Identification, Expression, and Functional Characterization of ScCaM in Response to Various Stresses in Sugarcane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Gene Isolation and Bioinformatics Analysis
2.3. Prokaryotic Expression in E. coli BL21 (DE3) Cells
2.4. Subcellular Localization Analysis
2.5. qRT-PCR Analysis
2.6. Phenotype and Expression Pattern Analysis of Arabidopsis thaliana Overexpressing ScCaM Gene under Different Exogenous Stresses
3. Results
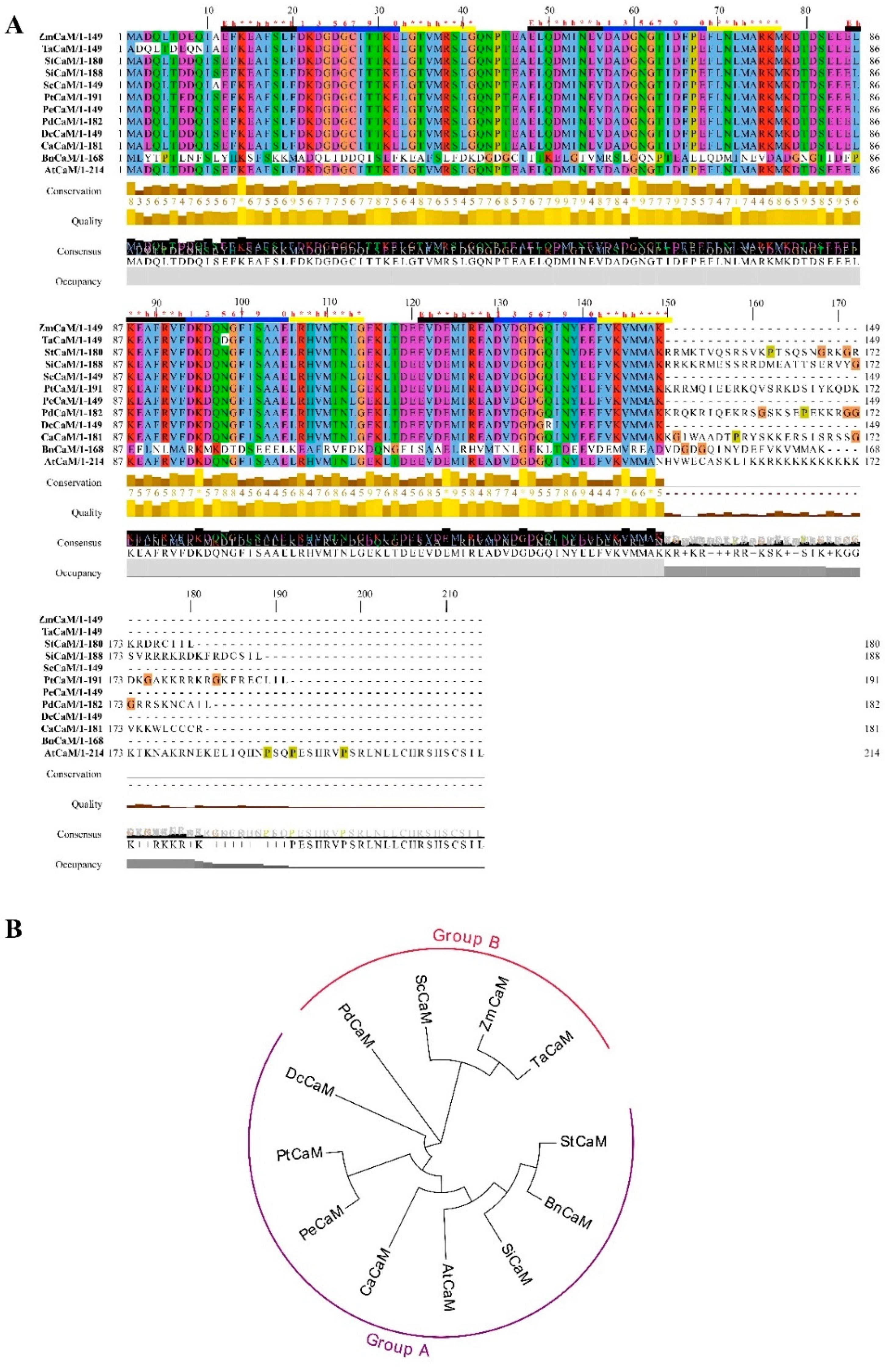
3.1. Identification and Sequence Features of ScCaM
3.2. Sequence and Phylogenetic Analysis of CaM Proteins
3.3. Expression of ScCaM in E. coli BL21 (DE3) Strain
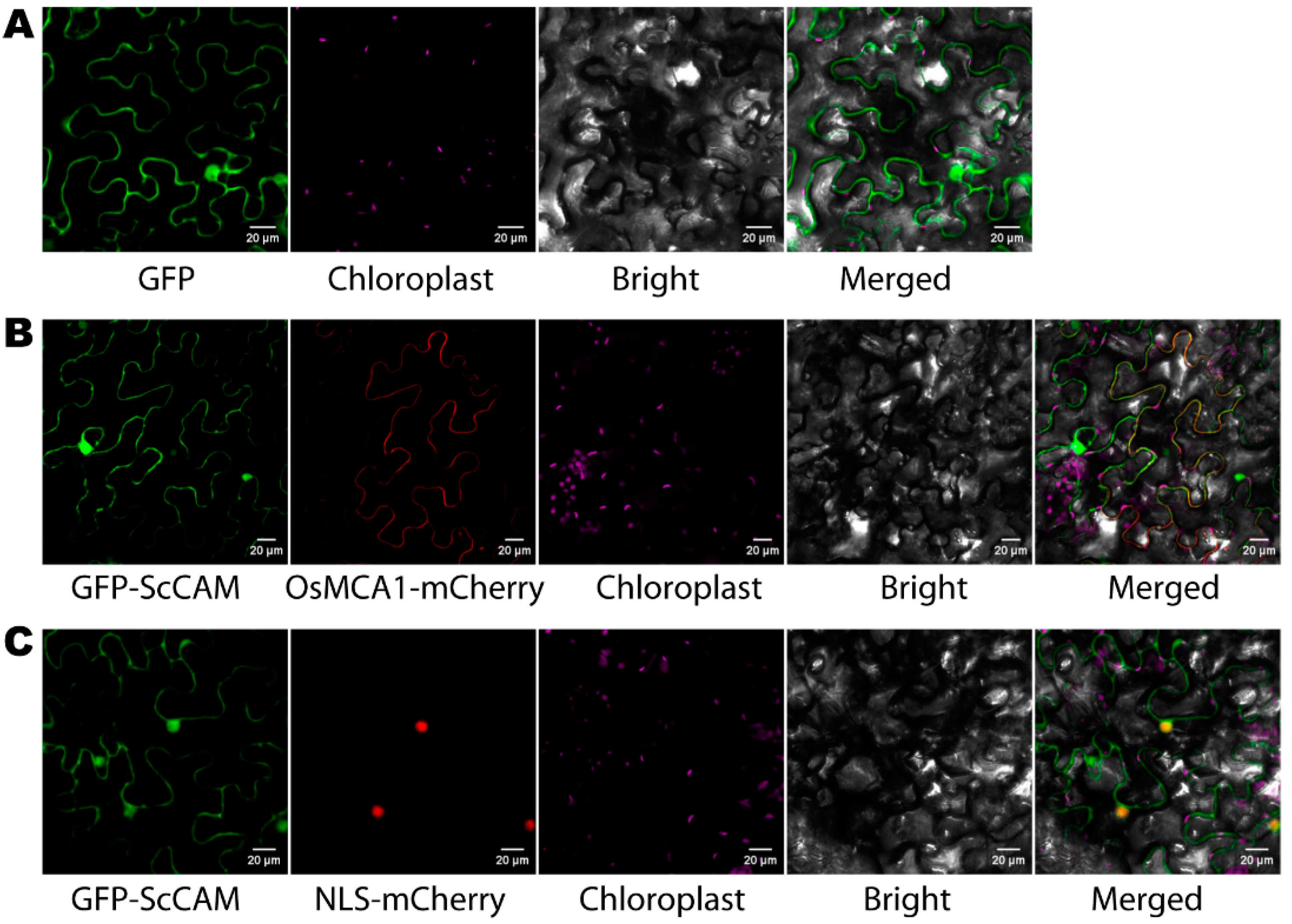
3.4. Subcellular Localization of ScCaM in N. benthamiana Cells
3.5. Gene Expression Patterns of ScCaM under Abiotic Stress and Phytohormone Stimulation in Sugarcane
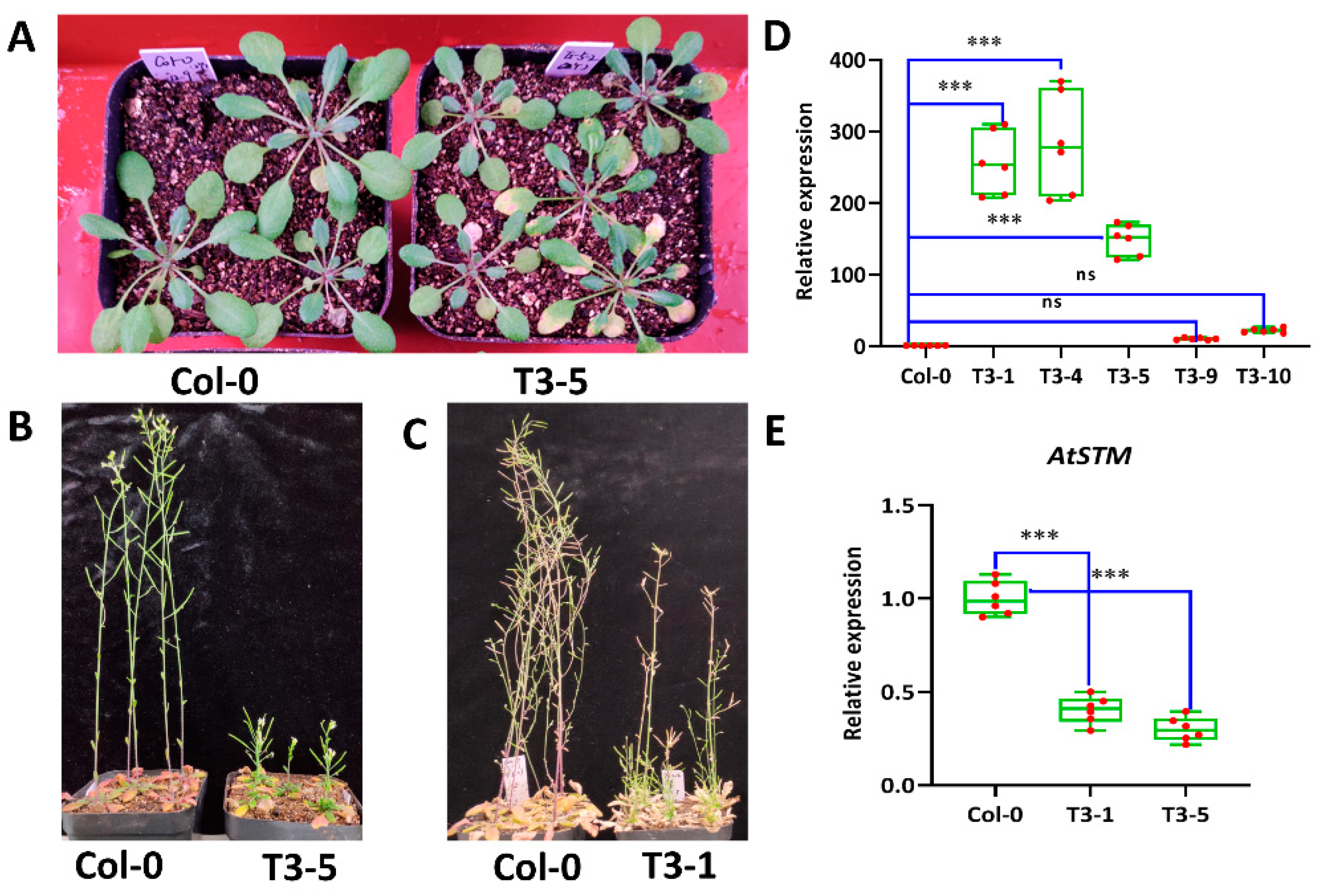
3.6. Overexpression of ScCaM Affect Growth and Development in A. thaliana
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Defalco, T.A.; Bender, K.W.; Snedden, W.A. Breaking the code: Ca2+ sensors in plant signalling. Biochem. J. 2010, 425, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Strynadka, N.C.; James, M.N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu. Rev. Biochem. 1989, 58, 951–998. [Google Scholar] [CrossRef]
- Chin, D.; Means, A.R. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000, 10, 322–328. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Kumar, P.; Bae, H. Genomics and evolutionary aspect of calcium signaling event in calmodulin and calmodulin-like proteins in plants. BMC Plant Biol. 2017, 17, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCormack, E.; Tsai, Y.-C.; Braam, J. Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci. 2005, 10, 383–389. [Google Scholar] [CrossRef]
