Extraction of Value-Added Minerals from Various Agricultural, Industrial and Domestic Wastes
Abstract
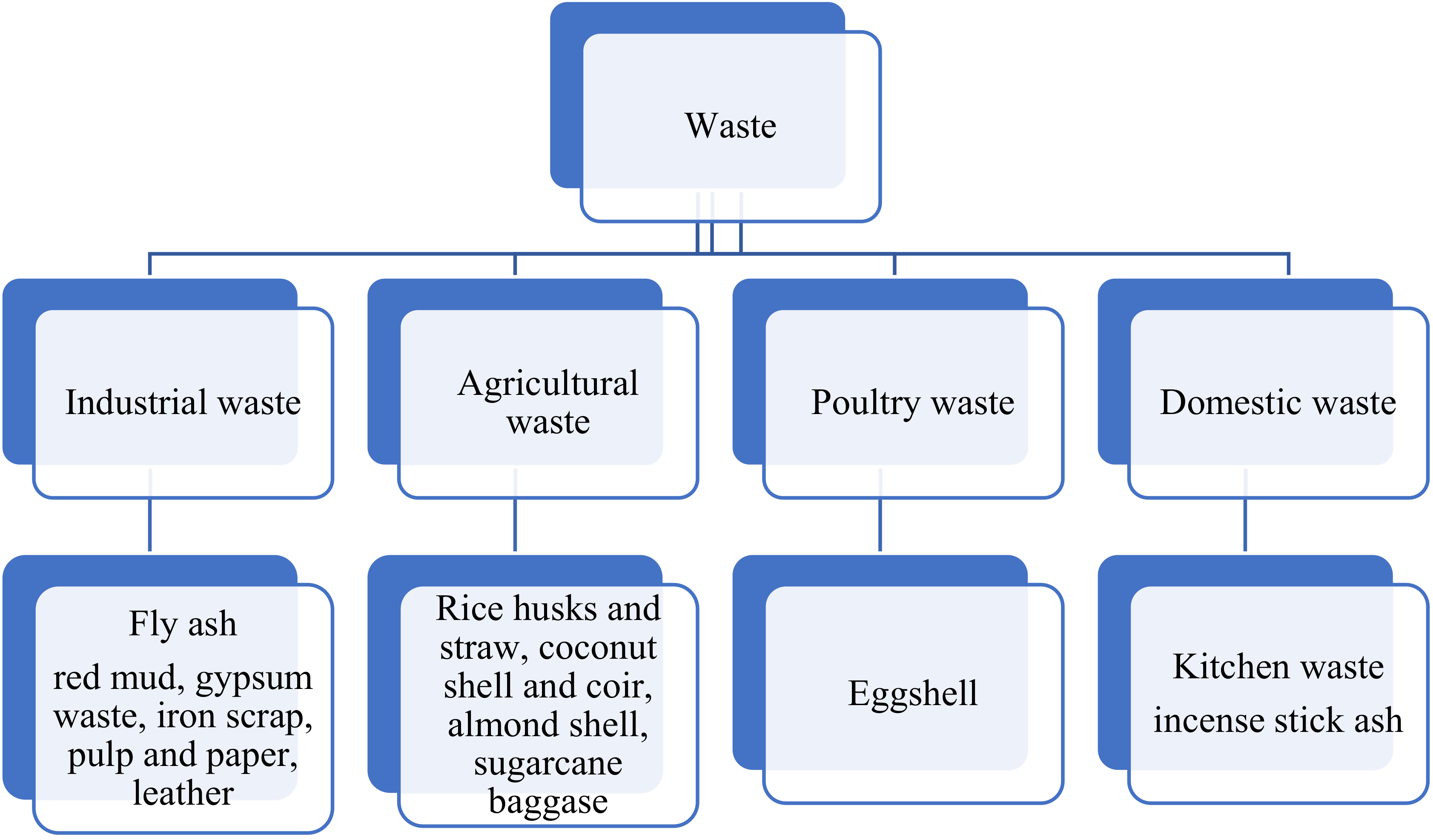
1. Introduction
2. Industrial Waste
2.1. Fly Ash
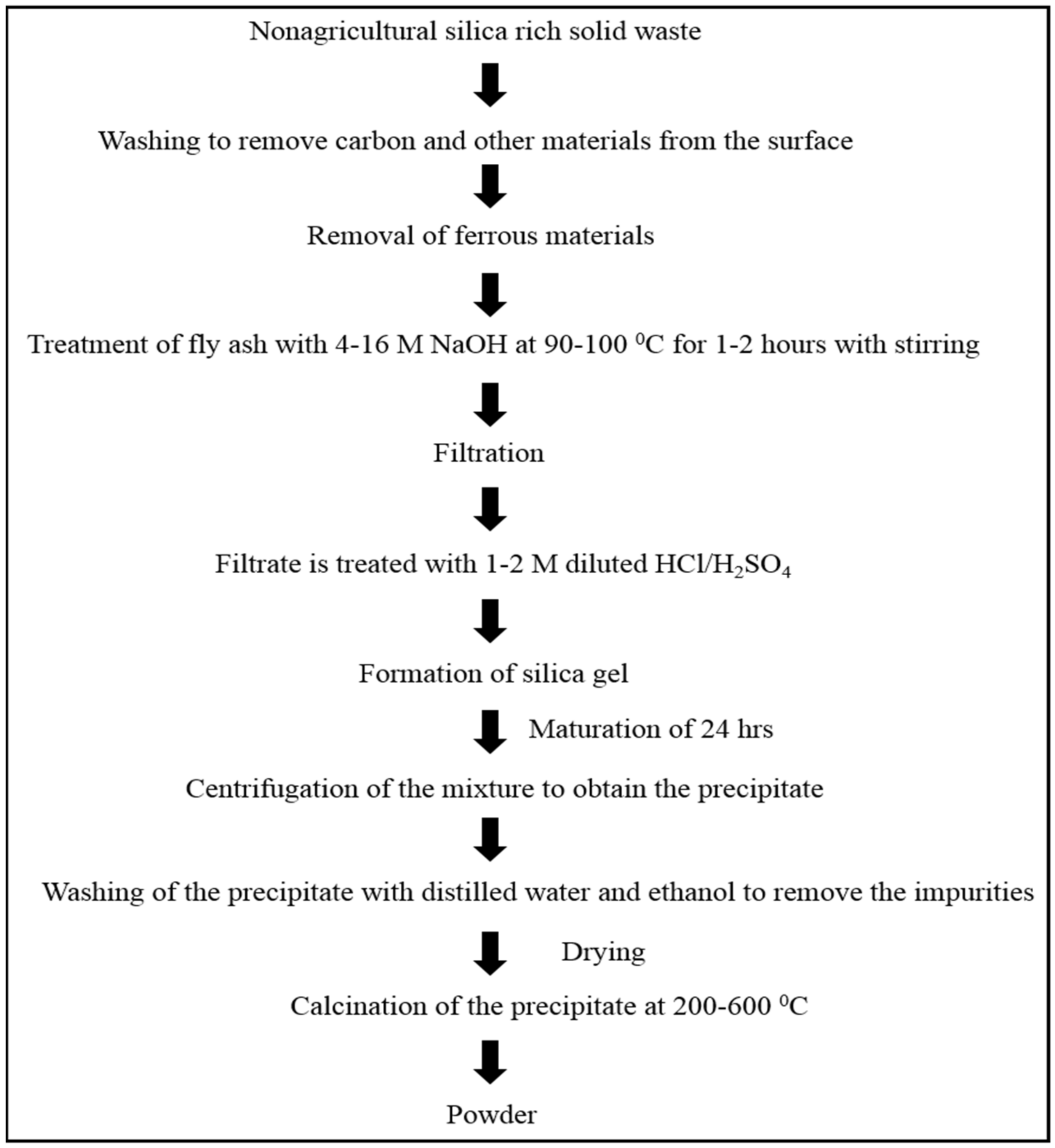
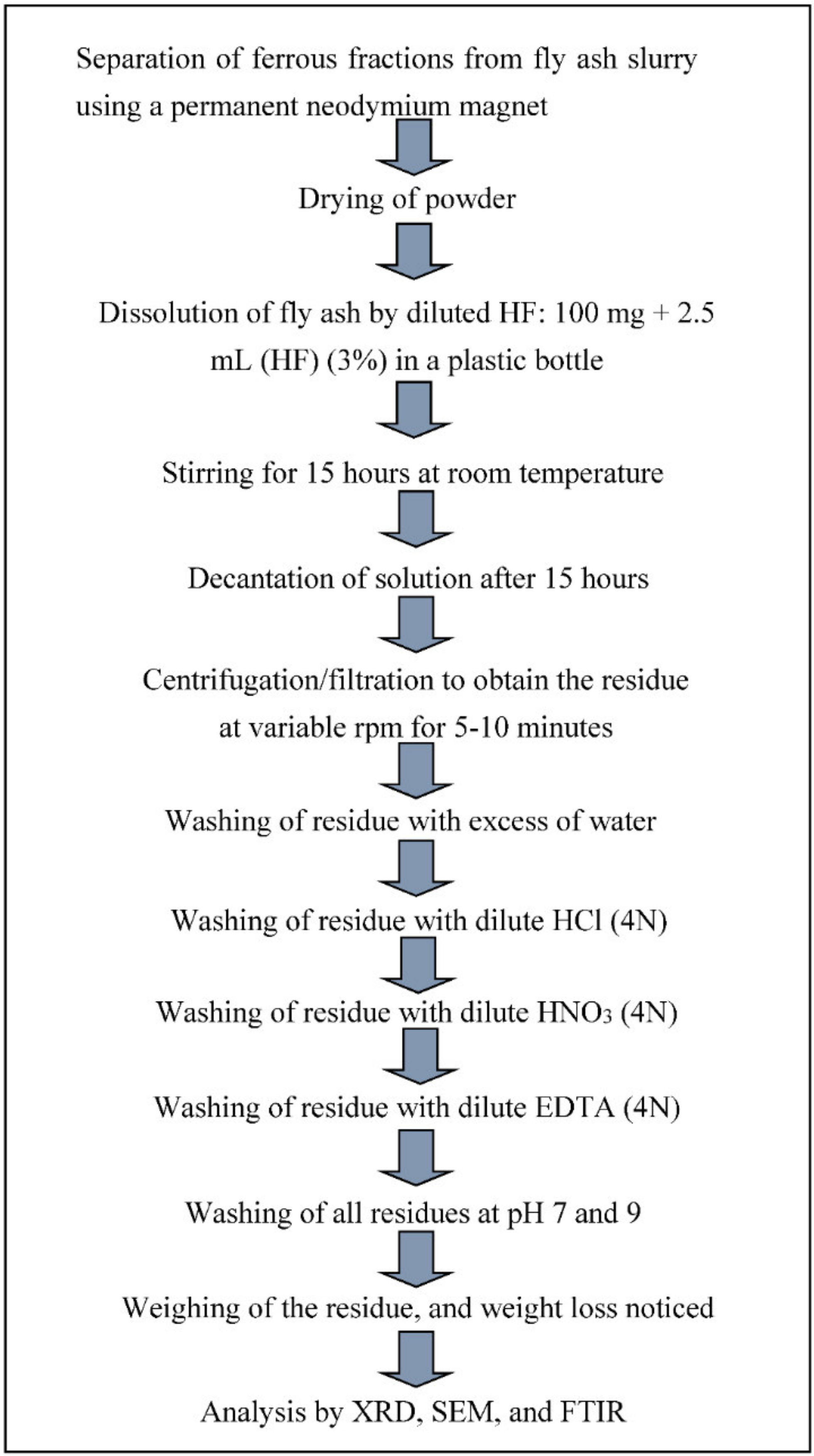
2.1.1. Recovery of Minerals from CFA
Silica
Alumina
Cenospheres
Mullite
Zeolites Synthesis from CFA
2.2. Red Mud
2.2.1. Recovery of Alumina from Red Mud
2.2.2. Applications of Red Mud
2.3. Iron Slags/Scraps
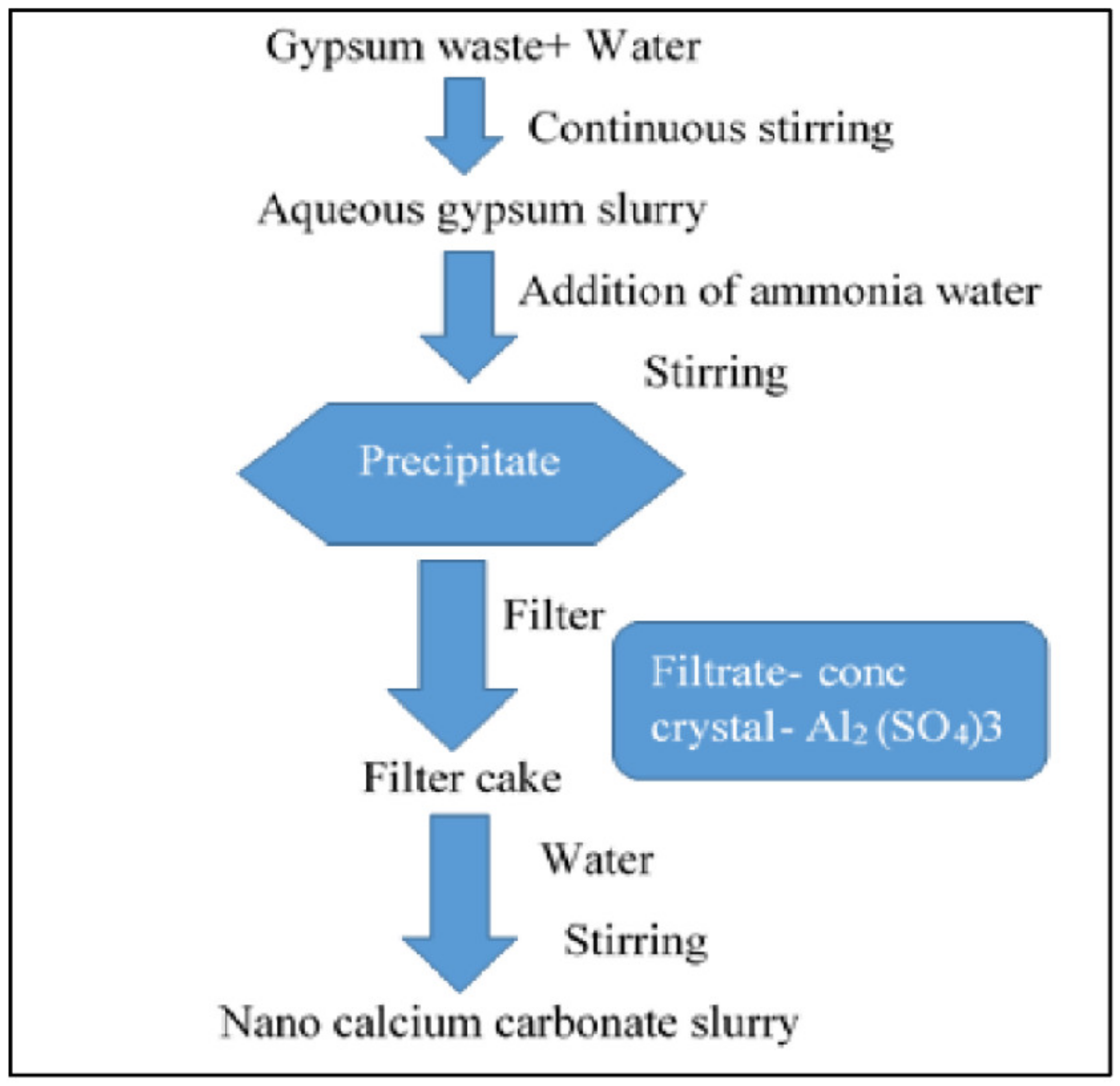
2.4. Gypsum Waste (CaSO4⋅ 2H2O)
2.5. Agricultural Waste
2.5.1. Rice Husks (RH) and the Recovery of Silica
2.5.2. Sugarcane Bagasse and the Recovery of Silica
2.5.3. Coconut Shells
Synthesis of Carbon Nanotubes from Coconut Shell Husk Ash
Corn Cobs as a Source of Activated Carbon
3. Domestic Waste
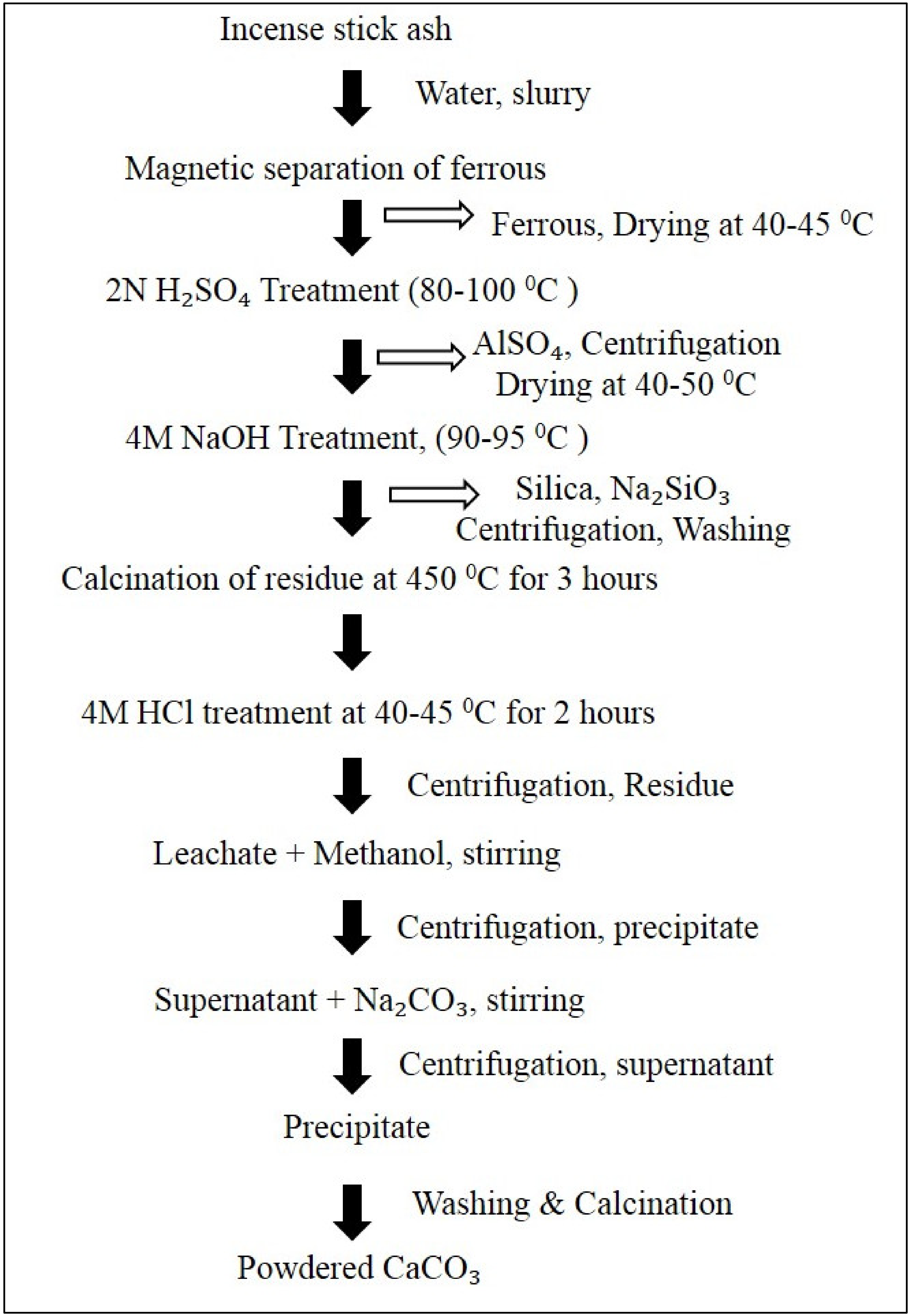
3.1. Incense Stick Ash (ISA)
3.2. Eggshell Waste
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, T.C.; Krishnaswamy, G.; Chi, D.S. Incense smoke: Clinical, structural and molecular effects on airway disease. Clin. Mol. Allergy 2008, 6, 3. [Google Scholar] [CrossRef]
- Khan, T.; Mubeen, U. Wheat Straw: A pragmatic overview. Curr. Res. J. Biol. Sci. 2012, 4, 673–675. [Google Scholar]
