Lecciana, a New Low-Vigour Olive Cultivar Suitable for Super High Density Orchards and for Nutraceutical EVOO Production
Abstract
:1. Introduction
2. Origin and Selection
3. Description
3.1. Materials and Methods
3.1.1. Site and Orchard
3.1.2. Molecular Markers
3.1.3. Morphological Markers
3.1.4. Genetic Sterilities and Blooming Phenogram
3.1.5. Ripening Indexes and Harvesting Time
3.1.6. Yield, Vigor and Yield Efficiencies
3.1.7. Oil Quality
3.2. Data Analysis
4. Results and Discussion
4.1. Molecular Markers
4.2. Morphological Markers
4.3. Genetic Sterilities and Blooming Phenogram
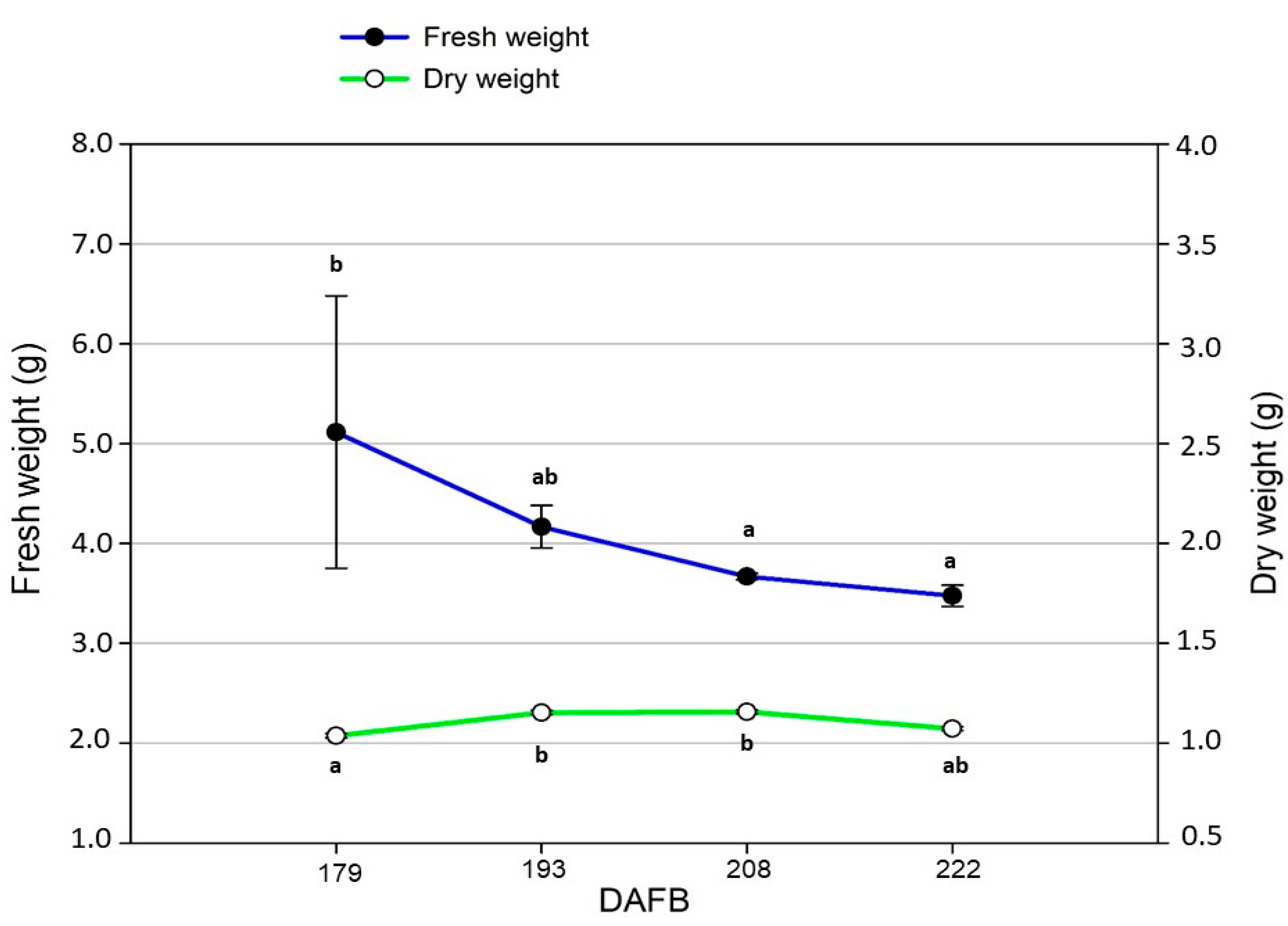
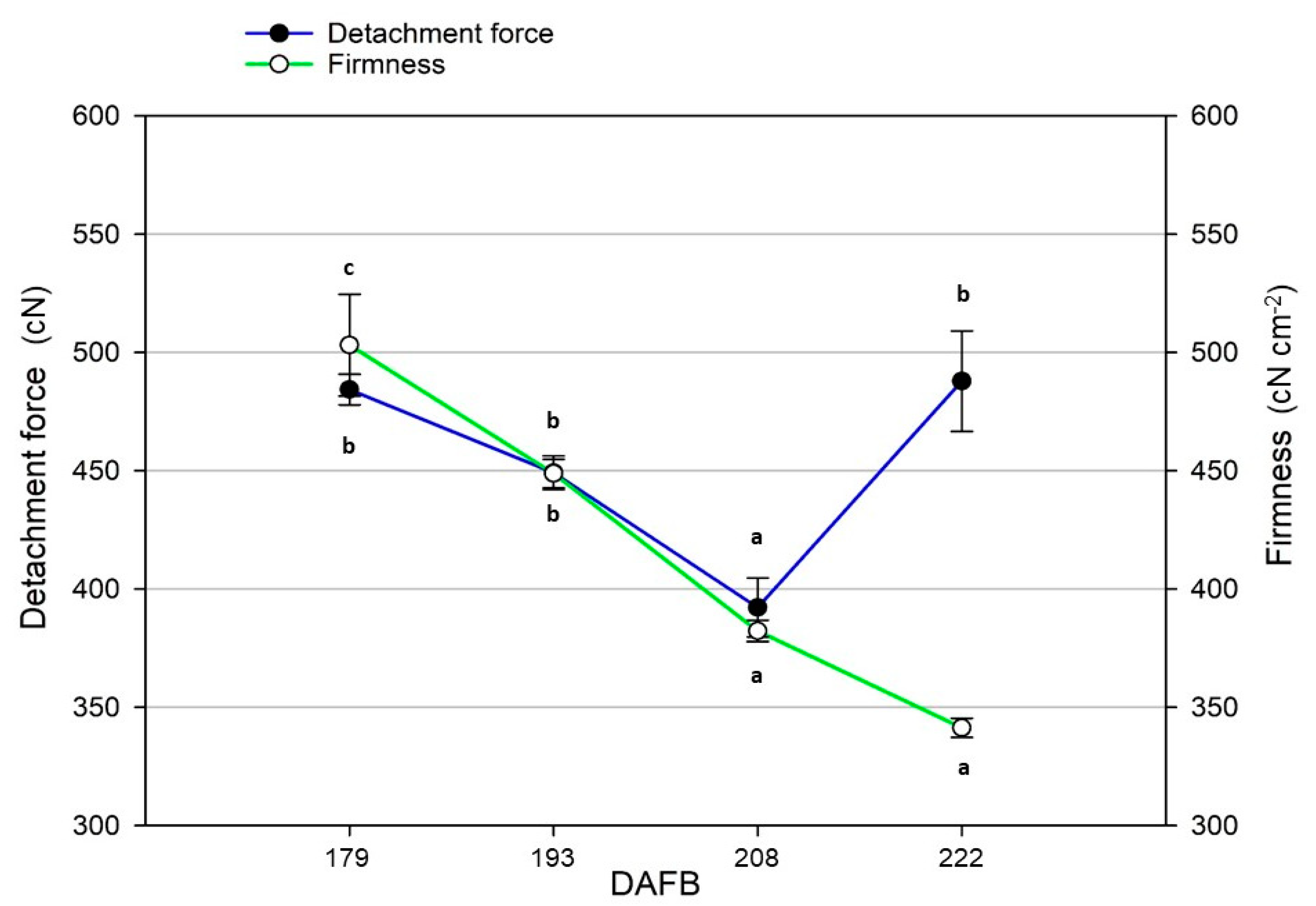
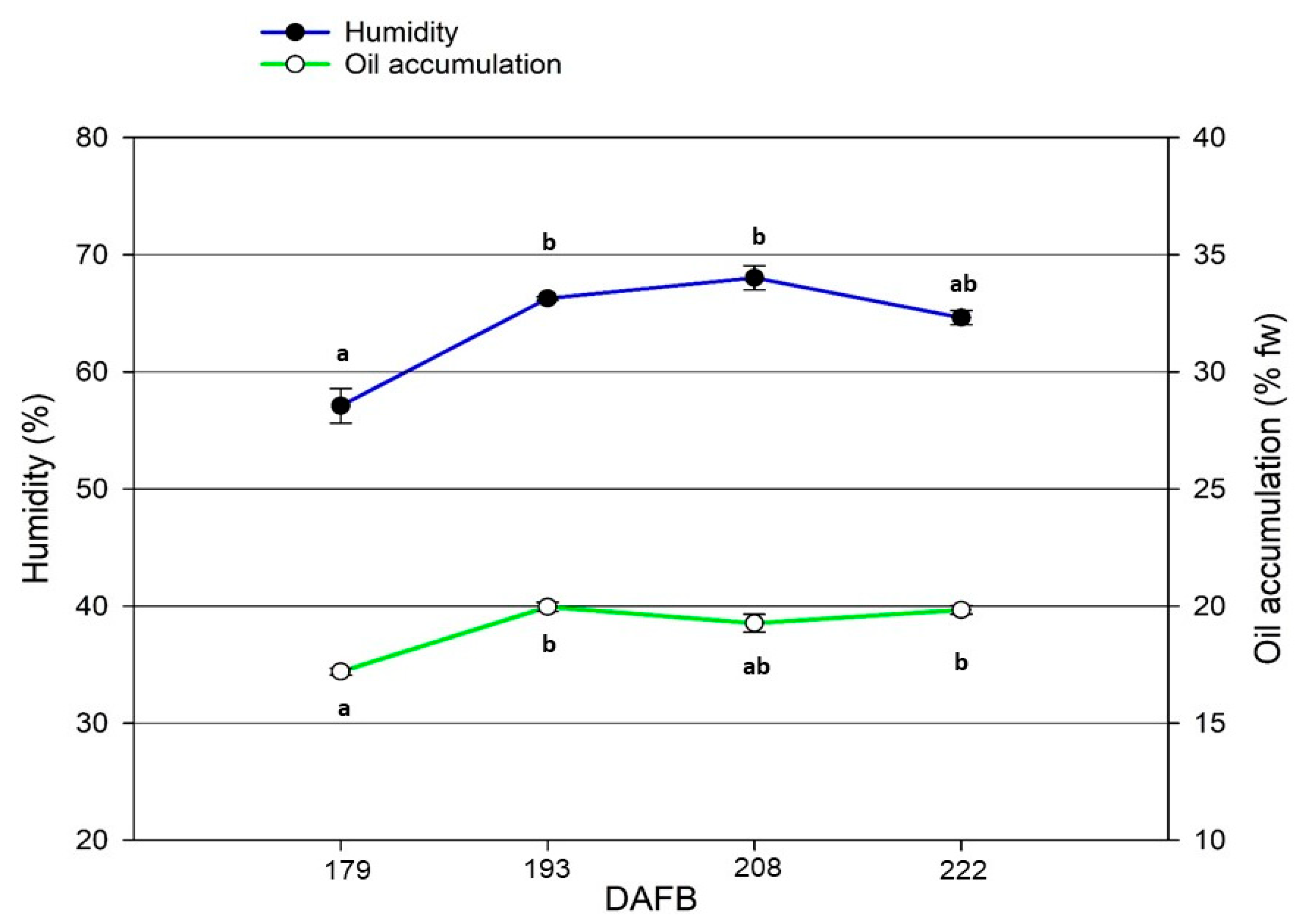
