Optimal Voluntary Vaccination of Adults and Adolescents Can Help Eradicate Hepatitis B in China
Abstract
1. Introduction
2. Model of HBV Dynamics
3. Game-Theoretical Model of Vaccination Decisions
4. Results
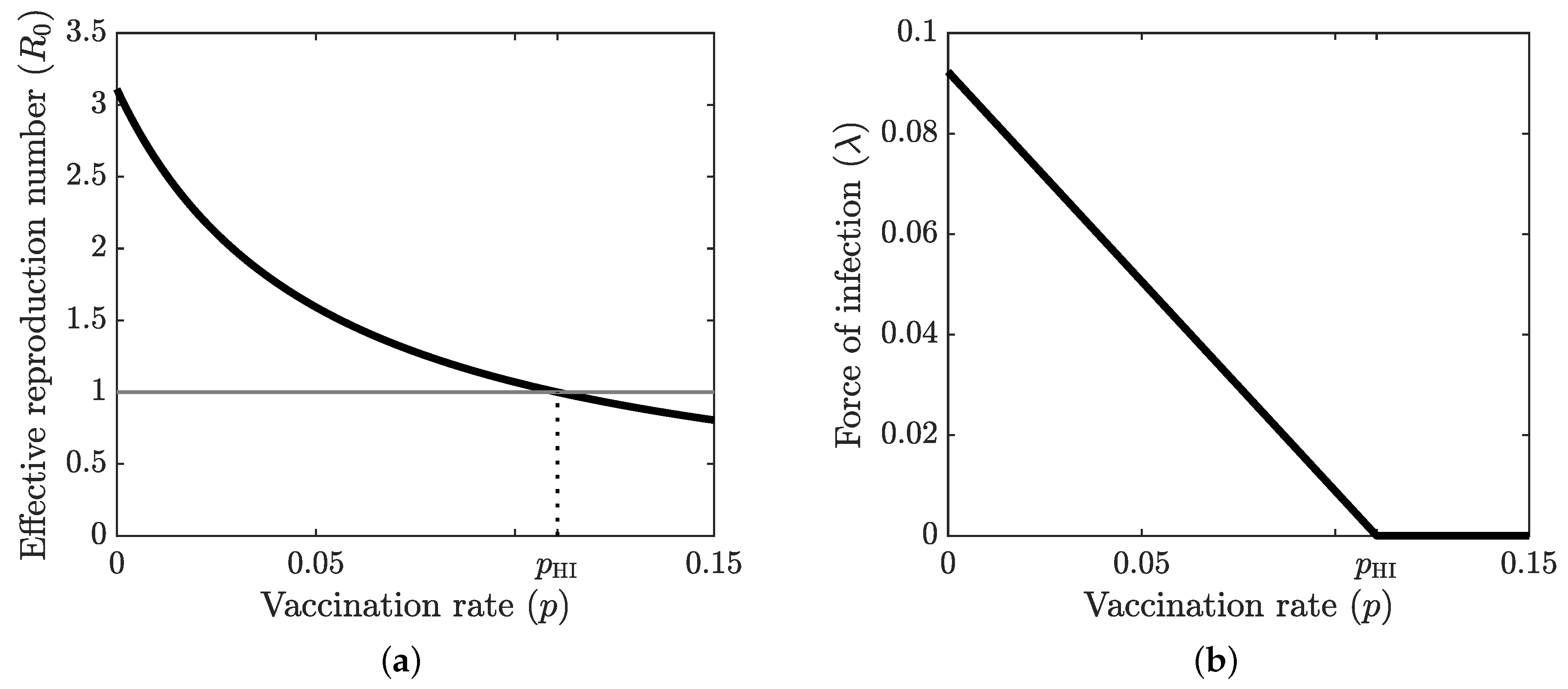
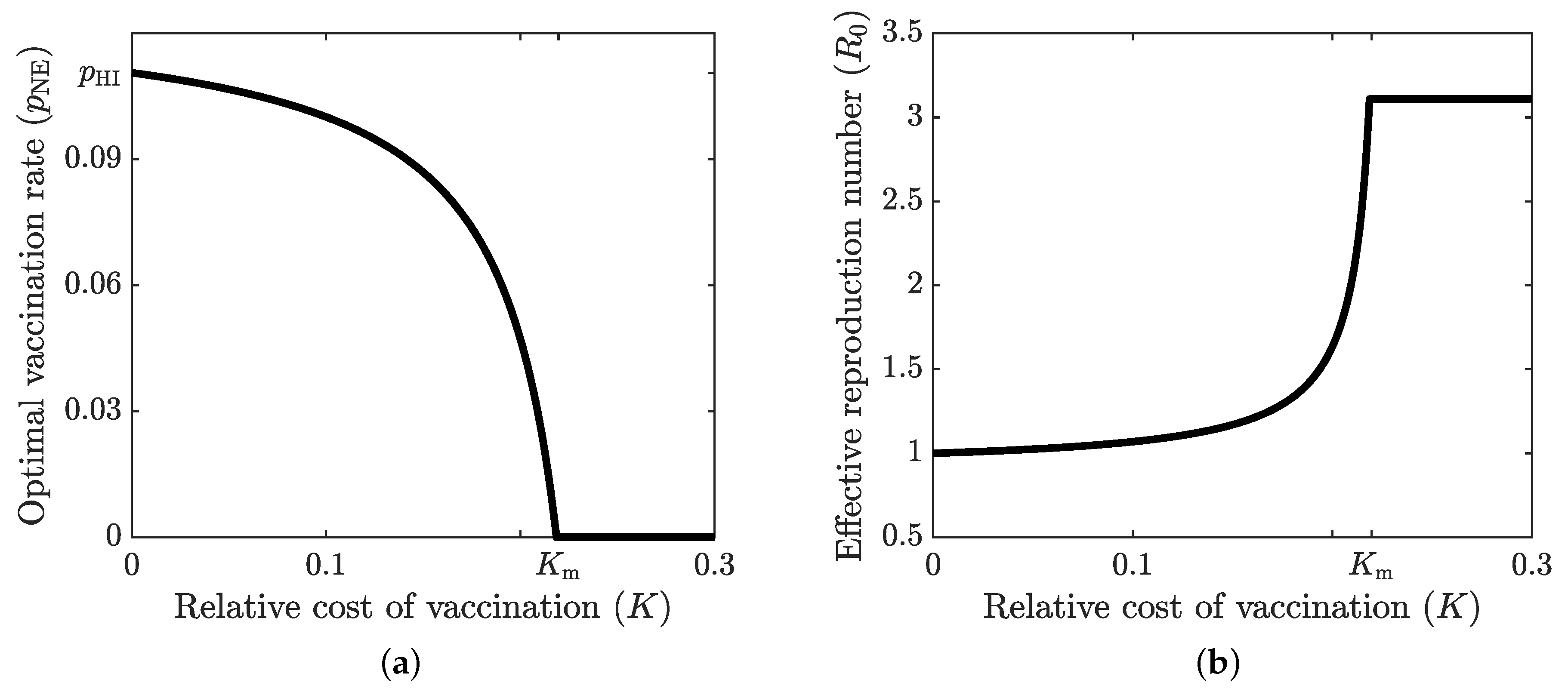
4.1. Optimal Vaccination Strategies
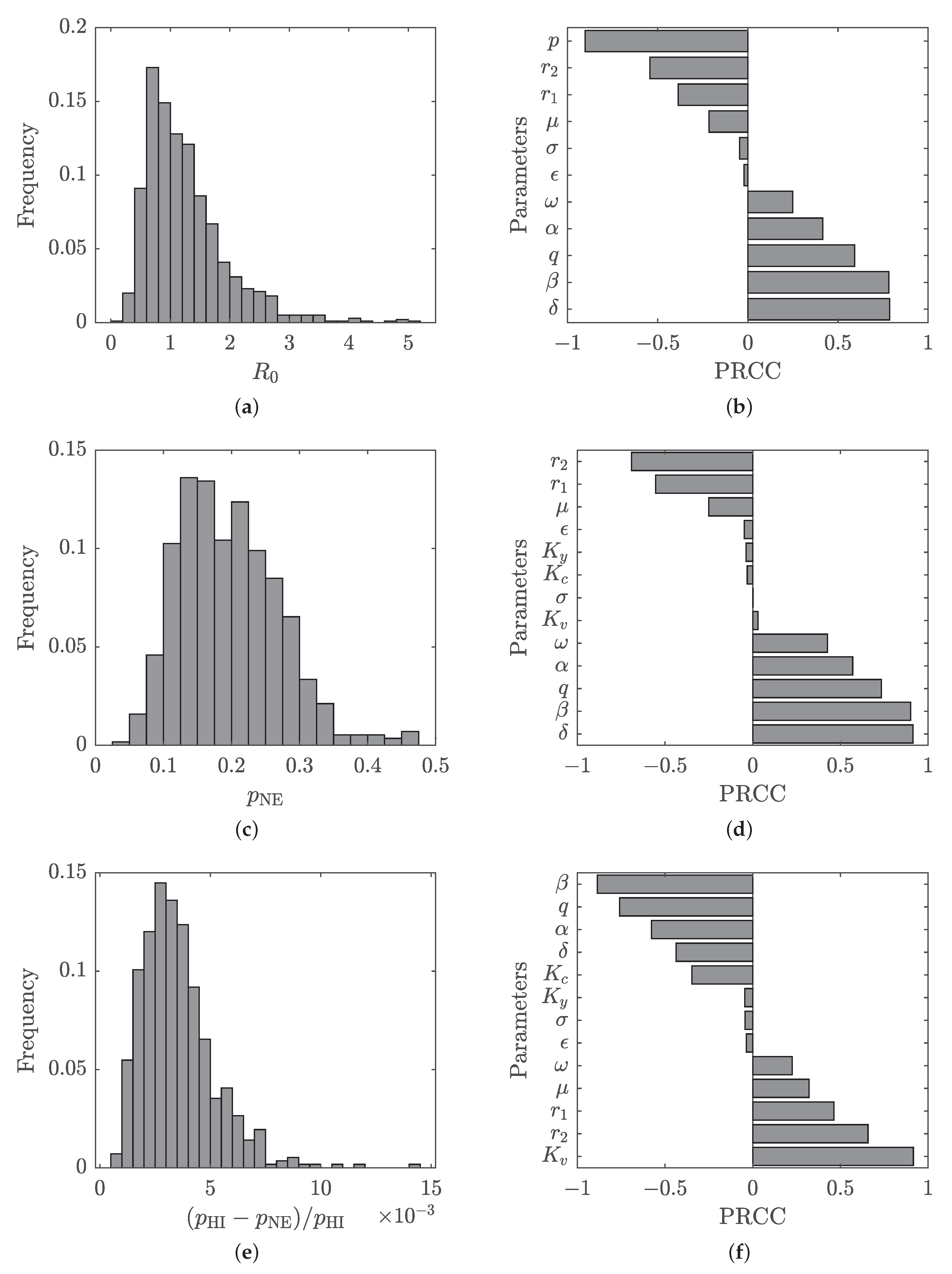
4.2. Sensitivity and Uncertainty Analysis
5. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- MacLachlan, J.; Locarnini, S.; Cowie, B. Estimating the global prevalence of hepatitis B. Lancet 2015, 386, 1515–1517. [Google Scholar] [CrossRef]
- Seeger, C.; Litwin, S.; Mason, W. Hepatitis B virus: Persistence and clearance. In Hepatitis B Virus in Human Diseases; Liaw, Y., Zoulim, F., Eds.; Humana Press: Cham, Switzerland, 2016; Chapter 6; pp. 123–145. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, W.; Ruan, S. Modeling the transmission dynamics and control of Hepatitis B virus in China. J. Theor. Biol. 2010, 262, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Liaw, Y. Natural history of hepatitis B infection. In Hepatitis B Virus in Human Diseases; Liaw, Y., Zoulim, F., Eds.; Humana Press: Cham, Switzerland, 2016; Chapter 11; pp. 217–248. [Google Scholar] [CrossRef]
- Margolis, H.S.; Alter, M.J.; Hadler, S.C. Hepatitis B: Evolving epidemiology and implications for control. In Seminars in Liver Disease; Thieme Medical Publishers, Inc.: Stuttgart, Germany, 1991; Volume 11, pp. 84–92. [Google Scholar]
- Shepard, C.; Simard, E.; Finelli, L.; Fiore, A.; Bell, B. Hepatitis B virus infection: Epidemiology and vaccination. Epidemiol. Rev. 2006, 28, 112–125. [Google Scholar] [CrossRef]
- Khan, T.; Zaman, G.; Chohan, M. The transmission dynamic and optimal control of acute and chronic hepatitis B. J. Biol. Dyn. 2017, 11, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liu, Z.; Gu, F. Epidemiology and prevention of hepatitis B virus infection. Int. J. Med. Sci. 2005, 2, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, I.I.; Tillmann, T.; Banerjee, A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Lok, A.S.; McMahon, B.J. Chronic hepatitis B. Hepatology 2007, 45, 507–539. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Bi, S.; Yang, W.; Wang, L.; Cui, G.; Cui, F.; Zhang, Y.; Liu, J.; Gong, X.; Chen, Y.; et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992–2005 in China. J. Infect. Dis. 2009, 200, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Cui, J.; Zhou, X. Dynamical behavior of a hepatitis B virus transmission model with vaccination. J. Theor. Biol. 2010, 265, 572–578. [Google Scholar] [CrossRef]
