil pollution. Environ. Pollut. 1996, 92, 213–217. [Google Scholar] [CrossRef]
- Gospodarek, J.; Petryszak, P.; Kołoczek, H. The effect of the bioremediation of soil contaminated with petroleum derivatives on the occurrence of epigeic and edaphic fauna. Bioremediat. J. 2016, 20, 38–53. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Jeliazkova, E.; Kovacheva, N.; Dzhurmanski, A. Metal uptake by medicinal plant species grown in soils contaminated by a smelter. Environ. Exp. Bot. 2008, 64, 207–216. [Google Scholar] [CrossRef]
- Wójcik, M.; Sugier, P.; Siebielec, G. Metal accumulation strategies in plants spontaneously inhabiting Zn-Pb waste deposits. Sci. Total Environ. 2014, 487, 313–322. [Google Scholar] [CrossRef]
- Dazy, M.; Jung, V.; Férard, J.-F.; Masfaraud, J.-F. Ecological recovery of vegetation on a coke-factory soil: Role of plant antioxidant enzymes and possible implications in site restoration. Chemosphere 2008, 74, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Maas, F.M.; De Kok, L.J.; Peters, J.; Kuiper, P.J.C. A Comparative Study on the Effects of H2S and SO2Fumigation on the Growth and Accumulation of Sulphate and Sulphydryl Compounds in Trifolium pratense L.,Glycine max Merr. and Phaseolus vulgaris L. J. Exp. Bot. 1987, 38, 1459–1469. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Nadgórska-Socha, A.; Kafel, A.; Kandziora-Ciupa, M.; Gospodarek, J.; Zawisza-Raszka, A. Accumulation of heavy metals and antioxidant responses in Vicia faba plants grown on monometallic contaminated soil. Environ. Sci. Pollut. Res. 2013, 20, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Nadgórska-Socha, A.; Ptasiński, B.; Kita, A. Heavy metal bioaccumulation and antioxidative responses in Cardaminopsis arenosa and Plantago lanceolata leaves from metalliferous and non-metalliferous sites: A field study. Ecotoxicology 2013, 22, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Kinnersley, R.; Scott, L. Aerial contamination of fruit through wet deposition and particulate dry deposition. J. Environ. Radioact. 2001, 52, 191–213. [Google Scholar] [CrossRef]
- Lin, A.; Zhang, X.; Zhu, Y.-G.; Zhao, F.-J. Arsenate-induced toxicity: Effects on antioxidative enzymes and DNA damage in Vicia faba. Environ. Toxicol. Chem. 2008, 27, 413. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination, Version 4.5; Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Regulation of the Minister of Environment of 5 September. J. Laws Repub. Pol. 2016, 1395.
- Abed, R.M.M.; Safi, N.M.D.; Köster, J.; de Beer, D.; El-Nahhal, Y.; Rullkötter, J.; Garcia-Pichel, F. Microbial Diversity of a Heavily Polluted Microbial Mat and Its Community Changes following Degradation of Petroleum Compounds. Appl. Environ. Microbiol. 2002, 68, 1674–1683. [Google Scholar] [CrossRef]
- Szarlip, P.; Stelmach, W.; Jaromin-Gleń, K.; Bieganowski, A.; Brzezińska, M.; Trembaczowski, A.; Hałas, S.; Łagód, G. Comparison of the dynamics of natural biodegradation of petrol and diesel oil in soil. DESALINATION Water Treat. 2014, 52, 3690–3697. [Google Scholar] [CrossRef]
- Njoku, L.; Akinola, M.O.; Busari, T.O. Effect of time of application of spent oil on the growth and performance of maize (Zea mays). Afr. J. Environ. Sci. Technol. 2012, 6, 67–71. [Google Scholar] [CrossRef]