4.4. Ripening Indexes and Harvesting Time
4.5. Yield, Vigor and Yield Efficiencies
4.6. Oil Quality
5. Conclusions
6. Patents, Diffusion and Availability
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clodoveo, M.L.; Camposeo, S.; De Gennaro, B.; Pascuzzi, S.; Roselli, L. In the ancient world, virgin olive oil was called “liquid gold” by Homer and “the great healer” by Hippocrates. Why has this mythic image been forgotten? Food Res. Int. 2014, 62, 1062–1068. [Google Scholar] [CrossRef]
- Lombardi, A.; Carlucci, D.; Cavallo, C.; De Gennaro, B.; Del Giudice, T.; Giannoccaro, G.; Paparella, A.; Roselli, L.; Vecchio, R.; Cicia, G. Do consumers understand health claims on extra-virgin olive oil? Food Res. Int. 2021, 143, 110267. [Google Scholar] [CrossRef] [PubMed]
- Lo Bianco, R.; Proietti, P.; Regni, L.; Caruso, T. Planting Systems for Modern Olive Growing: Strengths and Weaknesses. Agriculture 2021, 11, 494. [Google Scholar] [CrossRef]
- Pellegrini, G.; La Sala, P.; Camposeo, S.; Contò, F. Economic sustainability of the oil high and super-high density cropping systems in Italy. Glob. Bus. Econ. Rev. 2017, 19, 553–569. [Google Scholar] [CrossRef]
- Tous, J. Olive production systems and mechanization. Acta Hortic. 2011, 924, 169–184. [Google Scholar] [CrossRef]
- Olint. Available online: https://www.agromillora.com/olint/en/ (accessed on 30 August 2021).
