Shaping the Ecotone Zone in Forest Communities That Are Adjacent to Expressway Roads
Abstract
1. Introduction
2. Materials and Methods
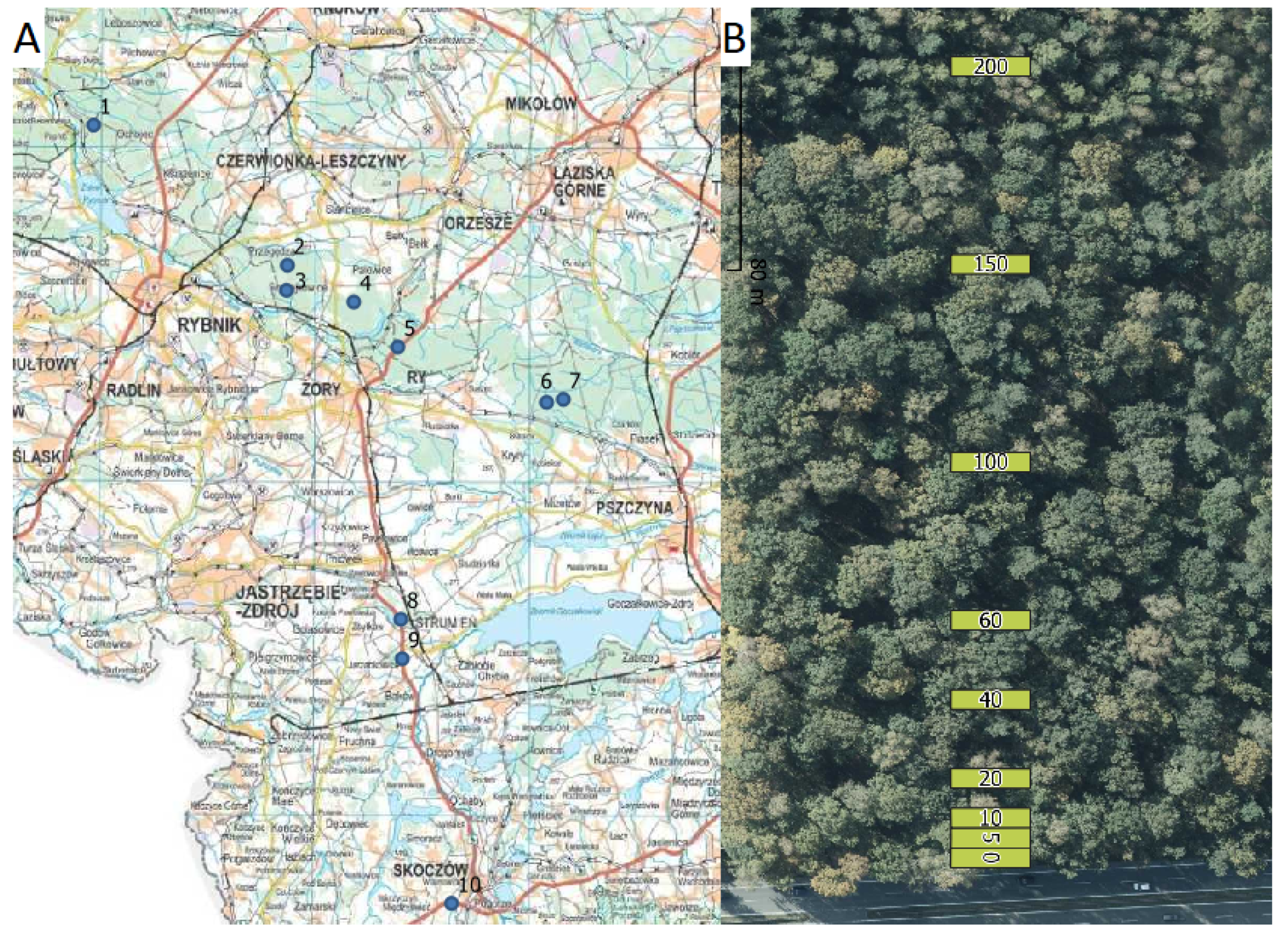
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lourenço, G.M.; Soares, G.R.; Santos, T.P.; Dáttilo, W.; Freitas, A.V.; Ribeiro, S.P. Equal but Different: Natural Ecotones Are Dissimilar to Anthropic Edges. PLoS ONE 2019, 14, e0213008. [Google Scholar] [CrossRef]
- Malanson, G.P.; Resler, L.M.; Tomback, D.F. Ecotone Response to Climatic Variability Depends on Stress Gradient Interactions. Clim. Chang. Responses 2017, 4, 1–8. [Google Scholar] [CrossRef][Green Version]
- Riitters, K.; Wickham, J.; Costanza, J.K.; Vogt, P. A Global Evaluation of Forest Interior Area Dynamics Using Tree Cover Data from 2000 to 2012. Landsc. Ecol. 2016, 31, 137–148. [Google Scholar] [CrossRef]
- Lloyd, K.M.; McQueen, A.A.; Lee, B.J.; Wilson, R.C.; Walker, S.; Wilson, J.B. Evidence on Ecotone Concepts from Switch, Environmental and Anthropogenic Ecotones. J. Veg. Sci. 2000, 11, 903–910. [Google Scholar] [CrossRef]
- Chen, J.; Franklin, J.F.; Spies, T.A. Contrasting Microclimates among Clearcut, Edge, and Interior of Old-Growth Douglas-Fir Forest. Agric. For. Meteorol. 1993, 63, 219–237. [Google Scholar] [CrossRef]
- Govaert, S.; Meeussen, C.; Vanneste, T.; Bollmann, K.; Brunet, J.; Cousins, S.A.; Diekmann, M.; Graae, B.J.; Hedwall, P.-O.; Heinken, T. Edge Influence on Understorey Plant Communities Depends on Forest Management. J. Veg. Sci. 2020, 31, 281–292. [Google Scholar] [CrossRef]
- Meeussen, C.; Govaert, S.; Vanneste, T.; Calders, K.; Bollmann, K.; Brunet, J.; Cousins, S.A.; Diekmann, M.; Graae, B.J.; Hedwall, P.-O. Structural Variation of Forest Edges across Europe. For. Ecol. Manag. 2020, 462, 117929. [Google Scholar] [CrossRef]
- Esseen, P.-A.; Hedström Ringvall, A.; Harper, K.A.; Christensen, P.; Svensson, J. Factors Driving Structure of Natural and Anthropogenic Forest Edges from Temperate to Boreal Ecosystems. J. Veg. Sci. 2016, 27, 482–492. [Google Scholar] [CrossRef]
- Magura, T.; Lövei, G.L.; Tóthmérész, B. Edge Responses Are Different in Edges under Natural versus Anthropogenic Influence: A Meta-Analysis Using Ground Beetles. Ecol. Evol. 2017, 7, 1009–1017. [Google Scholar] [CrossRef]