- Osuagwu, A.N.; Okigbo, A.U.; Ekpo, I.A.; Chukwurah, P.N.; Agbor, R.B. Effect of crude oil pollution on growth parameters, chlorophyll content and bulbils yield in air potato (Dioscorea bulbifera L.). Int. J. Appl. Sci. Technol. 2013, 3, 37–42. [Google Scholar]
- Lopes, A.; Piedade, M.T.F. Experimental study on the survival of the water hyacinth (Eichhornia crassipes (Mart.) Solms—Pontederiaceae) under different oil doses and times of exposure. Environ. Sci. Pollut. Res. 2014, 21, 13503–13511. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Wijatkowski, A.; Mikiciuk, M. Influence of diesel and biodiesel fuel-contaminated soil on microorganisms, growth and development of plants. Plant Soil Environ. 2016, 61, 189–194. [Google Scholar] [CrossRef]
- Małachowska-Jutsz, A.; Miksch, K. Rola ryzosfery roślin jedno- i dwuliściennych w usuwaniu WWA, TPH oraz frakcji ciężkich ze środowiska glebowego. Zesz. Nauk. Politech. Śląskiej. Inżynieria Sr. 2000, 45, 75–88. [Google Scholar]
- Wake, H. Oil refineries: A review of their ecological impacts on the aquatic environment. Estuar. Coast. Shelf Sci. 2005, 62, 131–140. [Google Scholar] [CrossRef]
- Odjegba, V.J.; Sadiq, A.O. Effects of spent engine oil on the growth parameters, chlorophyll and protein levels of Amaranthus hybridus L. Environmentalis 2002, 22, 23–28. [Google Scholar] [CrossRef]
- Vouillamoz, J.; Milke, M.W. Effect of compost in phytoremediation of diesel-contaminated soils. Water Sci. Technol. 2001, 43, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Ogboghodo, I.A.; Erebor, E.B.; Osemwota, I.O.; Isitekhale, H.H. The effects of application of poultry manure to crude oil polluted soils on maize (Zea mays) growth and soil properties. Environ. Monit. Assess. 2004, 96, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.C.; Camejo, D.; Fernández-García, N.; Rellán-Álvarez, R.; Marques, S.; Sevilla, F.; Jiménez, A. Effect of oil refinery sludges on the growth and antioxidant system of alfalfa plants. J. Hazard. Mater. 2009, 171, 879–885. [Google Scholar] [CrossRef]
- Dazy, M.; Béraud, E.; Cotelle, S.; Grévilliot, F.; Férard, J.-F.; Masfaraud, J.-F. Changes in plant communities along soil pollution gradients: Responses of leaf antioxidant enzyme activities and phytochelatin contents. Chemosphere 2009, 77, 376–383. [Google Scholar] [CrossRef]
- El Beltagi, H.S.; Mohamed, A.A. Changes in non-protein thiols, some antioxidant enzymes activity and ultrastructural alteration in radish plant (Raphanus sativus L.) grown under lead toxicity. Not. Bot. Horti Agrobot. Cluj Napoca 2010, 38, 76–85. [Google Scholar]
- Wang, C.; Tian, Y.; Wang, X.; Geng, J.; Jiang, J.; Yu, H.; Wang, C. Lead-contaminated soil induced oxidative stress, defense response and its indicative biomarkers in roots of Vicia faba seedlings. Ecotoxicology 2010, 19, 1130–1139. [Google Scholar] [CrossRef]
- Pongrac, P.; Zhao, F.-J.; Razinger, J.; Zrimec, A.; Regvar, M. Physiological responses to Cd and Zn in two Cd/Zn hyperaccumulating Thlaspi species. Environ. Exp. Bot. 2009, 66, 479–486. [Google Scholar] [CrossRef]