- Schnitzer, M.; Monreal, C.; Powell, E. Wheat straw biomass: A resource for high-value chemicals. J. Environ. Sci. Health 2014, 49, 51–67. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, C.; Davies, E.G.; Liu, Y. Agricultural Waste. Water Environ. Res. 2013, 85, 1377–1451. [Google Scholar] [CrossRef]
- Domínguez-Escribá, L.; Porcar, M. Rice straw management: The big waste. Biofuels Bioprod. Biorefining 2010, 4, 154–159. [Google Scholar] [CrossRef]
- Hasnain, M.H.; Javed, U.; Ali, A.; Zafar, M.S. Eco-friendly utilization of rice husk ash and bagasse ash blend as partial sand replacement in self-compacting concrete. Constr. Build. Mater. 2021, 273, 121753. [Google Scholar] [CrossRef]
- Ganiron, T.U., Jr. Sustainable Management of Waste Coconut Shells as Aggregates in Concrete Mixture. J. Eng. Sci. Technol. Rev. 2013, 6, 7–14. [Google Scholar] [CrossRef]
- Ahmad, T.; Danish, M.; Rafatullah, M.; Ghazali, A.; Sulaiman, O.; Hashim, R.; Ibrahim, M.N.M. The use of date palm as a potential adsorbent for wastewater treatment: A review. Environ. Sci. Pollut. Res. 2012, 19, 1464–1484. [Google Scholar] [CrossRef]
- Gómez, B.; Gullón, B.; Yáñez, R.; Parajó, J.C.; Alonso, J.L. Pectic Oligosacharides from Lemon Peel Wastes: Production, Purification, and Chemical Characterization. J. Agric. Food Chem. 2013, 61, 10043–10053. [Google Scholar] [CrossRef]