- Anifantis, A.S.; Camposeo, S.; Vivaldi, G.A.; Santoro, F.; Pascuzzi, S. Comparison of UAV photogrammetry and 3D modelling techniques with other currently used methods for estimating of the tree row volume of a super-high-density olive orchard. Agriculture 2019, 9, 233. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, G.; Ingrao, C.; Camposeo, S.; Tricase, C.; Contò, F.; Huisingh, D. Application of Water Footprint to olive growing systems in the Apulia region: A comparative assessment. J. Cleaner Prod. 2016, 112, 2407–2418. [Google Scholar] [CrossRef]
- Russo, G.; Vivaldi, G.A.; De Gennaro, B.; Camposeo, S. Environmental sustainability of different soil management techniques in a high-density olive orchard. J. Clean. Prod. 2015, 16, 498–508. [Google Scholar] [CrossRef]
- Garnett, T.; Appleby, M.C.; Balmford, A.; Bateman, I.J.; Benton, T.G.; Bloomer, P.; Burlingame, B.; Dawkins, M.; Dolan, L.; Fraser, D.; et al. Sustainable intensification in agriculture: Premises and policies. Science 2013, 341, 33–34. [Google Scholar] [CrossRef]
- Petersen, B.; Snapp, S. What is sustainable intensification? Views from experts. Land Use Policy 2015, 46, 1–10. [Google Scholar] [CrossRef]
- Tous, J.; Romero, A.; Hermoso, J.F.; Msallem, M.; Larbi, A. Olive orchard design and mechanization: Present and future. Acta Hortic. 2014, 1057, 231–246. [Google Scholar] [CrossRef]
- Connor, D.J.; Goméz-del-Campo, M.; Rousseaux, M.C.; Searles, P.S. Structure, management and productivity of hedgerow olive orchards: A review. Sci. Hortic. 2014, 169, 71–93. [Google Scholar] [CrossRef]
- Barranco, D.; Cimato, A.; Fiorino, P.; Rallo, L.; Touzani, A.; Castañeda, C.; Serafini, E.; Trujillo, I. World Catalogue of Olive Varieties, 1st ed.; Mundi Prensa: Madrid, Spain, 2000; p. 360. ISBN 978-84-8476-714-5. [Google Scholar]
- Rallo, L.; Barranco, D.; de la Rosa, R.; Léon, L. ‘Chiquitita’ olive. HortScience 2008, 43, 529–531. [Google Scholar] [CrossRef]
- Butler, J. New Olive Variety Launched by Agromillora. Olive Oil Times. 2014. Available online: https://www.oliveoiltimes.com/production/oliana-variety-launched-agromillora/39022 (accessed on 28 August 2021).