- Mishra, S.; Tripathi, R.; Srivastava, S.; Dwivedi, S.; Trivedi, P.K.; Dhankher, O.; Khare, A. Thiol metabolism play significant role during cadmium detoxification by Ceratophyllum demersum L. Bioresour. Technol. 2009, 100, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Teklić, T.; Hancock, J.T.; Engler, M.; Paradiković, N.; Cesar, V.; Lepeduš, H.; Stolfa, I.; Beslo, D. Antioxidative responses in radish (Raphanus sativus L.) plants stressed by copper and lead in nutrient solution and soil. Acta Biol. Crac. Ser. Bot. 2008, 50, 79–86. [Google Scholar]
- Merkl, N.; Schultze-Kraft, R.; Infante, C. Assessment Of Tropical Grasses And Legumes For Phytoremediation Of Petroleum-Contaminated Soils. Water Air Soil Pollut. 2005, 165, 195–209. [Google Scholar] [CrossRef]
- Gospodarek, J.; Nadgórska-Socha, A. Chemical composition of broad beans (Vicia faba L.) and development parameters of black bean aphid (Aphis fabae Scop.) under conditions of soil contamination with oil derivatives. J. Elementol. 2016, 21, 1359–1376. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. The Effect of Diesel Fuel on Common Vetch (Vicia Sativa L.) Plants. Environ. Geochem. Health 2003, 25, 123–130. [Google Scholar] [CrossRef]
- Riffaldi, R.; Levi-Minzi, R.; Cardelli, R.; Palumbo, S.; Saviozzi, A. Soil Biological Activities in Monitoring the Bioremediation of Diesel Oil-Contaminated Soil. Water Air Soil Pollut. 2006, 170, 3–15. [Google Scholar] [CrossRef]
- Moubasher, H.; Hegazy, A.; Mohamed, N.; Moustafa, Y.; Kabiel, H.; Hamad, A. Phytoremediation of soils polluted with crude petroleum oil using Bassia scoparia and its associated rhizosphere microorganisms. Int. Biodeterior. Biodegrad. 2015, 98, 113–120. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Ziółkowska, A. Effect of compost, bentonite and calcium oxide on content of some macroelements in plants from soil contaminated by petrol and diesel oil. J. Elementol. 2009, 14, 405–418. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Wyszkowska, J.; Ziółkowska, A. Effect of soil contamination with diesel oil on yellow lupine yield and macroelements content. Plant Soil Environ. 2011, 50, 218–226. [Google Scholar] [CrossRef]
- Nwaichi, E.O.; Wegwu, M.O.; Nwosu, U.L. Distribution of selected carcinogenic hydrocarbon and heavy metals in an oil-polluted agriculture zone. Environ. Monit. Assess. 2014, 186, 8697–8706. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Bordoloi, N.K. Bioremediation and reclamation of soil contaminated with petroleum oil hydrocarbons by exogenously seeded bacterial consortium: A pilot-scale study. Environ. Sci. Pollut. Res. 2011, 18, 471–478. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2015/1005 of 25 June 2015 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Lead in Certain Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R1005&from=EN (accessed on 25 October 2015).
- Commission Regulation (EU) No 488/2014 of 12 May 2014 Amending Regulation (EC) No 1881/2006 as regards Maximum Levels of Cadmium in Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014R0488&from=ET (accessed on 10 December 2014).
- Kaszycki, P.; Szumilas, P.; Kołoczek, H. Biopreparat przeznaczony do likwidacji środowiskowych skażeń węglowodorami i ich pochodnym. Inżynieria Ekol. 2001, 4, 15–22. [Google Scholar]