- Landge, A.; VShahade, I.; Topare, N. Application of Biosorbent Prunus Amygdalus (Almond) Nut Shell Carbon on Treatment of Cr (VI) Contaminated Waste Water. In Proceedings of the 66th Annual Session of Indian Institute of Chemical Engineers, Pune, Indian, 27–30 December 2013; pp. 1–5. [Google Scholar]
- Zhang, Z.; Gonzalez, A.M.; Davies, E.; Liu, Y. Agricultural Wastes. Water Environ. Res. 2012, 84, 1386–1406. [Google Scholar] [CrossRef]
- Mohomane, S.; Linganiso, L.Z.; Linganiso, E.C.; Motaung, T.E.; Songca, S.P. The application of fly ash as industrial waste material in building construction industries. In “Waste-To-Profit”(W-T-P); Nova Science Publishers Inc.: New York, NY, USA, 2018; pp. 181–202. [Google Scholar]
- Nordin, N.; Abdullah, M.M.A.B.; Tahir, M.F.M.; Sandu, A.V.; Hussin, K. Utilization of fly ash waste as construction material. Int. J. Conserv. Sci. 2016, 7, 161–166. [Google Scholar]
- Rai, S.; Wasewar, K.L.; Lataye, D.H.; Mishra, R.S.; Puttewar, S.; Chaddha, M.J.; Mahindiran, P.; Mukhopadhyay, J. Neutralization of red mud with pickling waste liquor using Taguchi’s design of experimental methodology. Waste Manag. Res. 2012, 30, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, D.Y. Physical and Chemical Properties of Sintering Red Mud and Bayer Red Mud and the Implications for Beneficial Utilization. Materials 2012, 5, 1800–1810. [Google Scholar] [CrossRef]