- Fontanazza, G.; Bartolozzi, F. Vergati, G. FS-17. Riv. Frut. 1998, 5, 61. [Google Scholar]
- Sonnoli, A. Una nuova varietà di olivo a dimensione ridotta. Olivae 2001, 88, 46–49. [Google Scholar]
- Camposeo, S.; Godini, A. Preliminary observations about the performance of 13 varieties according to the super high density oliveculture training system in Apulia (southern Italy). Adv. Hortic. Sci. 2010, 24, 16–20. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Polverigiani, S.; Sirugo, M.; Neri, D. Damage to several olive cultivars by two over-the-row harvesters in high-density orchards. Acta Hortic. 2018, 1199, 415–419. [Google Scholar] [CrossRef]
- Vivaldi, G.A.; Strippoli, G.; Pascuzzi, S.; Stellacci, A.M.; Camposeo, S. Olive genotypes cultivated in an adult high-density orchard respond differently to canopy restraining by mechanical and manual pruning. Sci. Hortic. 2015, 192, 391–399. [Google Scholar] [CrossRef]
- Godini, A.; Vivaldi, G.A.; Camposeo, S. Olive cultivars field-tested in super high-density system in southern Italy. Calif. Agric. 2011, 65, 39–40. [Google Scholar] [CrossRef] [Green Version]
- Roselli, L.; Clodoveo, M.L.; Corbo, F.; De Gennaro, B. Are health claims a useful tool to segment the category of extra-virgin olive oil? Threats and opportunities for the Italian olive oil supply chain. Trends Food Sci. Technol. 2017, 68, 176–181. [Google Scholar] [CrossRef]
- De Gennaro, B.C.; Roselli, L.; Bimbo, F.; Carlucci, D.; Cavallo, C.; Cicia, G.; Del Giudice, T.; Lombardi, A.; Paparella, A.; Vecchio, R. Do Italian consumers value health claims on extra-virgin olive oil? J. Func. Foods 2021, 81, 104461. [Google Scholar] [CrossRef]
- Servili, M. The phenolic compounds: A commercial argument in the economic war to come on the quality of olive oil? OCL 2014, 21, D509. [Google Scholar] [CrossRef] [Green Version]
- Clodoveo, M.L.; Camposeo, S.; Amirante, R.; Dugo, G.; Cicero, N.; Boskou, D. Research and innovative approaches to obtain virgin olive oils with a higher level of bioactive constituents. In Olives and Olive Oil Bioactive Constituents; Boskou, D., Ed.; AOCS Press: Urbana, IL, USA, 2015; pp. 179–216. ISBN 978-1-630670-41-2. [Google Scholar]
- Allalout, A.; Krichène, D.; Methenni, K.; Taamalli, A.; Oueslati, I.; Daoud, D.; Zarrouk, M. Characterization of virgin olive oil from super intensive Spanish and Greek varieties grown in northern Tunisia. Sci. Hort. 2009, 120, 77–83. [Google Scholar] [CrossRef]
- Polari, J.J.; Mori, M.; Wang, S.C. Virgin olive oils from super-high-density orchards in California: Impact of cultivar, harvest time, and crop season on quality and chemical composition. Eur. J. Lipid Sci. Technol. 2021, 123, 2000180. [Google Scholar] [CrossRef]
- Bouaziz, M.; Jemai, H.; Khabou, W.; Sayadi, S. Oil content, phenolic profiling and antioxidant potential of Tunisian olive drupes. J. Sci. Food Agric. 2010, 90, 1750–1758. [Google Scholar] [CrossRef]
- Gutiérrez-Rosales, F.; Rios, J.J.; Gomez-Rey, M.L. Main polyphenols in the bitter taste of virgin olive oil. Structural confirmation by on-line high-performance liquid chromatography electrospray ionization mass spectrometry. J. Agric. Food Chem. 2003, 51, 6021–6025. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Barranco, D.; Caballero, J.M.; Martin, A.; Rallo, L.; Del Rio, C.; Tous, J.; Trujillo, I. Variedades de Olivo en Espana, 2nd ed.; Mundi-Prensa: Madrid, Spain, 2004; p. 47. ISBN 978-84-8476-192-1. [Google Scholar]
- Farinelli, D.; Tombesi, S. Performance and oil quality of ‘Arbequina’ and four Italian olive cultivars under super high density hedgerow planting system cultivated in central Italy. Sci. Hortic. 2015, 192, 97–107. [Google Scholar] [CrossRef]
- León, L.; De La Rosa, R.; Barranco, D.; Rallo, L. Breeding for early bearing in olive. HortScience 2007, 42, 499–502. [Google Scholar] [CrossRef] [Green Version]
- Spadoni, A.; Sion, S.; Gadaleta, S.; Savoia, M.; Piarulli, L.; Fanelli, V.; Di Rienzo, V.; Taranto, F.; Miazzi, M.M.; Montemurro, C. A simple and rapid method for genomic DNA extraction and microsatellite analysis in tree plants. J. Agric. Sci. Technol. 2019, 21, 1215–1226. [Google Scholar]
- De la Rosa, R.; James, C.M.; Tobutt, K.R. Isolation and characterization of polymorphic microsatellites in olive (Olea europaea L.) and their transferability to other genera in the Oleaceae. Mol. Ecol. Notes 2002, 2, 265–267. [Google Scholar] [CrossRef]
- Cipriani, G.; Marrazzo, M.T.; Marconi, R.; Cimato, A.; Testolin, R. Microsatellite markers isolated in olive (Olea europaea L.) are suitable for individual finger-printing and reveal polymorphism within ancient cultivars. Theor. Appl. Genet. 2002, 104, 223–228. [Google Scholar] [CrossRef]
- Saddoud Deddabi, O.; Montemurro, C.; Ben Maachia, S.; Ben Amar, F.; Fanelli, V.; Gadaleta, S.; El Riachy, M.; Chehade, A.; Siblini, M.; Boucheffa, S.; et al. A hot spot of olive biodiversity in the Tunisian oasis of Degache. Diversity 2020, 12, 358. [Google Scholar] [CrossRef]
- UPOV. 2011. Available online: https://www.upov.int (accessed on 20 September 2021).