- Harper, K.A.; Macdonald, S.E. Quantifying Distance of Edge Influence: A Comparison of Methods and a New Randomization Method. Ecosphere 2011, 2, 1–17. [Google Scholar] [CrossRef]
- Harper, K.A.; Macdonald, S.E.; Mayerhofer, M.S.; Biswas, S.R.; Esseen, P.-A.; Hylander, K.; Stewart, K.J.; Mallik, A.U.; Drapeau, P.; Jonsson, B.-G. Edge Influence on Vegetation at Natural and Anthropogenic Edges of Boreal Forests in C Anada and F Ennoscandia. J. Ecol. 2015, 103, 550–562. [Google Scholar] [CrossRef]
- Pawłowski, B. Skład i budowa zbiorowisk roślinnych oraz metody ich badania. In Szata Roślinna Polski; PWN: Warszawa, Poland, 1972; Volume 1, pp. 237–269. [Google Scholar]
- Harper, K.A.; Macdonald, S.E. Structure and Composition of Riparian Boreal Forest: New Methods for Analyzing Edge Influence. Ecology 2001, 82, 649–659. [Google Scholar] [CrossRef]
- Jager Eckehart, J.; Muller, F.; Ritz, C.; Welk, E.; Wesche, K. Exkursionsflora von Deutschland. Gefäßpflanzen: Atlasband: Mit 3000 Abgebildeten Arten; 13. Auflage.; Springer Spektrum: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-49709-8. [Google Scholar]
- Szafer, W.; Kulczynski, S.; Pawlowski, B. Rosliny Polskie: Czesc II; Panstwowe Wydawnictwo Naukowe: Warszawa, Poland, 1988; ISBN 978-83-01-05287-4. [Google Scholar]
- Ellenberg, H.; Leuschner, C. Vegetation Mitteleuropas Mit Den Alpen. 6 .Aufl; Ulmer Verlag: Stuttgart, Germany, 2010; ISBN 978-3-8252-8104-5. [Google Scholar]
- Brothers, T.S.; Spingarn, A. Forest Fragmentation and Alien Plant Invasion of Central Indiana Old-Growth Forests. Conserv. Biol. 1992, 6, 91–100. [Google Scholar] [CrossRef]
- Chmura, D. The Slope Aspect Affects the Heterogeneity and Growth of Ground Flora Vegetation in Deciduous Temperate Forest. Pol. J. Ecol. 2008, 56, 463–470. [Google Scholar]
- Developer Core Team, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P.; Moretti, M. Improving Indicator Species Analysis by Combining Groups of Sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Henry, M.; Stevens, M.H.H. Vegan: Community Ecology Package. Ordination Methods, Diversity Analysis and Other Functions for Community and Vegetation Ecologists. R Package Ver. 2015, 2–3. Available online: https://www.worldagroforestry.org/publication/vegan-community-ecology-package-ordination-methods-diversity-analysis-and-other (accessed on 21 October 2021).