- La Verde, V.; Dominici, P.; Astegno, A. Towards understanding plant calcium signaling through calmodulin-like proteins: A biochemical and structural perspective. Int. J. Mol. Sci. 2018, 19, 1331. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Ali, G.S.; Simons, K.A.; Hou, J.; Yang, T.; Reddy, A.S.N.; Poovaiah, B.W. Ca2+/calmodulin regulates salicylic-acid-mediated plant immunity. Nature 2009, 457, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Poovaiah, B.W. Ca2+/calmodulin is critical for brassinosteroid biosynthesis and plant growth. Nature 2005, 437, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; El-Habbak, M.H.; Havens, W.M.; Singh, A.; Zheng, D.; Vaughn, L.; Haudenshield, J.S.; Hartman, G.L.; Korban, S.S.; Ghabrial, S.A. Overexpression of GmCaM4 in soybean enhances resistance to pathogens and tolerance to salt stress. Mol. Plant Pathol. 2014, 15, 145–160. [Google Scholar] [CrossRef]
- Kushwaha, R.; Singh, A.; Chattopadhyay, S. Calmodulin 7 plays an important role as transcriptional regulator in Arabidopsis seedling development. Plant Cell 2008, 20, 1747–1759. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Y.; Wang, X.J.; Xue, J.; Xia, N.; Deng, L.; Cai, G.L.; Tang, C.L.; Wei, G.L.; Huang, L.L.; Kang, Z.S. Cloning and expression analysis of a novel calmodulin isoform TaCaM5 from wheat. Acta Agron. Sin. 2010, 36, 953–960. [Google Scholar] [CrossRef]
- Su, W.; Zhang, C.; Feng, J.; Feng, A.; You, C.; Ren, Y.; Wang, D.; Sun, T.; Su, Y.; Xu, L.; et al. Genome-wide identification, characterization and expression analysis of the carotenoid cleavage oxygenase (CCO) gene family in Saccharum. Plant Physiol. Biochem. 2021, 162, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.A.; Carvalho, R.F.; Cia, M.C.; Gratão, P.L. Sugarcane under pressure: An overview of biochemical and physiological studies of abiotic stress. Trop. Plant Biol. 2011, 4, 42–51. [Google Scholar] [CrossRef]