- Zhu, D.; Guo, N.; Wang, J.; Nicholas, S.; Wang, Z.; Zhang, G.; Shi, L.; Wangen, K.R. Socioeconomic inequality in hepatitis B vaccination of rural adults in China. Hum. Vaccines Immunother. 2018, 14, 464–470. [Google Scholar] [CrossRef]
- Wang, H.; Men, P.; Xiao, Y.; Gao, P.; Lv, M.; Yuan, Q.; Chen, W.; Bai, S.; Wu, J. Hepatitis B infection in the general population of China: A systematic review and meta-analysis. BMC Infect. Dis. 2019, 19, 811. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, S.; Wang, Q.; Shen, H.; Zhang, M.; Zhang, Y.; Yan, D.; Liu, M. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21–49 years in rural China: A population-based, cross-sectional study. Lancet Infect. Dis. 2016, 16, 80–86. [Google Scholar] [CrossRef]
- Zhang, C.; Ke, W.; Gao, Y.; Zhou, S.; Liu, L.; Ye, X.; Yao, Z.; Yang, Y. Cost-effectiveness analysis of antiviral therapies for hepatitis B e antigen-positive chronic hepatitis B patients in China. Clin. Drug Investig. 2015, 35, 197–209. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Yang, D. Antiviral therapy for chronic hepatitis B in China. Med. Microbiol. Immunol. 2015, 204, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Toy, M.; Hutton, D.; McCulloch, K.; Romero, N.; Revill, P.A.; Penicaud, M.C.; So, S.; Cowie, B.C. The price tag of a potential cure for chronic hepatitis B infection: A cost threshold analysis for USA, China and Australia. In Liver International; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021. [Google Scholar]
- Liang, P.; Zu, J.; Zhuang, G. A literature review of mathematical models of hepatitis B virus transmission applied to immunization strategies from 1994 to 2015. J. Epidemiol. 2018, 28, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Liao, L.E.; Perelson, A.S. Within-host mathematical models of hepatitis B virus infection: Past, present, and future. Curr. Opin. Syst. Biol. 2019, 18, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zu, J. The review of differential equation models of HBV infection dynamics. J. Virol. Methods 2019, 266, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kamyad, A.V.; Akbari, R.; Heydari, A.A.; Heydari, A. Mathematical modeling of transmission dynamics and optimal control of vaccination and treatment for hepatitis B virus. Comput. Math. Methods Med. 2014, 2014, 475451. