Re-Use of Silico-Manganese Slag
Abstract
:1. Introduction
2. Materials and Methods
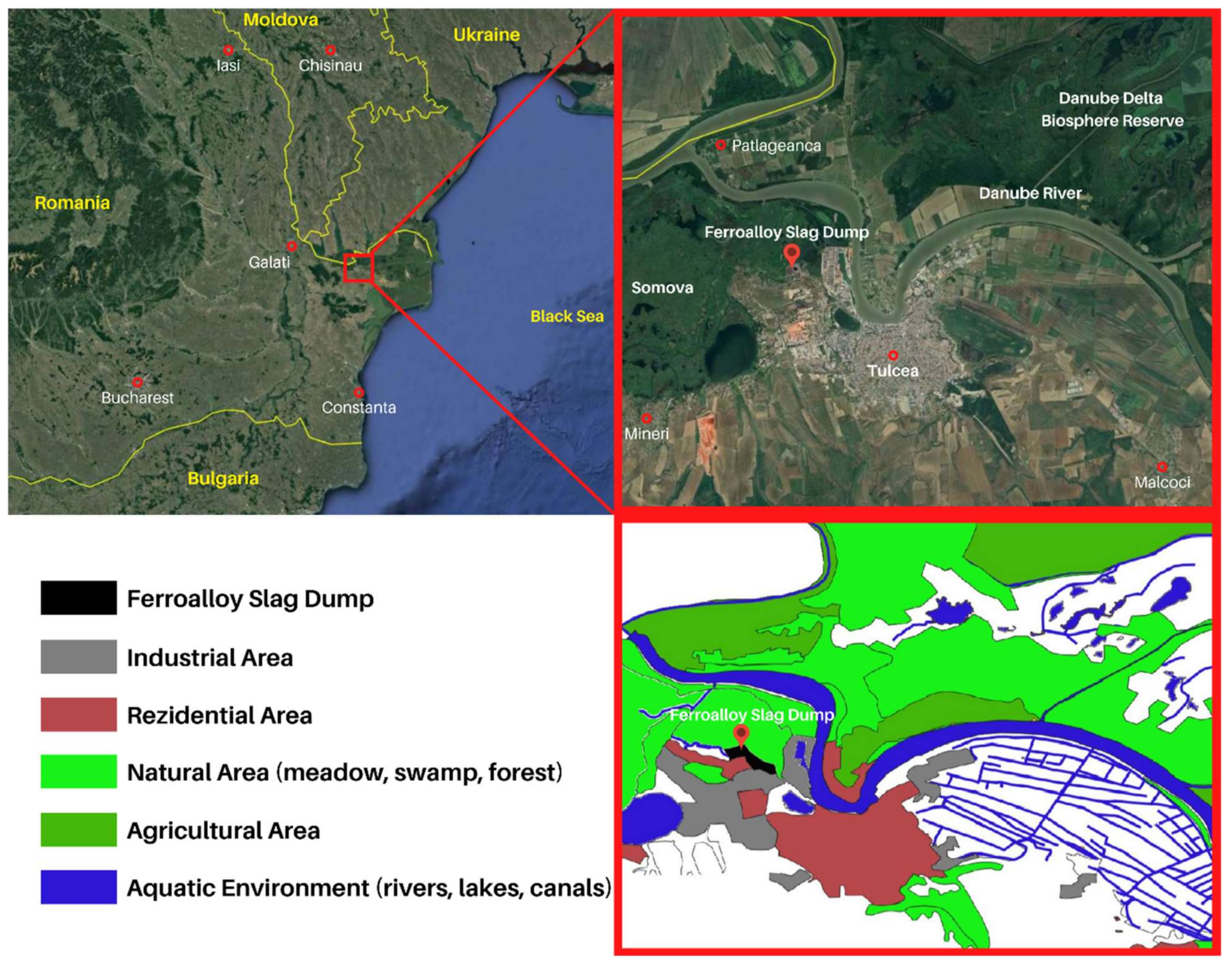
2.1. Area Description and Sampling
2.2. Sample Preparation and Extraction Procedure
2.3. Characterization Techniques
3. Results and Discussions
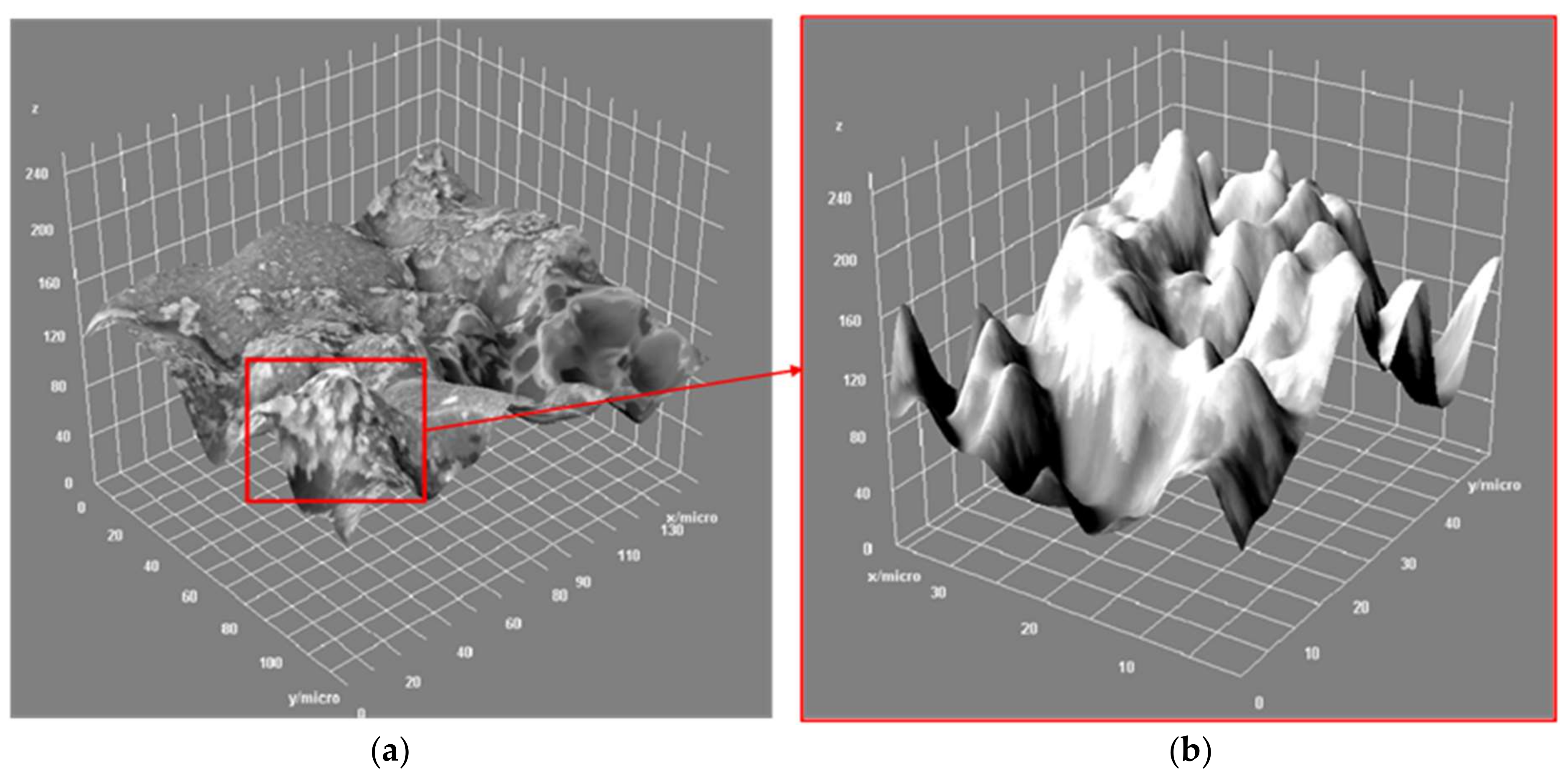
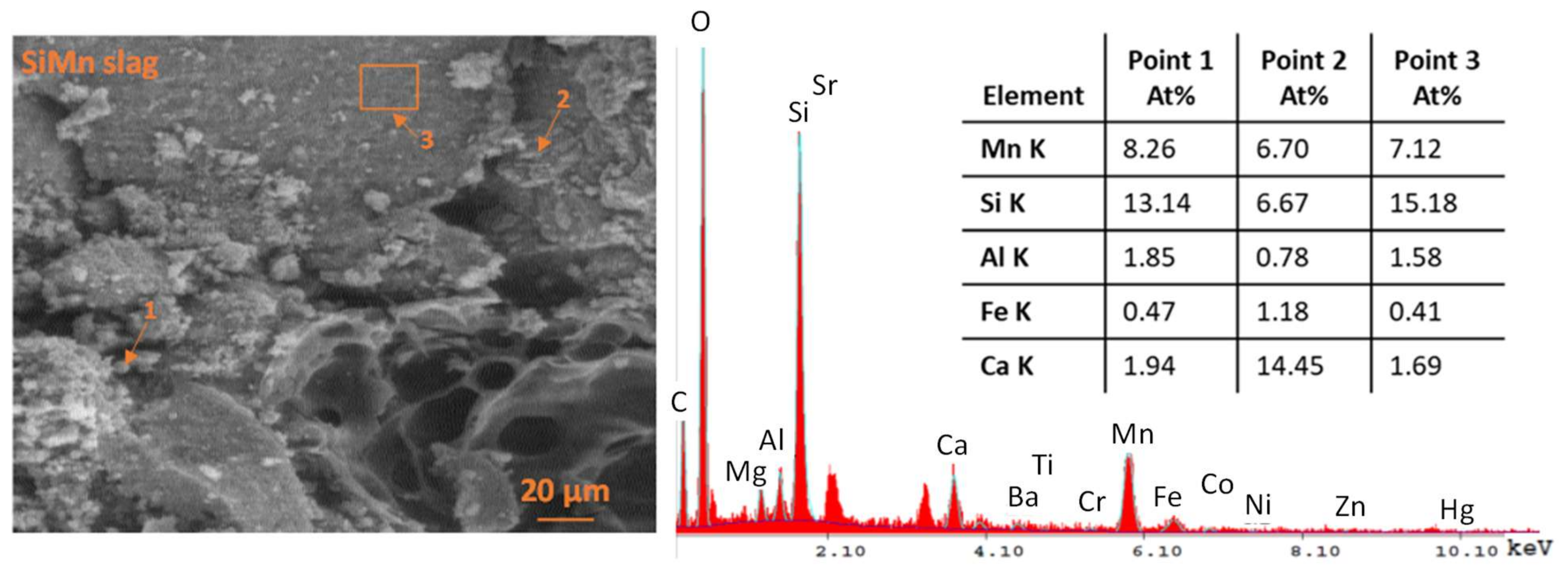
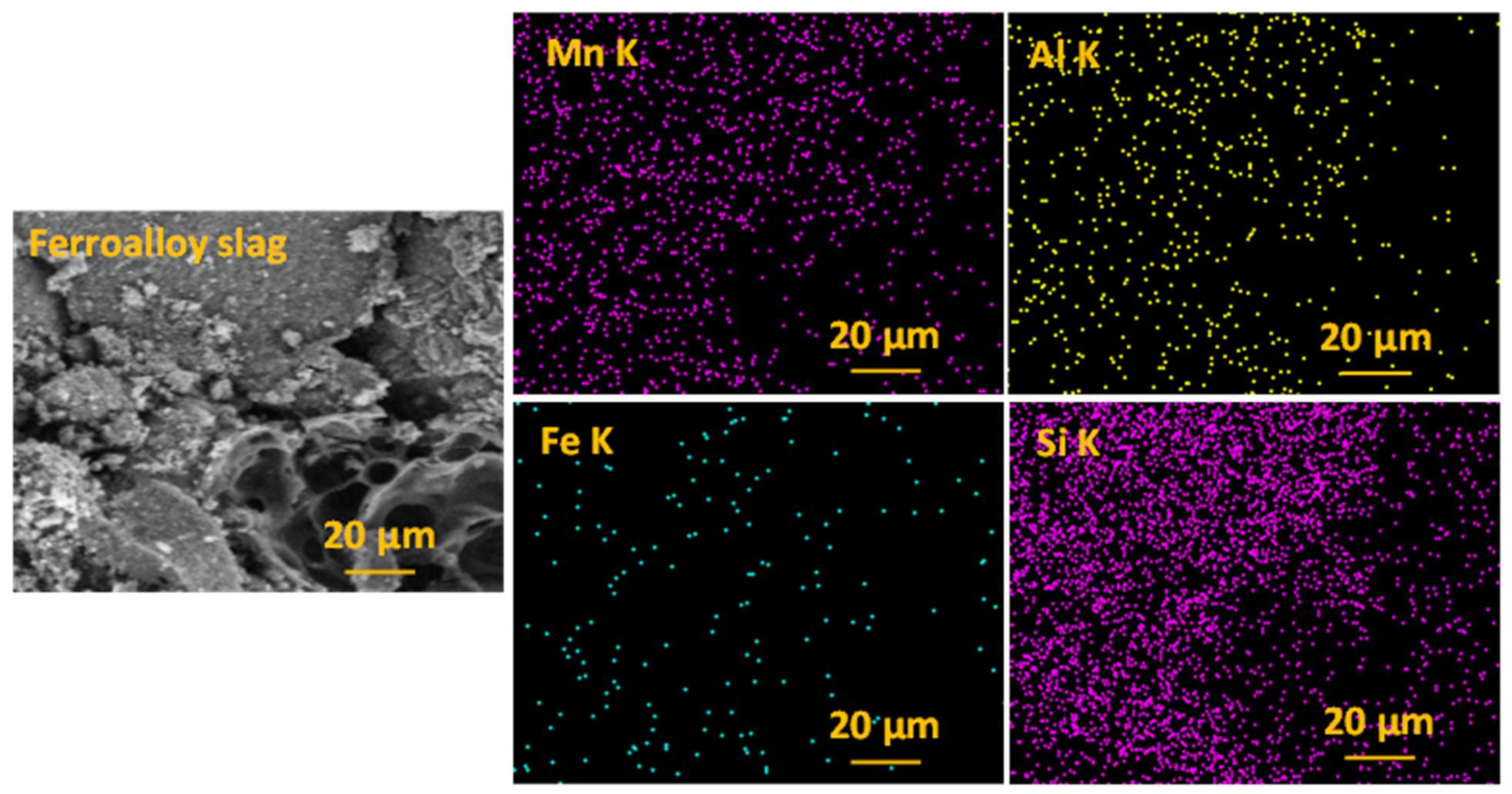
Morphological and Elemental Composition of the Slag
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mavroudis, I.; Petrides, F.; Karantali, E.; Chatzikonstantinou, S.; McKenna, J.; Ciobica, A.; Iordache, A.-C.; Dobrin, R.; Trus, C.; Kazis, D. A voxel-wise meta-analysis on the cerebellum in essential tremor. Med. Lith. 2021, 57, 264. [Google Scholar]
- Statescu, C.; Honceriu, C.; Jurcau, R.N.; Trus, C. An Original Study on the Correlations Between Magnesium and Depression and their Cardiovascular Relevance. Rev. Chim. 2019, 70, 4102–4104. [Google Scholar] [CrossRef]