- Wang, L.; Tsuda, K.; Sato, M.; Cohen, J.D.; Katagiri, F.; Glazebrook, J. Arabidopsis CaM binding protein CBP60g contributes to MAMP-induced SA accumulation and is involved in disease resistance against Pseudomonas syringae. PLoS Pathog. 2009, 5, e1000301. [Google Scholar] [CrossRef] [Green Version]
- Parvin, S.; Lee, O.R.; Sathiyaraj, G.; Khorolragchaa, A.; Kim, Y.J.; Devi, B.S.; Yang, D.C. Interrelationship between calmodulin (CaM) and H2O2 in abscisic acid-induced antioxidant defense in the seedlings of Panax ginseng. Mol. Biol. Rep. 2012, 39, 7327–7338. [Google Scholar] [CrossRef]
- Que, Y.; Xu, L.; Liu, J.; Guo, J.; Zheng, Y.; Xu, J.; Yuan, Z.; Chen, P.; Chen, R. Construction of full-length cDNA library from sugarcane leaves and the corresponding EST analysis. J. Agric. Biotechnol. 2009, 17, 843–850. [Google Scholar]
- Su, W.; Zhang, C.; Wang, D.; Ren, Y.; Sun, T.; Feng, J.; Su, Y.; Xu, L.; Shi, M.; Que, Y. The CaCA superfamily genes in Saccharum: Comparative analysis and their functional implications in response to biotic and abiotic stress. BMC Genom. 2021, 22, 549. [Google Scholar] [CrossRef]
- Wu, G.; Liu, S.; Zhao, Y.; Wang, W.; Kong, Z.; Tang, D. ENHANCED DISEASE RESISTANCE4 associates with CLATHRIN HEAVY CHAIN2 and modulates plant immunity by regulating relocation of EDR1 in Arabidopsis. Plant Cell 2015, 27, 857–873. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kurusu, T.; Nishikawa, D.; Yamazaki, Y.; Gotoh, M.; Nakano, M.; Hamada, H.; Yamanaka, T.; Iida, K.; Nakagawa, Y.; Saji, H.; et al. Plasma membrane protein OsMCA1 is involved in regulation of hypo-osmotic shock-induced Ca2+ influx and modulates generation of reactive oxygen species in cultured rice cells. BMC Plant Biol. 2012, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Zhao, T.; Deng, L.; Lv, D.; Zhang, X.; Pan, X.; Xu, J.; Long, G. Visualizing the Essential Role of Complete Virion Assembly Machinery in Efficient Hepatitis C Virus Cell-to-Cell Transmission by a Viral Infection-Activated Split-Intein-Mediated Reporter System. J. Virol. 2017, 91, e01720-16. [Google Scholar] [CrossRef] [Green Version]