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Y. The analysis and application of an HBV model. Appl. Math. Model. 2012, 36, 1302–1312. [Google Scholar] [CrossRef]
- O’Leary, C.; Hong, Z.; Zhang, F.; Dawood, M.; Smart, G.; Kaita, K.; Wu, J. A mathematical model to study the effect of hepatitis B virus vaccine and antivirus treatment among the Canadian Inuit population. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 63. [Google Scholar] [CrossRef] [PubMed]
- Thornley, S.; Bullen, C.; Roberts, M. Hepatitis B in a high prevalence New Zealand population: A mathematical model applied to infection control policy. J. Theor. Biol. 2008, 254, 599–603. [Google Scholar] [CrossRef]
- Medley, G.F.; Lindop, N.A.; Edmunds, W.J.; Nokes, D.J. Hepatitis-B virus endemicity: Heterogeneity, catastrophic dynamics and control. Nat. Med. 2001, 7, 619. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, W.; Medley, G.; Nokes, D. The transmission dynamics and control of Hepatitis B virus in The Gambia. Stat. Med. 1996, 15, 2215–2233. [Google Scholar] [CrossRef]
- Williams, J.; Nokes, D.; Medley, G.; Anderson, R. The transmission dynamics of hepatitis B in the UK: A mathematical model for evaluating costs and effectiveness of immunization programmes. Epidemiol. Infect. 1996, 116, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xu, Z.; Lu, Y. A mathematical model of hepatitis B virus transmission and its application for vaccination strategy in China. Int. J. Epidemiol. 2000, 29, 744–752. [Google Scholar] [CrossRef]
- Kretzschmar, M.; Mangen, M.J.; van de Laar, M.; de Wit, A. Model based analysis of hepatitis B vaccination strategies in the Netherlands. Vaccine 2009, 27, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Ruan, S.; Zhang, W. An age-structured model for the transmission dynamics of hepatitis B. SIAM J. Appl. Math. 2010, 70, 3121–3139. [Google Scholar] [CrossRef]