- Statescu, C.; Honceriu, C.; Trus, C. Does Magnesium Deficient Diet and its Associated Metabolic Dysfunctions Induces Anxiety-like Symptoms Further cardiovascular relevance. Rev. Chim. 2019, 70, 3579–3581. [Google Scholar] [CrossRef]
- Das, B.; Prakash, S.; Reddy, P.S.R.; Misra, V.N. An overview of utilization of slag and sludge from steel industries. Resour. Conserv. Recycl. 2007, 50, 40–57. [Google Scholar] [CrossRef]
- Ayala, J.; Fernandez, B. Recovery of manganese from silicomanganese slag by means of a hydrometallurgical process. Hydrometallurgy 2015, 158, 68–73. [Google Scholar] [CrossRef]
- Huaiwei, Z.; Hong, X. An overview for the utilization of wastes from stainless steel industries. Resour. Conserv. Recycl. 2011, 55, 745–754. [Google Scholar] [CrossRef]
- Koros, P.J. Dusts, scale, slags, sludges. Not wastes, but sources of profits. Metall. Mater. Trans. B 2003, 34, 769–779. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Alquzweeni, S.S.; Naji, L.A.; Naushad, M. Predominant Mechanisms in the Treatment of Wastewater Due to Interaction of Benzaldehyde and Iron Slag Byproduct. Int. J. Environ. Res. Pub. Health 2020, 17, 226. [Google Scholar] [CrossRef] [Green Version]