- McAinsh, M.R.; Pittman, J.K. Shaping the calcium signature. New Phytol. 2009, 181, 275–294. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Yadav, D.; Khan, A.L.; Hashem, A.; Abd Allah, E.F.; Al-Harrasi, A. Molecular players of EF-hand containing calcium signaling event in plants. Int. J. Mol. Sci. 2019, 20, 1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperschneider, J.; Catanzariti, A.-M.; DeBoer, K.; Petre, B.; Gardiner, D.M.; Singh, K.B.; Dodds, P.N.; Taylor, J.M. LOCALIZER: Subcellular localization prediction of both plant and effector proteins in the plant cell. Sci. Rep. 2017, 7, 44598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dauwalder, M.; Roux, S.J.; Hardison, L. Distribution of calmodulin in pea seedlings: Immunocytochemical localization in plumules and root apices. Planta 1986, 168, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yan, S.; Zhou, H.; Dong, R.; Lei, J.; Chen, C.; Cao, B. Overexpression of CsCaM3 improves high temperature tolerance in cucumber. Front. Plant Sci. 2018, 9, 797. [Google Scholar] [CrossRef]
- Nie, H.; Zhao, C.; Wu, G.; Wu, Y.; Chen, Y.; Tang, D. SR1, a calmodulin-binding transcription factor, modulates plant defense and ethylene-induced senescence by directly regulating NDR1 and EIN3. Plant Physiol. 2012, 158, 1847–1859. [Google Scholar] [CrossRef] [Green Version]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. Signal transduction. The calcium entry pas de deux. Science 2000, 287, 1604–1605. [Google Scholar] [CrossRef]