- Bauch, C.; Earn, D. Vaccination and the theory of games. Proc. Natl. Acad. Sci. USA 2004, 101, 13391–13394. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, A.; Maiwand, S.; Ngo, M.; Putalapattu, V.; Rychtář, J.; Taylor, D. Game-theoretical model of retroactive Hepatitis B vaccination in China. Bull. Math. Biol. 2020, 82, 80. [Google Scholar] [CrossRef] [PubMed]
- Scheckelhoff, K.; Ejaz, A.; Erovenko, I.V. A game-theoretic model of optimal clean equipment usage to prevent hepatitis C among injecting drug users. Preprint 2019. [Google Scholar]
- Cheng, E.; Gambhirrao, N.; Patel, R.; Zhowandai, A.; Rychtář, J.; Taylor, D. A game-theoretical analysis of Poliomyelitis vaccination. J. Theor. Biol. 2020, 499, 110298. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.R.; Foster, A.O.; Feagins, D.A.; Rowell, J.T.; Erovenko, I.V. Optimal voluntary and mandatory insect repellent usage and emigration strategies to control the chikungunya outbreak on Reunion Island. PeerJ 2020, 8, e10151. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Machado, J.; Sanchez, E.; Erovenko, I.V. Optimal vaccination strategies to reduce endemic levels of meningitis in Africa. Preprint 2019. [Google Scholar]
- Bankuru, S.V.; Kossol, S.; Hou, W.; Mahmoudi, P.; Rychtář, J.; Taylor, D. A Game-theoretic Model of Monkeypox to Assess Vaccination Strategies. PeerJ 2020, 8, e9272. [Google Scholar] [CrossRef] [PubMed]
- Kobe, J.; Pritchard, N.; Short, Z.; Erovenko, I.V.; Rychtář, J.; Rowell, J.T. A Game-Theoretic Model of Cholera with Optimal Personal Protection Strategies. Bull. Math. Biol. 2018, 80, 2580–2599. [Google Scholar] [CrossRef] [PubMed]
- Brettin, A.; Rossi-Goldthorpe, R.; Weishaar, K.; Erovenko, I.V. Ebola could be eradicated through voluntary vaccination. R. Soc. Open Sci. 2018, 5, 171591. [Google Scholar] [CrossRef]
- Dorsett, C.; Oh, H.; Paulemond, M.L.; Rychtář, J. Optimal repellent usage to combat Dengue Fever. Bull. Math. Biol. 2016, 78, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Broom, M.; Rychtář, J.; Spears-Gill, T. The game-theoretical model of using insecticide-treated bed-nets to fight Malaria. Appl. Math. 2016, 7, 852–860. [Google Scholar] [CrossRef]
- Sykes, D.; Rychtář, J. A game-theoretic approach to valuating Toxoplasmosis vaccination strategies. Theor. Popul. Biol. 2015, 105, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Crawford, K.; Lancaster, A.; Oh, H.; Rychtář, J. A voluntary use of insecticide-treated cattle can eliminate African Sleeping Sickness. Lett. Biomath. 2015, 2, 91–101. [Google Scholar] [CrossRef]