- Seifi, E.; Guerin, J.; Kaiser, B.; Sedgley, M. Flowering and fruit set in olive: A review. J. Plant Physiol. 2015, 5, 1263–1272. [Google Scholar] [CrossRef]
- Camposeo, S.; Ferrara, G.; Palasciano, M.; Godini, A. About the biological behavior of cv. Coratina. Acta Hort. 2012, 949, 129–133. [Google Scholar] [CrossRef]
- Camposeo, S.; Vivaldi, G.A.; Gattullo, C.E. Ripening indices and harvesting times of different olive cultivars for continuous harvest. Sci. Hortic. 2013, 151, 1–10. [Google Scholar] [CrossRef]
- Farinelli, D.; Tombesi, S.; Famiani, F.; Tombesi, A. The fruit detachment force/fruit weight ratio can be used to predict the harvesting yield and the efficiency of trunk shakers on mechanical olive harvesting. Acta Hortic. 2012, 965, 61–64. [Google Scholar] [CrossRef]
- Sola-Guirado, R.R.; Aragon-Rodriguez, F.; Castro-Garcia, S.; Gil-Ribes, J. The vibration behaviour of hedgerow olive trees in response to mechanical harvesting with straddle harvester. Biosyst. Engin. 2019, 184, 81–89. [Google Scholar] [CrossRef]
- Baldoni, L.; Cultrera, N.G.; Mariotti, R.; Ricciolini, C.; Arcioni, S.; Vendramin, G.G.; Buonamici, A.; Porceddu, A.; Sarri, V.; Ojeda, M.A.; et al. A consensus list of microsatellite markers for olive genotyping. Mol. Breed. 2009, 24, 213–231. [Google Scholar] [CrossRef]
- Montemurro, C.; Dambruoso, G.; Bottalico, G.; Sabetta, W. Self-incompatibility assessment of some Italian olive genotypes (Olea europaea L.) and cross-derived seedling selection by SSR markers on seed endosperms. Front. Plant Sci. 2019, 10, 451. [Google Scholar] [CrossRef] [Green Version]
- Camposeo, S.; Vivaldi, G.A. Yield, harvesting efficiency and oil chemical quality of cultivars Arbequina and Arbosana harvested by straddle machine in two Apulian growing areas. Acta Hortic. 2018, 1199, 397–402. [Google Scholar] [CrossRef]
- Caruso, T.; Campisi, G.; Marra, F.P.; Camposeo, S.; Vivaldi, G.A.; Proietti, P.; Nasini, L. Growth and yields of the cultivar Arbequina in high density planting systems in three different olive growing areas in Italy. Acta Hortic. 2014, 1057, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Gomez-del-Campo, M.; Connor, D.J.; Trentacoste, E.R. Long-term effect of intra-row spacing on growth and productivity of super-high density hedgerow olive orchards (cv. Arbequina). Front. Plant Sci. 2017, 8, 1790. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.B.; Collins, G.; Sedgley, M. Sexual compatibility within and between olive cultivars. J. Hortic. Sci. Biotechnol. 2002, 77, 665–673. [Google Scholar] [CrossRef]
- Alagna, F.; Caceres, M.E.; Pandolfi, S.; Collani, S.; Mousavi, S.; Mariotti, R.; Cultrera, N.G.M.; Baldoni, L.; Barcaccia, G. The Paradox of self-fertile varieties in the context of self-incompatible genotypes in olive. Front. Plant Sci. 2019, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, R.; Pandolfi, S.; De Cauwer, I.; Saumitou-Laprade, P.; Vernet, P.; Rossi, M.; Baglivo, F.; Baldoni, L.; Mousavi, S. Diallelic self-incompatibility is the main determinant of fertilization patterns in olive orchards. Evol. Appl. 2021, 14, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Rallo, L.; Cuevas, J. Frutification y produccion. In El cultivo del Olivo, 7th ed.; Barranco, D., Fernandez-Escobar, R., Rallo, L., Eds.; Mundi Prensa: Madrid, Spain, 2017; pp. 162–163. ISBN 978-84-8476-714-5. [Google Scholar]