- Santamaria, A.; Gonzalez, J.J.; Losanez, M.M.; Skaf, M.; Ortega-Lopez, V. The design of self-compacting structural mortar containing steelmaking slags as aggregate. Cem. Concr. Comp 2020, 111, 103627. [Google Scholar] [CrossRef]
- Qasrawi, H. The use of steel slag aggregate to enhance the mechanical properties of recycled aggregate concrete and retain the environment. Constr. Build Mater. 2014, 54, 298–304. [Google Scholar] [CrossRef]
- Desa, U.N. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: Geneva, Switzerland, 2015; p. 15-16301 (E). Available online: https://sdgs.un.org/2030agenda (accessed on 30 August 2021).
- Buruiana, D.L.; Bordei, M. Industrial waste recovery solutions for rehabilitation of affected. Metal. Int. 2011, 16, 155–158. [Google Scholar]
- Buruiana, D.L.; Lefter, D.; Tiron, G.L.; Balta, S.; Bordei, M. The influence on the environment factors of waste disposal in undeveloped areas. Int. Multi. Sci. Geoco. 2015, 2, 573–579. [Google Scholar]
- Buruiana, D.L.; Lefter, D.; Tiron, G.L.; Balta, S.; Bordei, M. Toxicity of heavy metals on the environment and human health. Int. Multi. Sci. Geoco. 2015, 27, 565–571. [Google Scholar]
- Buruiana, D.L.; Balta, S.; Iticescu, C.; Georgescu, L.P.; Humeniuc, I.I. Determining the concentration of heavy metals in the soils near slag landfills. Rev. Rom. Mat. 2016, 46, 108–114. [Google Scholar]
- Shelburne, W.M.; Degroot, D.J. The use of waste & recycled materials in highway construction. Civ. Eng. Pract. 1998, 13, 5–16. [Google Scholar]
- Groot, D.R.; Kazad, D.M.; Pollmann, H.; Villiers, J.P.R.; Redtmann, T.; Steenkamp, J. The recovery of manganese and generation of a valuable residue from ferromanganese slags by hydrometallurgical route. In Proceedings of the Thirteenth International Ferroalloys Congress, Efficient Technologies in Ferroalloy Industry, Almaty, Kazakhstan, 9–13 June 2013; pp. 1051–1060. [Google Scholar]
- Sully, A.H. Metallurgy of the Rarer Metals Manganese; Butterworths Scientific Publications: London, UK, 1955; pp. 7–10. [Google Scholar]
- Urane, D. Process for the Manufacture of Ferromanganese. Australian Patent US3433628A, 18 March 1969. [Google Scholar]
- Kim, B.-S.; Jeong, S.-B.; Jeong, M.-H.; Ryu, J.-W. Upgrading of manganese from waste silicomanganese slag by a mechanical separation process. Mater. Trans. 2011, 135–923. [Google Scholar] [CrossRef] [Green Version]
- Aleksandrov, A.A.; Dashevskii, V.Y.; Leontev, L.I. Manganese extraction from slag obtained in the production of refined manganese ferroalloys. Steel Transl. 2013, 43, 661–665. [Google Scholar] [CrossRef]
- Naganoor, P.C.; Prasanna, A.S.R.; Shivaprasad, K.H.; Bhat, K.L. Extraction of manganese from Ferro-manganese slag. Process. Fines 2000, 2, 300–306. [Google Scholar]
- Bodor, M.; Balta, S.; Pintilie, S.C.; Lazar, A.L.; Buruiana, D. Studies regarding the distribution and composition of particulate matters in the air of an industrialized city. Int. Multi. Sci. Geoco. 2016, 2, 587–592. [Google Scholar]
- ICPDR, 2019, International Commission for the Protection of the Danube River. Flyer Reprint. Available online: https://www.icpdr.org/main/icpdr/danube-river-protection-convention (accessed on 28 August 2021).