- Fortunato, A.; Glasser, C.; Watson, J.; Lu, Y.; Rychtář, J.; Taylor, D. Mathematical modeling of the use of insecticide treated nets for elimination of visceral leishmaniasis in Bihar, India. R. Soc. Open Sci. 2021, 8, 201960. [Google Scholar] [CrossRef] [PubMed]
- Bauch, C.; Anonychuk, A.; Pham, B.; Gilca, V.; Duval, B.; Krahn, M. Cost-utility of universal hepatitis A vaccination in Canada. Vaccine 2007, 25, 8536–8548. [Google Scholar] [CrossRef] [PubMed]
- Bauch, C.; Rao, A.S.S.; Pham, B.; Krahn, M.; Gilca, V.; Duval, B.; Chen, M.; Tricco, A. A dynamic model for assessing universal Hepatitis A vaccination in Canada. Vaccine 2007, 25, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Anonychuk, A.M.; Tricco, A.C.; Bauch, C.T.; Ba’Pham; Gilca, V.; Duval, B.; John-Baptiste, A.; Woo, G.; Krahn, M. Cost-effectiveness analyses of hepatitis A vaccine. Pharmacoeconomics 2008, 26, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.L.; Piraveenan, M.; Pattison, P.; Prokopenko, M. Game theoretic modelling of infectious disease dynamics and intervention methods: A review. J. Biol. Dyn. 2020, 14, 57–89. [Google Scholar] [CrossRef]
- Acosta-Alonzo, C.B.; Erovenko, I.V.; Lancaster, A.; Oh, H.; Rychtář, J.; Taylor, D. High endemic levels of typhoid fever in rural areas of Ghana may stem from optimal voluntary vaccination behavior. Proc. R. Soc. A 2020, 476, 20200354. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Issa, H.; Rychtář, J.; Taylor, D.; Umana, N. A voluntary use of insecticide treated nets can stop the vector transmission of Chagas disease. PLoS Neglected Trop. Dis. 2020, 14, e0008833. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, Y.; Tanimoto, J. Realistic decision-making processes in a vaccination game. Phys. A Stat. Mech. Its Appl. 2018, 494, 236–241. [Google Scholar] [CrossRef]
- Kabir, K.A.; Jusup, M.; Tanimoto, J. Behavioral incentives in a vaccination-dilemma setting with optional treatment. Phys. Rev. E 2019, 100, 062402. [Google Scholar] [CrossRef] [PubMed]
- Kabir, K.A.; Tanimoto, J. Modelling and analysing the coexistence of dual dilemmas in the proactive vaccination game and retroactive treatment game in epidemic viral dynamics. Proc. R. Soc. A 2019, 475, 20190484. [Google Scholar] [CrossRef]