- Rotondi, A.; Magli, M.; Morrone, L.; Alfei, B.; Pannelli, G. Italian National database of monovarietal extra virgin olive oils. In The Mediterranean Genetic Code. Grapevine and Olive; Sladonja, B., Ed.; IntechOpen: London, UK, 2013; pp. 179–200. ISBN 978-953-51-1607-5. [Google Scholar] [CrossRef] [Green Version]
- Borges, T.H.; Pereira, J.A.; Cabrera-Vique, C.; Seiquer, I. Study of the antioxidant potential of Arbequina extra virgin olive oils from Brazil and Spain applying combined models of simulated digestion and cell culture markers. J. Funct. Foods 2017, 37, 209–218. [Google Scholar] [CrossRef]
- Marino, G.; Macaluso, L.; Marra, F.P.; Ferguson, L.; Marchese, A.; Campisi, G.; Volo, P.; Laudicina, V.A.; Caruso, T. Horticultural performance of 23 Sicilian olive genotypes in hedgerow systems: Vegetative growth, productive potential and oil quality. Sci. Hortic. 2017, 217, 217–225. [Google Scholar] [CrossRef]
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean olive orchards under climate change: A review of future impacts and adaptation strategies. Agronomy 2021, 11, 56. [Google Scholar] [CrossRef]
- Freixa, E.; Gil, J.M.; Tous, J.; Hermoso, J.F. Comparative study of the economic viability of high- and super-high-density olive orchards in Spain. Acta Hortic. 2011, 924, 247–254. [Google Scholar] [CrossRef]





| Genotype | EMO90 | GAPU101 | ||
|---|---|---|---|---|
| Arbosana (♀) | 181 | 183 | 181 | 187 |
| Leccino (♂) | 183 | 189 | 195 | 197 |
| Lecciana (F1) | 183 | 183 | 181 | 197 |
| Tree | Vigor (1) | Weak |
| Growth habit (2) | Upright | |
| Canopy density (3) | Medium | |
| Leaf | Shape (7) | Elongated |
| Blade longitudinal curvature (9) | Straight | |
| Inflorescence | Length (11) | Short |
| Fruit | Length (14) | Medium |
| Width (15) | Narrow | |
| Weight (16) | Medium | |
| Shape (17) | Elliptic | |
| Size of lenticels (20) | Medium | |
| Number of lenticels (21) | Few | |
| Over color at full maturity (22) | Dark violet | |
| Symmetry in position A (23) | Weakly asymmetric | |
| Shape of apex in position A (24) | Rounded | |
| Nipple (25) | Absent | |
| Shape of base (26) | Truncate | |
| Stone | Shape in position B (28) | Elliptic |
| Length (29) | Medium | |
| Width in position B (30) | Narrow | |
| Weight (32) | Medium | |
| Symmetry in position A (33) | Weakly asymmetric | |
| Number of grooves on basal end (35) | Between 7 and 10 | |
| Shape of apex in position A (37) | Acute | |
| Mucron (38) | Present | |
| Rugosity of surface (40) | Strong |
| Open Flowers (%) | 2015 | 2016 |
|---|---|---|
| 10 | May 3 | April 29 |
| 50 | May 12 | May 9 |
| 90 | May 16 | May 15 |