| Ingredient Name | Petrol | Diesel Fuel | Engine Oil |
|---|---|---|---|
| Gasoline | ≥50 | ||
| Toluene | 5–30 | ||
| Benzene | 0.1 | ||
| Methyl t-amyl ether (TAME) | ≤15 | ||
| 2-ethoxy-2-methylpropane (ETBE) | ≤15 | ||
| Tert-butyl methyl ether | ≤15 | ||
| Propan 2-ol | ≤10 | ||
| Izobutyl alcohol | <10 | ||
| Tert-butyl alcohol | ≤7 | ||
| Ethanol | ≤5 | ||
| Methanol | <3 | ||
| Olefin hydrocarbons | <18 1 | ||
| Aromatic hydrocarbons | <35 1 | ||
| Polycyclic aromatic hydrocarbons (PAHs) | <8 1 | ||
| Diesel fuel | ≥90 | ||
| Heavy paraffin distillates, treated with hydrogen (crude oil); base oil— unspecified 01-2119484627-25 | ~43 | ||
| Heavy distillates from hydrocracking; base oil—unspecified 01-2119486951-26 | ~35 | ||
| Highly refined mineral oil | ~4.5 | ||
| Zinc salts of mixed esters of O,O-bis (sec-Bu I 1,3-dimethylbutyl) phosphorodition acid 01-2119657973-23-0000 | 0.54–1.0 | ||
| Benzenesulfonic acid, methyl mono-C20-26- branched alkyl derivatives, calcium salts | 0.54–1.05 | ||
| Benzenesulfonic acid, methyl mono-C20-24- branched alkyl derivatives, calcium salts | 0.11–0.54 |
| Ingredient Name | Petrol | Diesel Fuel | Engine Oil |
|---|---|---|---|
| Cd | <0.1 1 | <0.1 | 0.23 (±0.01) |
| Cu | 0.13 (±0.03) | <0.1 | 11.30 (±0.70) |
| Mn | <0.1 | <0.1 | 1.90 (±0.70) |
| Ni | <0.1 | <0.1 | 3.10 (±0.70) |
| Pb | <0.1 | <0.1 | 3.90 (±0.10) |
| Zn | 0.61 (±0.08) | <0.1 | 865.00 (±21.00) |
| Treatment | Gasoline Range Hydrocarbons (C6–C12) | Mineral Oil Hydrocarbons (C12–C36) |
|---|---|---|
| EO 0R 1 | <0.8 | 600 |
| EO R | <0.8 | 520 |
| DF 0R | <0.8 | 370 |
| DF R | <0.8 | 190 |
| P 0R | <0.8 | 9.5 |
| P R | <0.8 | <6 |
| C 0R | <0.8 | <6 |
| C R | <0.8 | <6 |
| Treatment | Height of Plant (cm) | Number of Shoots per Plant | Mass of Shoots (g per Plant) | Mass of Leaves (g per Plant) | Mass of Pods (g per Plant) | Mass of Seeds (g per Plant) |
|---|---|---|---|---|---|---|
| EO 0R | 63.55 (±11.4) ab 1 | 2.08 (±0.9) ab | 54.78 (±14.0) bc | 39.20 (±13.4) ab | 45.84 (±21.3) b | 11.18 (±2.3) ab |
| EO R | 64.92 (±6.4) ab | 2.25 (±0.8) ab | 51.62 (±9.1) b | 38.84 (±8.3) a | 61.12 (±12.7) bc | 10.83 (±4.5) ab |
| DF 0R | 51.73 (±7.1) a | 1.83 (±0.7) a | 36.17 (±12.3) a | 28.41 (±8.2) a | 19.78 (±6.8) a | 4.86 (±2.6) a |
| DF R | 78.59 (±5.0) ab | 2.00 (±0.4) ab | 66.44 (±13.2) bc | 48.86 (±14.1) ab | 57.42 (±19.6) bc | 12.72 (±5.7) ab |
| P 0R | 55.56 (±5.3) a | 2.50 (±1.0) ab | 62.84 (±14.3) bc | 42.95 (±13.0) ab | 53.69 (±21.1) bc | 15.82 (±8.2) ab |
| P R | 84.98 (±10.0) b | 2.08 (±0.7) ab | 79.01(±15.1) c | 53.06 (±14.2) ab | 53.61 (±24.3) bc | 14.98 (±4.6) ab |
| C 0R | 50.92 (±8.6) a | 2.42 (±0.8) b | 53.16 (±6.1) b | 42.68 (±10.1) ab | 63.25 (±23.5) c | 17.02 (±8.4) ab |
| C R | 94.66 (±6.4) b | 1.92 (±0.7) ab | 88.36 (±18.3) d | 64.92 (±12.1) b | 64.29 (±22.2) c | 17.14 (±5.1) b |
| Treatment | N | C | S |
|---|---|---|---|
| EO 0R | 48.71 (±2.89) c 1 | 418.48 (±1.89) c | 3.95 (±0.02) e |
| EO R | 47.21 (±1.27) c | 419.28 (±0.08) c | 2.91 (±0.01) d |
| DF 0R | 38.02 (±7.20) a | 411.53 (±6.92) ab | 2.17 (±0.04) bc |
| DF R | 49.02 (±0.45) c | 418.16 (±0.26) c | 2.29 (±0.01) c |
| P 0R | 45.29 (±2.50) bc | 415.15 (±0.71) bc | 1.77 (±0.01) ab |
| P R | 38.52 (±0.41) a | 407.53 (±1.27) a | 1.38 (±0.08) a |