- Borecki, T.; Stepien, E.; Miscicki, S.; Nowakowska, J.; Wojcik, R. Wplyw Drog Szybkiego Ruchu Na Wybrane Elementy Taksacyjne Drzewostanow Sosnowych. Sylwan 1997, 141, 37–48. [Google Scholar]
- Marcantonio, M.; Rocchini, D.; Geri, F.; Bacaro, G.; Amici, V. Biodiversity, Roads, & Landscape Fragmentation: Two Mediterranean Cases. Appl. Geogr. 2013, 42, 63–72. [Google Scholar]
- Suárez-Esteban, A.; Delibes, M.; Fedriani, J.M. Unpaved Road Verges as Hotspots of Fleshy-Fruited Shrub Recruitment and Establishment. Biol. Conserv. 2013, 167, 50–56. [Google Scholar] [CrossRef]
- Delgado, J.D.; Arroyo, N.L.; Arévalo, J.R.; Fernández-Palacios, J.M. Edge Effects of Roads on Temperature, Light, Canopy Cover, and Canopy Height in Laurel and Pine Forests (Tenerife, Canary Islands). Landsc. Urban Plan. 2007, 81, 328–340. [Google Scholar] [CrossRef]
- Corney, P.M.; DUC, M.L.; Smart, S.M.; Kirby, K.J.; Bunce, R.G.H.; Marrs, R.H. Relationships between the Species Composition of Forest Field-Layer Vegetation and Environmental Drivers, Assessed Using a National Scale Survey. J. Ecol. 2006, 94, 383–401. [Google Scholar] [CrossRef]
- Hawbaker, T.J.; Radeloff, V.C.; Clayton, M.K.; Hammer, R.B.; Gonzalez-Abraham, C.E. Road Development, Housing Growth, and Landscape Fragmentation in Northern Wisconsin: 1937–1999. Ecol. Appl. 2006, 16, 1222–1237. [Google Scholar] [CrossRef]
- Bernhardt, M.; Fischer, A.; Kirchner, M.; Jakobi, G. Impact of Motorways on Adjacent Coniferous Forest Communities. In Eco-Complexity and Dynamics of the Cultural Landscape, Proceedings of the 34th Annual Conference of the Ecological Society of Germany, Austria and Switzerland, Giessen, Germany, 1–17 September 2004; p. 90.