| Position (m) | Flowers Per Inflorescence (n) | Gynosterility (%) |
|---|---|---|
| 1.5–2.0 | 15.8 ± 2.3 ns | 1.68 ± 0.8 c |
| 1.0–1.5 | 14.4 ± 2.0 ns | 5.01 ± 2.1 a |
| 0.5–1.0 | 13.2 ± 2.1 ns | 2.56 ± 1.9 b |
| mean | 14.5 ± 2.5 | 3.08 ± 2.2 |
| Pollination Type | Exposure | Fruit Set (%) | Mean |
|---|---|---|---|
| Self | Est | 0.10 ± 0.05 b | 0.10 ± 0.05 b |
| West | 0.09 ± 0.04 b | ||
| Open | Est | 2.92 ± 0.67 a | 3.24 ± 0.78 a |
| West | 3.56 ± 0.83 a |
| Parameters | Lecciana | Arbequina | |
|---|---|---|---|
| Canopy volume (m3·tree−1) | 6.6 ± 1.0 | 5.7 ± 0.6 | * |
| Fruit yield (kg·tree−1) | 6.1 ± 1.1 | 5.0 ± 1.2 | * |
| c Fruit yield (kg·tree−1) | 23.3 ± 3.4 | 19.7 ± 2.1 | * |
| Trunk section area (cm2) | 48.5 ± 0.5 | 47.0 ± 0.7 | * |
| Fruit yield efficiency (kg·cm−2) | 0.48 ± 0.05 | 0.42 ± 0.08 | * |
| Oil content (% fw) | 19.3 ± 1.2 | 20.5 ± 1.5 | * |
| Olive Moisture (%) | 58.4 ± 2.4 | 57.2 ± 2.5 | ns |
| c Oil yield (kg fw·tree−1) | 4.50 ± 0.59 | 4.04 ± 0.36 | * |
| Oil yield efficiency (kg dw·cm−2) | 0.20 ± 0.03 | 0.20 ± 0.04 | ns |
| Fatty Acids | Lecciana | Arbequina | Italian | |
|---|---|---|---|---|
| Palmitic acid (C16:0; %) | 15.1 ± 0.3 | 16.9 ± 1.0 | ns | 12.01 |
| Palmitoleic acid (C16:1; %) | 1.1 ± 0.1 | 2.0 ± 0.3 | * | 1.1 |
| Stearic acid (C18:0; %) | 2.3 ± 0.4 | 1.6 ± 0.4 | * | 2.2 |
| Oleic acid (C18:1; %) | 73.7 ± 2.4 | 70.2 ± 1.0 | ns | 73.0 |
| Linoleic acid (C18:2; %) | 6.2 ± 0.1 | 13.2 ± 1.5 | * | 10.3 |
| Linolenic acid (C18:3; %) | 0.6 ± 0.0 | 0.6 ± 0.2 | ns | 0.3 |
| Ripening Time (DAFB) | Polyphenols (mg·kg−1) | Fruitiness (n/7.0) | |
|---|---|---|---|
| 2015 | 2016 | ||
| 177 | 180 | 442.5 ± 6.6 c | 4.1 ± 0.3 b |
| 191 | 194 | 470.3 ± 1.5 a | 4.5 ± 0.3 a |
| 206 | 209 | 458.1 ± 5.4 b | 4.4 ± 0.2 a |
| 220 | 223 | 451.0 ± 9.3 b | 4.3 ± 0.3 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camposeo, S.; Vivaldi, G.A.; Montemurro, C.; Fanelli, V.; Cunill Canal, M. Lecciana, a New Low-Vigour Olive Cultivar Suitable for Super High Density Orchards and for Nutraceutical EVOO Production. Agronomy 2021, 11, 2154. https://doi.org/10.3390/agronomy11112154
Camposeo S, Vivaldi GA, Montemurro C, Fanelli V, Cunill Canal M. Lecciana, a New Low-Vigour Olive Cultivar Suitable for Super High Density Orchards and for Nutraceutical EVOO Production. Agronomy. 2021; 11(11):2154. https://doi.org/10.3390/agronomy11112154
Chicago/Turabian StyleCamposeo, Salvatore, Gaetano Alessandro Vivaldi, Cinzia Montemurro, Valentina Fanelli, and Marisa Cunill Canal. 2021. "Lecciana, a New Low-Vigour Olive Cultivar Suitable for Super High Density Orchards and for Nutraceutical EVOO Production" Agronomy 11, no. 11: 2154. https://doi.org/10.3390/agronomy11112154
APA StyleCamposeo, S., Vivaldi, G. A., Montemurro, C., Fanelli, V., & Cunill Canal, M. (2021). Lecciana, a New Low-Vigour Olive Cultivar Suitable for Super High Density Orchards and for Nutraceutical EVOO Production. Agronomy, 11(11), 2154. https://doi.org/10.3390/agronomy11112154