| C 0R | 44.56 (±1.41) bc | 419.55 (±2.62) c | 2.41 (±0.02) c |
| C R | 42.32 (±4.90) ab | 415.00 (±0.48) bc | 2.22 (±0.01) c |
| Treatment | Mg | Ca | K | P |
|---|---|---|---|---|
| EO 0R | 1.88 (±0.11) a 1 | 11.31 (±0.47) b | 12.02 (±0.66) bc | 8.01 (±0.20) a |
| EO R | 1.82 (±0.02) a | 12.36 (±0.01) b | 14.07 (±0.44) d | 8.82 (±0.39) a |
| DF 0R | 1.94 (±0.06) a | 8.12 (±0.26) a | 10.61 (±0.28) a | 8.52 (±0.17) a |
| DF R | 2.54 (±0.09) b | 12.64 (±0.25) b | 18.77 (±0.35) e | 11.67 (±0.31) b |
| P 0R | 2.46 (±0.08) b | 15.47 (±0.16) c | 10.76 (±0.34) ab | 7.83 (±0.30) a |
| P R | 2.62 (±0.25) b | 19.30 (±0.20) d | 11.95 (±0.71) bc | 8.25 (±0.71) a |
| C 0R | 2.55 (±0.03) b | 15.61 (±1.30) c | 12.88 (±1.30) cd | 11.98 (±1.74) b |
| C R | 2.88 (±0.05) c | 17.96 (±0.02) d | 10.93 (±0.02) ab | 8.11 (±0.56) a |
| Treatment | Fe | Zn | Pb | Mn | Cd | Cu | Ni |
|---|---|---|---|---|---|---|---|
| EO 0R | 321.65 (±4.0) a 1 | 174.10 (±7.0) bc | 6.81 (±0.1) cd | 85.68 (±2.3) b | 1.60 (±0.1) d | 8.16 (±0.1) a | 1.33 (±0.1) a |
| EO R | 385.84 (±12.8) cd | 208.56 (±5.2) d | 4.14 (±2.5) ab | 70.30 (±7.2) a | 1.75 (±0.2) d | 15.10 (±0.5) d | 1.41 (±0.1) a |
| DF 0R | 394.09 (±10.9) cd | 123.27 (±3.0) a | 5.73 (±0.2) bc | 113.54 (±2.9) cd | 1.14 (±0.2) b | 12.10 (±0.4) b | 1.27 (±0.1) a |
| DF R | 409.08 (±9.3) d | 181.01 (±3.1) c | 8.26 (±0.7) d | 90.21 (±3.6) b | 1.38 (±0.1) c | 13.92 (±0.4) c | 1.43 (±0.2) a |
| P 0R | 353.97 (±21.9) b | 180.68 (±6.0) c | 6.04 (±0.5) c | 125.69 (±2.7) d | 1.74 (±0.1) d | 14.98 (±0.7) d | 1.47 (±0.2) a |
| P R | 450.19 (±23.0) e | 178.57 (±17.5) c | 4.04 (±0.1) ab | 103.49 (±12.7) c | 1.36 (±0.1) c | 16.61 (±1.0) e | 1.47 (±0.1) a |
| C 0R | 368.00 (±17.2) bc | 161.69 (±14.5) b | 3.71 (±0.6) a | 118.34 (±13.4) d | 0.85 (±0.1) a | 13.95 (±0.2) c | 1.25 (±0.1) a |
| C R | 439.13 (±14.4) e | 243.63 (±3.7) e | 4.11 (±0.7) ab | 179.29 (±4.7) e | 0.88 (±0.1) a | 12.41 (±0.1) b | 1.45 (±0.1) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gospodarek, J.; Rusin, M.; Kandziora-Ciupa, M.; Nadgórska-Socha, A. The Subsequent Effects of Soil Pollution by Petroleum Products and Its Bioremediation on the Antioxidant Response and Content of Elements in Vicia faba Plants. Energies 2021, 14, 7748. https://doi.org/10.3390/en14227748
Gospodarek J, Rusin M, Kandziora-Ciupa M, Nadgórska-Socha A. The Subsequent Effects of Soil Pollution by Petroleum Products and Its Bioremediation on the Antioxidant Response and Content of Elements in Vicia faba Plants. Energies. 2021; 14(22):7748. https://doi.org/10.3390/en14227748
Chicago/Turabian StyleGospodarek, Janina, Milena Rusin, Marta Kandziora-Ciupa, and Aleksandra Nadgórska-Socha. 2021. "The Subsequent Effects of Soil Pollution by Petroleum Products and Its Bioremediation on the Antioxidant Response and Content of Elements in Vicia faba Plants" Energies 14, no. 22: 7748. https://doi.org/10.3390/en14227748
APA StyleGospodarek, J., Rusin, M., Kandziora-Ciupa, M., & Nadgórska-Socha, A. (2021). The Subsequent Effects of Soil Pollution by Petroleum Products and Its Bioremediation on the Antioxidant Response and Content of Elements in Vicia faba Plants. Energies, 14(22), 7748. https://doi.org/10.3390/en14227748