- Geraldo, R.H.; Pinheiro, S.M.; Silva, J.S.; Andrade, H.M.; Dweck, J.; Gonçalves, J.; Camarini, G. Gypsum plaster waste recycling: A potential environmental and industrial solution. J. Clean. Prod. 2017, 164, 288–300. [Google Scholar] [CrossRef]
- Romero-Hermida, I.; Morales-Flórez, V.; Santos, A.; Villena, A.; Esquivias, L. Technological Proposals for Recycling Industrial Wastes for Environmental Applications. Minerals 2014, 4, 746–757. [Google Scholar] [CrossRef]
- Ahmadi, M.; Bayati, N.; Babaei, A.; Teymouri, P. Sludge characterization of an industrial wastewater treatmnt plant, Iran. Iran. J. Health Sci. 2013, 1, 10–18. [Google Scholar]
- Shreekant, R.; Vardhan, A.M.H. Utilization of Iron Ore Waste in Brick Making For the Construction Industry. Int. J. Earth Sci. Eng. 2016, 9, 450–455. [Google Scholar]
- Awuchi, C.G.; Igwe, S.V. Industrial waste management: Brief survey and advice to cottage, small, and medium scale industries in Uganda. Int. J. Adv. Acad. Res. 2017, 3, 26–43. [Google Scholar]
- Fecheyr-Lippens, D.; Nallapaneni, A.; Shawkey, M. Exploring the Use of Unprocessed Waste Chicken Eggshells for UV-Protective Applications. Sustainability 2017, 9, 232. [Google Scholar] [CrossRef]