- Kuga, K.; Tanimoto, J.; Jusup, M. To vaccinate or not to vaccinate: A comprehensive study of vaccination-subsidizing policies with multi-agent simulations and mean-field modeling. J. Theor. Biol. 2019, 469, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Arefin, M.R.; Masaki, T.; Kabir, K.A.; Tanimoto, J. Interplay between cost and effectiveness in influenza vaccine uptake: A vaccination game approach. Proc. R. Soc. A 2019, 475, 20190608. [Google Scholar] [CrossRef] [PubMed]
- Arefin, M.R.; Kabir, K.A.; Tanimoto, J. A mean-field vaccination game scheme to analyze the effect of a single vaccination strategy on a two-strain epidemic spreading. J. Stat. Mech. Theory Exp. 2020, 2020, 033501. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.; Xia, C. Role of vaccine efficacy in the vaccination behavior under myopic update rule on complex networks. Chaos Solitons Fractals 2020, 130, 109425. [Google Scholar] [CrossRef]
- Kabir, K.A.; Tanimoto, J. Evolutionary game theory modelling to represent the behavioural dynamics of economic shutdowns and shield immunity in the COVID-19 pandemic. R. Soc. Open Sci. 2020, 7, 201095. [Google Scholar] [CrossRef] [PubMed]
- Kabir, K.A.; Tanimoto, J. Analysis of individual strategies for artificial and natural immunity with imperfectness and durability of protection. J. Theor. Biol. 2021, 509, 110531. [Google Scholar] [CrossRef] [PubMed]
- Kuga, K.; Tanaka, M.; Tanimoto, J. Pair approximation model for the vaccination game: Predicting the dynamic process of epidemic spread and individual actions against contagion. Proc. R. Soc. A 2021, 477, 20200769. [Google Scholar] [CrossRef]
- Kabir, K.A.; Tanimoto, J. Cost-efficiency analysis of voluntary vaccination against n-serovar diseases using antibody-dependent enhancement: A game approach. J. Theor. Biol. 2020, 503, 110379. [Google Scholar] [CrossRef] [PubMed]
- World Bank. Life Expectancy at Birth. 2019. Available online: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=CN (accessed on 18 December 2019).
- Alter, M.J. Epidemiology and prevention of Hepatitis B. Semin. Liver Dis. 2003, 23, 39–46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Damme, P. Long-term protection after hepatitis B vaccine. J. Infect. Dis. 2016, 214, 1–3. [Google Scholar] [CrossRef]