- Murariu, G.; Iticescu, C.; Murariu, A.; Rosu, B.; Munteanu, D.; Buruiana, D.L. Assessment of Water Quality State Dynamics Using Adaptive Filtering Methods and Neural Networks Approaching Case study—Danube River in Galati area. Rev. Chim. 2019, 70, 1914–1919. [Google Scholar] [CrossRef]
- Buruiana, D.L. Economic impact analysis of the possibility to recycle wastes from ship building. Int. Multi. Sci. Geoco. 2012, 4, 685–692. [Google Scholar]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotox. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Peel, J.L.; Richard, H.; Valerie, G.; Armistead, G.R.; Lucas, N. Impact of Nitrogen and Climate Change Interactions on Ambient Air Pollution and Human Health. Biogeochemistry 2013, 114, 121–123. [Google Scholar] [CrossRef] [Green Version]
- Petrescu, T. Report on the Environmental Impact Study. 2009. Available online: https://www.eib.org/attachments/pipeline/20110247_eia_ro.pdf (accessed on 1 September 2021).
- Makedonski, L.; Peycheva, K.; Stancheva, M. Determination of heavy metals in selected black sea fish species. Food Contr. 2017, 72, 313–318. [Google Scholar] [CrossRef]
- Simionov, I.-A.; Cristea, D.S.; Petrea, S.-M.; Mogodan, A.; Nicoara, M.; Plavan, G.; Baltag, E.S.; Jijie, R.; Strungaru, S.-A. Preliminary investigation of lower Danube pollution caused by potentially toxic metals. Chemosphere 2021, 264, 128496. [Google Scholar] [CrossRef] [PubMed]
- Strungaru, S.-A.; Nicoara, M.; Teodosiu, C.; Baltag, E.; Ciobanu, C.; Plavan, G. Patterns of toxic metals bioaccumulation in a cross-border freshwater reservoir. Chemosphere 2018, 207, 192–202. [Google Scholar] [CrossRef]


| Element | 80 µm | 315 µm | ||||
|---|---|---|---|---|---|---|
| Initial | Calcinated | Leached | Initial | Calcinated | Leached | |
| Mn | 67.10 | 74.55 | 44.10 | 67.58 | 68.88 | 53.46 |
| Si | 25.64 | 18.39 | 37.70 | 25.20 | 16.06 | 25.11 |
| Fe | 4.19 | 5.08 | 15.14 | 4.30 | 10.96 | 19.38 |
| Zn | 1.37 | 1.62 | 0.59 | 1.15 | 3.31 | 1.06 |
| Zr | 0.13 | 0.11 | 0.15 | 0.12 | 0.13 | 0.12 |
| Others | 1.57 | 0.25 | 2.85 | 1.65 | 0.66 | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buruiana, D.L.; Obreja, C.-D.; Herbei, E.E.; Ghisman, V. Re-Use of Silico-Manganese Slag. Sustainability 2021, 13, 11771. https://doi.org/10.3390/su132111771
Buruiana DL, Obreja C-D, Herbei EE, Ghisman V. Re-Use of Silico-Manganese Slag. Sustainability. 2021; 13(21):11771. https://doi.org/10.3390/su132111771
Chicago/Turabian StyleBuruiana, Daniela Laura, Cristian-Dragos Obreja, Elena Emanuela Herbei, and Viorica Ghisman. 2021. "Re-Use of Silico-Manganese Slag" Sustainability 13, no. 21: 11771. https://doi.org/10.3390/su132111771
APA StyleBuruiana, D. L., Obreja, C.-D., Herbei, E. E., & Ghisman, V. (2021). Re-Use of Silico-Manganese Slag. Sustainability, 13(21), 11771. https://doi.org/10.3390/su132111771