- Chmura, D.; Sierka, E. The Invasibility of Deciduous Forest Communities after Disturbance: A Case Study of Carex Brizoides and Impatiens Parviflora Invasion. For. Ecol. Manag. 2007, 242, 487–495. [Google Scholar] [CrossRef]
- Florianová, A.; Münzbergová, Z. Invasive Impatiens Parviflora Has Negative Impact on Native Vegetation in Oak-Hornbeam Forests. Flora 2017, 226, 10–16. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Gawroński, S. Effect of Litter Removal on Species Richness and Acidification of a Mixed Oak-Pine Woodland. Biol. Conserv. 2002, 106, 389–398. [Google Scholar] [CrossRef]
- Mizera, P.; Grajewski, S.M. Efekt Brzegowy Drogi a Występowanie Krzewinek z Rodziny Ericaceae i Zmienność PH Gleb w Puszczy Noteckiej. Infrastrukt. Ekol. Teren. Wiej. 2016, 867–881. [Google Scholar] [CrossRef]
- Šálek, L.; Zahradník, D.; Marušák, R.; Jeřábková, L.; Merganič, J. Forest Edges in Managed Riparian Forests in the Eastern Part of the Czech Republic. For. Ecol. Manag. 2013, 305, 1–10. [Google Scholar] [CrossRef]
- Cayuela, L.; Murcia, C.; Hawk, A.A.; Fernández-Vega, J.; Oviedo-Brenes, F. Tree Responses to Edge Effects and Canopy Openness in a Tropical Montane Forest Fragment in Southern Costa Rica. Trop. Conserv. Sci. 2009, 2, 425–436. [Google Scholar] [CrossRef]
- Oosterhoorn, M.; Kappelle, M. Vegetation Structure and Composition along an Interior-Edge-Exterior Gradient in a Costa Rican Montane Cloud Forest. For. Ecol. Manag. 2000, 126, 291–307. [Google Scholar] [CrossRef]
- Russell, W.H.; McBride, J.R.; Carnell, K. Edge Effects and the Effective Size of Old-Growth Coast Redwood Preserves. In Proceedings: Wilderness Science in a Time of Change. Proc. RMRS-P-000; Cole, D.N., McCool, F.S., Eds.; Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2000; pp. 128–136. [Google Scholar]
- Palik, B.J.; Murphy, P.G. Disturbance versus Edge Effects in Sugar-Maple/Beech Forest Fragments. For. Ecol. Manag. 1990, 32, 187–202. [Google Scholar] [CrossRef]
- Matlack, G.R.; Litvaitis, J.A. Forest Edges. Maint. Biodivers. For. Ecosyst. 1999, 210, 233. [Google Scholar]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics: Updated Edition.; John Wiley and Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Harper, K.A.; Drapeau, P.; Lesieur, D.; Bergeron, Y. Forest Structure and Composition at Fire Edges of Different Ages: Evidence of Persistent Structural Features on the Landscape. For. Ecol. Manag. 2014, 314, 131–140. [Google Scholar] [CrossRef]
- Hofmeister, J.; Hošek, J.; Brabec, M.; Hédl, R.; Modrỳ, M. Strong Influence of Long-Distance Edge Effect on Herb-Layer Vegetation in Forest Fragments in an Agricultural Landscape. Perspect. Plant Ecol. Evol. Syst. 2013, 15, 293–303. [Google Scholar] [CrossRef]
- Bueno, A.S.; Bruno, R.S.; Pimentel, T.P.; Sanaiotti, T.M.; Magnusson, W.E. The Width of Riparian Habitats for Understory Birds in an Amazonian Forest. Ecol. Appl. 2012, 22, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, E.N.; Asner, G.P.; Keller, M.; Knapp, D.E.; Oliveira, P.J.; Silva, J.N. Forest Fragmentation and Edge Effects from Deforestation and Selective Logging in the Brazilian Amazon. Biol. Conserv. 2008, 141, 1745–1757. [Google Scholar] [CrossRef]


| Code | Name of Variable | Type |
|---|---|---|
| TrDHa | Tree density per hectare | Numerical |
| ShD | Shrub density | Numerical |
| DsD | Dwarf shrub density | Numerical |