- Meng, X.; Deng, D. Trash to Treasure: Waste Eggshells Used as Reactor and Template for Synthesis of Co9S8 Nanorod Arrays on Carbon Fibers for Energy Storage. Chem. Mater. 2016, 28, 3897–3904. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Manuspiya, H. A critical review of eggshell waste: An effective source of hydroxyapatite as photocatalyst. J. Met. Mater. Miner. 2018, 28, 124–135. [Google Scholar]
- Adeoye, A.; Man, H.C.; Soom, M.; Thamer, A.M.; Oluwakunmi, A.C. Poultry waste generation, management and the environment: A case of Minna, North Central Nigeria. J. Solid Waste Technol. Manag. 2015, 41, 146–156. [Google Scholar] [CrossRef]
- Bolan, N.S.; Szogi, A.A.; Chuasavathi, T.; Seshadri, B.; Rothrock, M.J.; Panneerselvam, P. Uses and management of poultry litter. World’s Poult. Sci. J. 2010, 66, 673–698. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mohamed-Mansour, M.S. Solid waste issue: Sources, composition, disposal, recycling and valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Latake, T.; Kulkarni, A.; Bhosale, S. Disposal of Solid Waste For Landfilling In Karad City A Review. In International Journal for Research in Applied Science and Engineering Technology; Seventh Sense Research Group: Thanjavur, India, 2016; p. 4. ISSN 2395-0072. [Google Scholar]