- Dai, C.; Lee, Y.; Lee, I.C.; Nam, H.G.; Kwak, J.M. Calmodulin 1 Regulates Senescence and ABA Response in Arabidopsis. Front. Plant Sci. 2018, 9, 803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, J.A.; Moan, E.I.; Medford, J.I.; Barton, M.K. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 1996, 379, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Endrizzi, K.; Moussian, B.; Haecker, A.; Levin, J.Z.; Laux, T. The SHOOT MERISTEMLESS gene is required for maintenance of undifferentiated cells in Arabidopsis shoot and floral meristems and acts at a different regulatory level than the meristem genes WUSCHEL and ZWILLE. Plant J. 1996, 10, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.K.; Poethig, R.S. Formation of the shoot apical meristem in Arabidopsis thaliana: An analysis of development in the wild type and in the shoot meristemless mutant. Development 1993, 119, 823–831. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, C.; Su, W.; Wu, G.; Fu, X.; Que, Y.; Luo, J. Identification, Expression, and Functional Characterization of ScCaM in Response to Various Stresses in Sugarcane. Agronomy 2021, 11, 2153. https://doi.org/10.3390/agronomy11112153
Liu J, Zhang C, Su W, Wu G, Fu X, Que Y, Luo J. Identification, Expression, and Functional Characterization of ScCaM in Response to Various Stresses in Sugarcane. Agronomy. 2021; 11(11):2153. https://doi.org/10.3390/agronomy11112153
Chicago/Turabian StyleLiu, Jinxian, Chang Zhang, Weihua Su, Guangheng Wu, Xianyu Fu, Youxiong Que, and Jun Luo. 2021. "Identification, Expression, and Functional Characterization of ScCaM in Response to Various Stresses in Sugarcane" Agronomy 11, no. 11: 2153. https://doi.org/10.3390/agronomy11112153
APA StyleLiu, J., Zhang, C., Su, W., Wu, G., Fu, X., Que, Y., & Luo, J. (2021). Identification, Expression, and Functional Characterization of ScCaM in Response to Various Stresses in Sugarcane. Agronomy, 11(11), 2153. https://doi.org/10.3390/agronomy11112153