| ShC | Shrub cover | Percent cover |
| DsC | Dwarf shrubs cover | Percent cover |
| VpC | Herb vascular plant cover | Percent cover |
| TrH | Trees Shannon-Wiener index | Numerical |
| ShH | Shrubs Shannon-Wiener index | Numerical |
| DsH | Dwarf shrubs Shannon-Wiener index | Numerical |
| VpH | Vascular plants Shannon-Wiener index | Numerical |
| TrG | Mean tree girth | Numerical |
| TrHeight | Mean tree height | Numerical |
| ShSr | Shrub species richness | Integer |
| DsSr | Dwarf shrub species richness | Integer |
| VpSr | Vascular plant species richness | Integer |
| TrSr | Tree species richness | Integer |
| Forest Interior (n = 161) | Forest Edge (n = 108) | Total (n = 269) | |
|---|---|---|---|
| Number of species | 36 | 54 | 65 |
| Species turnover: | |||
| Forest edge | 0.444 | - | - |
| Total | 0.093 | 0.287 | - |
| Mean (min, max) | 0.71 (0; 10) | 0.74 (0; 1) | 0.80 (0; 1) |
| Forest Interior | Forest Margin | ||
|---|---|---|---|
| IndVal | IndVal | ||
| Pteridium aquilinum | 0.700 *** | Carex brizoides | 0.610 *** |
| Trientalis europaea | 0.650 *** | Frangula alnus | 0.506 *** |
| Quercus petraea | 0.488 *** | Impatiens parviflora | 0.419 *** |
| Calamagrostis villosa | 0.476 *** | Rubus sp. | 0.419 *** |
| Fagus sylvatica | 0.423 *** | Populus tremula | 0.386 *** |
| Pinus sylvestris | 0.305 ** | Sambucus nigra | 0.385 *** |
| Vaccinium vitis-idaea | 0.284 ** | Padus avium | 0.373 *** |
| Maianthemum bifolium | 0.209 * | Corylus avellana | 0.347 *** |
| - | Acer platanoides | 0.289 *** | |
| - | Athyrium filix-femina | 0.272 ** | |
| - | Acer pseudoplatanus | 0.272 *** | |
| - | Ulmus glabra | 0.272 ** | |
| - | Quercus robur | 0.262 * | |
| - | Galeobdolon luteum | 0.236 ** | |
| - | Viola reichenbachiana | 0.236 ** | |
| - | Stellaria media | 0.215 * | |
| - | Dryopteris filix-mas | 0.213 * | |
| - | Lysimachia vulgaris | 0.192 * | |
| CCA1 | CCA2 | Pseudo-F | Pr (>F) | |
|---|---|---|---|---|
| eigenvalues | 0.4942 | 0.2901 | - | - |
| distance | 0.6034 | 0.79555 | 8.5903 | 0.001 |
| slope | −0.9250 | 0.37538 | 9.8854 | 0.001 |
| aspect | −0.3526 | −0.06156 | 2.6460 | 0.001 |
| DCA1 | DCA2 | r2 | Pr (>r) | |
| eigenvalues | 0.6314 | 0.5178 | - | - |
| herbs | 0.03278 | −0.99946 | 0.0668 | 0.001 |
| shrubs | 0.94226 | 0.33488 | 0.1509 | 0.001 |
| dwarfs | −0.72471 | 0.68906 | 0.4917 | 0.001 |
| L | −0.99501 | 0.09976 | 0.0546 | 0.001 |
| T | −0.30663 | 0.95183 | 0.0301 | 0.028 |
| K | −0.62909 | −0.77733 | 0.0106 | NS |
| F | 0.74783 | −0.66389 | 0.0990 | 0.001 |
| R | 0.99998 | −0.00672 | 0.6353 | 0.001 |
| N | 0.92743 | 0.37400 | 0.5578 | 0.001 |
| S | −0.19523 | 0.98076 | 0.2270 | 0.001 |
| H | −0.24658 | 0.96912 | 0.2486 | 0.001 |
| E | −0.26915 | 0.96310 | 0.0739 | 0.001 |
| Community | - | - | 0.5403 | 0.001 |
| Variable | Coefficients of Model | Wald Test Statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | df | t | p-Value | Chisq | Df | p-Value | ||
| TrDHa | (Intercept) | 1592.88 | 317.54 | 102.00 | 5.02 | 0.00 | |||
| distance | −0.73 | 0.26 | 102.00 | −2.85 | 0.01 | 8.1002 | 1 | 0.004426 | |
| Dist = MARGIN | −177.97 | 315.09 | 102.00 | −0.57 | NS | 0.319 | 1 | NS | |
| ShD | (Intercept) | 4.04 | 0.80 | 11.30 | 5.02 | 0.000 | |||
| distance | 0.00 | 0.00 | 11.68 | −3.68 | 0.003 | 13.549 | 1 | 0.000233 | |
| Dist = MARGIN | −1.54 | 0.81 | 10.45 | −1.90 | NS | 3.6049 | 1 | NS | |