- Lo, K.J.; Tsai, Y.T.; Lee, S.D.; Wu, T.C.; Wang, J.Y.; Chen, G.H.; Yeh, C.L.; Chiang, B.N.; Yeh, S.H.; Goudeau, A. Immunoprophylaxis of infection with hepatitis B virus in infants born to hepatitis B surface antigen-positive carrier mothers. J. Infect. Dis. 1985, 152, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Hyams, K.C. Risks of chronicity following acute hepatitis B virus infection: A review. Clin. Infect. Dis. 1995, 20, 992–1000. [Google Scholar] [CrossRef]
- Hutton, D.W.; So, S.K.; Brandeau, M.L. Cost-effectiveness of nationwide Hepatitis B catch-up vaccination among children and adolescents in China. Hepatology 2010, 51, 405–414. [Google Scholar] [CrossRef]
- Toy, M.; Hutton, D.W.; So, S.K. Cost-effectiveness and cost thresholds of generic and brand drugs in a national chronic Hepatitis B treatment program in China. PLoS ONE 2015, 10, e0139876. [Google Scholar] [CrossRef] [PubMed]
- Hethcote, H. The Mathematics of Infectious Diseases. SIAM Rev. 2000, 42, 599–653. [Google Scholar] [CrossRef]
- van den Driessche, P.; Watmough, J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002, 180, 29–48. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, J.; Wangen, K.R. Hepatitis B vaccination coverage rates among adults in rural China: Are economic barriers relevant? Vaccine 2014, 32, 6705–6710. [Google Scholar] [CrossRef] [PubMed]
- Blower, S.; Dowlatabadi, H. Sensitivity and uncertainty analysis of complex models of disease transmission: An HIV model, as an example. Int. Stat. Rev. 1994, 62, 229–243. [Google Scholar] [CrossRef]
- Saltelli, A.; Tarantola, S.; Campolongo, F.; Ratto, M. Sensitivity Analysis in Practice: A Guide to Assessing Scientific Models; Wiley Online Library: Hoboken, NJ, USA, 2004; Volume 1. [Google Scholar]
- McKay, M.D.; Beckman, R.J.; Conover, W.J. A comparison of three methods for selecting values of input variables in the analysis of output from a computer code. Technometrics 2000, 42, 55–61. [Google Scholar] [CrossRef]
- Marino, S.; Hogue, I.B.; Ray, C.J.; Kirschner, D.E. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 2008, 254, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, D. Uncertainty and Sensitivity Functions and Implementation. 2020. Available online: http://malthus.micro.med.umich.edu/lab/usanalysis.html (accessed on 9 January 2021).
- Bruce, M.G.; Bruden, D.; Hurlburt, D.; Zanis, C.; Thompson, G.; Rea, L.; Toomey, M.; Townshend-Bulson, L.; Rudolph, K.; Bulkow, L.; et al. Antibody levels and protection after hepatitis B vaccine: Results of a 30-year follow-up study and response to a booster dose. J. Infect. Dis. 2016, 214, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Jijón, S.; Supervie, V.; Breban, R. Prevention of treatable infectious diseases: A game-theoretic approach. Vaccine 2017, 35, 5339–5345. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, J.; Zhu, D.; Leng, A.; Wangen, K. Hepatitis B-related knowledge and vaccination in association with discrimination against hepatitis B in rural China. Hum. Vaccines Immunother. 2016, 12, 70–76. [Google Scholar] [CrossRef] [PubMed]

| Symbol | Description | Base Value | Range | Source |
|---|---|---|---|---|
| Death rate and birth rate | [0.01, 0.015] | [63] | ||
| Proportion of failed immunization | 0.12 | [0, 0.2] | [11] | |
| Incubation rate | 3 | [1, 6] | [64] | |
| Rate of loss of immunity | 0.033 | [0.01, 0.067] | [65] | |
| Recovery rate from acute infections | 4 | [3, 5] | [19] | |
| Recovery rate of carriers | 0.025 | [0.005, 0.025] | [27] | |
| Probability of transmission from carrier mothers | 0.78 | [0.7, 0.9] | [66] | |
| q | Probability of an acute infection becoming chronic | 0.04 | [0.02, 0.06] | [67] |
| p | Vaccination rate | varies | [0, 0.3] | assumed |
| Transmission rate | 10 | [5, 15] | [27] | |
| Reduction factor for transmission from carriers | 0.16 | [0.1, 0.2] | [27] | |
| Cost of vaccination | $3 | [1, 5] | [68] | |
| Cost of acute infection | $300 | [100, 500] | [69] | |
| Cost of chronic infection | $1000 | [100, 2000] | [18] | |
| Expected cost of infection | $1895 | Equation (11) | ||
| K | Relative cost of infection | 0.0016 | ||
| Optimal vaccination rate | varies | Equation (16) | ||
| Vaccination rate needed for herd immunity | varies | Equation (15) | ||
| Minimal K for which | varies | Equation (20) | ||
| Force of infection | varies | Equation (1) | ||
| Probability of infection if unvaccinated | varies | Equation (12) | ||
| Probability of infection if vaccinated | varies | Equation (13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheckelhoff, K.; Ejaz, A.; Erovenko, I.V.; Rychtář, J.; Taylor, D. Optimal Voluntary Vaccination of Adults and Adolescents Can Help Eradicate Hepatitis B in China. Games 2021, 12, 82. https://doi.org/10.3390/g12040082
Scheckelhoff K, Ejaz A, Erovenko IV, Rychtář J, Taylor D. Optimal Voluntary Vaccination of Adults and Adolescents Can Help Eradicate Hepatitis B in China. Games. 2021; 12(4):82. https://doi.org/10.3390/g12040082
Chicago/Turabian StyleScheckelhoff, Kristen, Ayesha Ejaz, Igor V. Erovenko, Jan Rychtář, and Dewey Taylor. 2021. "Optimal Voluntary Vaccination of Adults and Adolescents Can Help Eradicate Hepatitis B in China" Games 12, no. 4: 82. https://doi.org/10.3390/g12040082
APA StyleScheckelhoff, K., Ejaz, A., Erovenko, I. V., Rychtář, J., & Taylor, D. (2021). Optimal Voluntary Vaccination of Adults and Adolescents Can Help Eradicate Hepatitis B in China. Games, 12(4), 82. https://doi.org/10.3390/g12040082