| DsD | (Intercept) | 3.78 | 2.06 | 27.44 | 1.83 | NS | |||
| distance | 0.00 | 0.00 | 38.09 | −0.71 | NS | 0.5081 | 1 | NS | |
| Dist = MARGIN | −2.34 | 2.22 | 19.39 | −1.05 | NS | 1.1079 | 1 | NS | |
| ShC | (Intercept) | 77.44 | 14.34 | 14.96 | 5.40 | 0.00 | |||
| Distance | −0.04 | 0.01 | 15.90 | −3.86 | 0.0014 | 14.908 | 1 | 0.000113 | |
| Dist = MARGIN | −26.73 | 14.63 | 13.52 | −1.83 | NS | 3.3373 | 1 | NS | |
| DsC | (Intercept) | 25.14 | 16.93 | 23.56 | 1.48 | NS | |||
| distance | −0.01 | 0.01 | 32.32 | −0.44 | NS | 0.1926 | 1 | NS | |
| Dist = MARGIN | −12.75 | 18.13 | 16.81 | −0.70 | NS | 0.4944 | 1 | NS | |
| VpC | (Intercept) | 65.12 | 20.49 | 34.02 | 3.18 | 0.00 | |||
| distance | 0.01 | 0.02 | 56.76 | 0.33 | NS | 0.1091 | 1 | NS | |
| Dist = MARGIN | 6.85 | 22.73 | 21.17 | 0.30 | NS | 0.0908 | 1 | NS | |
| TrH | (Intercept) | 0.49 | 0.11 | 11.21 | 4.35 | 0.00111 | |||
| distance | 0.00 | 0.00 | 11.72 | −1.85 | NS | 3.4099 | 1 | NS | |
| Dist = MARGIN | −0.06 | 0.11 | 10.28 | −0.52 | NS | 0.2688 | 1 | NS | |
| ShH | (Intercept) | 0.65 | 0.15 | 15.80 | 4.50 | 0.000 | |||
| distance | 0.00 | 0.00 | 16.86 | −2.63 | 0.018 | 6.8907 | 1 | 0.008665 | |
| Dist = MARGIN | −0.33 | 0.15 | 14.23 | −2.22 | 0.043 | 4.912 | 1 | 0.026671 | |
| DsH | (Intercept) | 0.08 | 0.03 | 12.41 | 2.66 | 0.020 | |||
| distance | 0.00 | 0.00 | 12.76 | −2.05 | NS | 4.1856 | 1 | 0.04077 | |
| Dist = MARGIN | −0.07 | 0.03 | 11.51 | −2.51 | 0.0284 | 6.2761 | 1 | 0.01224 | |
| VpH | (Intercept) | 0.34 | 0.15 | 17.60 | 2.20 | 0.0411 | |||
| distance | 0.00 | 0.00 | 19.35 | 0.69 | NS | 0.48 | 1 | NS | |
| Dist = MARGIN | −0.02 | 0.16 | 15.37 | −0.13 | NS | 0.0156 | 1 | NS | |
| TrG | (Intercept) | 36.02 | 8.14 | 102.00 | 4.43 | 0.00 | |||
| distance | 0.04 | 0.01 | 102.00 | 5.57 | 0.00 | 30.98 | 1 | 0.00 | |
| Dist = MARGIN | 11.04 | 8.08 | 102.00 | 1.37 | NS | 1.8677 | 1 | NS | |
| TrHeight | (Intercept) | 14.15 | 2.20 | 11.22 | 6.44 | 0.00 | |||
| distance | 0.01 | 0.00 | 11.39 | 3.96 | 0.00 | 15.708 | 1 | 0.0001 | |
| Dist = MARGIN | −0.90 | 2.19 | 10.31 | −0.41 | NS | 0.1675 | 1 | NS | |
| DsD | (Intercept) | 3.78 | 2.06 | 27.44 | 1.83 | NS | |||
| distance | 0.00 | 0.00 | 38.09 | −0.71 | NS | 0.5081 | 1 | NS | |
| Dist = MARGIN | −2.34 | 2.22 | 19.39 | −1.05 | NS | 1.1079 | 1 | NS | |
| ShSr | (Intercept) | 6.14 | 1.06 | 13.35 | 5.77 | 0.00 | |||
| distance | 0.00 | 0.00 | 13.99 | −3.34 | 0.00 | 11.14 | 1 | 0.00 | |
| Dist = MARGIN | −2.83 | 1.08 | 12.22 | −2.62 | 0.02 | 6.88 | 1 | 0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czaja, J.; Wilczek, Z.; Chmura, D. Shaping the Ecotone Zone in Forest Communities That Are Adjacent to Expressway Roads. Forests 2021, 12, 1490. https://doi.org/10.3390/f12111490
Czaja J, Wilczek Z, Chmura D. Shaping the Ecotone Zone in Forest Communities That Are Adjacent to Expressway Roads. Forests. 2021; 12(11):1490. https://doi.org/10.3390/f12111490
Chicago/Turabian StyleCzaja, Justyna, Zbigniew Wilczek, and Damian Chmura. 2021. "Shaping the Ecotone Zone in Forest Communities That Are Adjacent to Expressway Roads" Forests 12, no. 11: 1490. https://doi.org/10.3390/f12111490
APA StyleCzaja, J., Wilczek, Z., & Chmura, D. (2021). Shaping the Ecotone Zone in Forest Communities That Are Adjacent to Expressway Roads. Forests, 12(11), 1490. https://doi.org/10.3390/f12111490