- Ali, S.M.; Pervaiz, A.; Afzal, B.; Hamid, N.; Yasmin, A. Open dumping of municipal solid waste and its hazardous impacts on soil and vegetation diversity at waste dumping sites of Islamabad city. J. King Saudi Univ. Sci. 2014, 26, 59–65. [Google Scholar] [CrossRef]
- Sakawi, Z.; Mastura, S.; Jaafar, O.; Mahmud, M. Community perception of odour pollution from landfills. Res. J. Environ. Earth Sci. 2017, 3, 142–145. [Google Scholar]
- De Moraes, C.M.; Stanczyk, N.M.; Betz, H.S.; Pulido, H.; Sim, D.G.; Read, A.F.; Mescher, M.C. Malaria-induced changes in host odors enhance mosquito attraction. Proc. Natl. Acad. Sci. USA 2014, 111, 11079–11084. [Google Scholar] [CrossRef]
- Sijakova Ivanova, T.; Panov, Z.; Blazev, K.; Zajkova, V. Investigation of fly ash heavy metals content and physico chemical properties from thermal power plant, Republic of Macedonia. Int. J. Eng. Sci. Technol. (IJEST) 2011, 3, 8219–8225. [Google Scholar]
- Ozden, B.; Brennan, C.; Landsberger, S. Investigation of bauxite residue (red mud) in terms of its environmental risk. J. Radioanal. Nucl. Chem. 2019, 319, 339–346. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Bui, T.H.; Pham, V.T.H.Q.; Do, M.Q.; Hoang, M.D.; Le, V.Q. Leaching Behavior and Immobilization of Heavy Metals in Geopolymer Synthesized from Red Mud and Fly Ash. In Key Engineering Materials; Trans Tech Publications Ltd.: Zurich, Switzerland, 2018; pp. 518–522. [Google Scholar]
- Vongdala, N.; Tran, H.D.; Xuan, T.D.; Teschke, R.; Khanh, T.D. Heavy Metal Accumulation in Water, Soil, and Plants of Municipal Solid Waste Landfill in Vientiane, Laos. Int. J. Environ. Res. Public Health 2019, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwari, M.; Swamy, C.N. Waste to wealth-Agriculture solid waste management study. Poll. Res. 2010, 29, 513–517. [Google Scholar]
- Jain, A.K.; Gupta, V.K.; Bhatnagar, A.; Bhatnagar, A. Utilisation of Industrial Waste Products as Adsorbents for the Removal of Dyes. J. Hazard. Mater. 2003, 101, 31–42. [Google Scholar] [CrossRef]
- Sulyman, M.; Namiesnik, J.; Gierak, A. Low-cost Adsorbents Derived from Agricultural By-products/Wastes for Enhancing Contaminant Uptakes from Wastewater: A Review. Pol. J. Environ. Stud. 2017, 26, 479–510. [Google Scholar] [CrossRef]
- Krishnappa, V. Properties and Application of Geopolymer Masonry Units. In International Journal of Civil Engineering EFES; Seventh Sense Research Group: Thanjavur, India, 2015; pp. 117–119. ISSN 2348-8352. [Google Scholar]
- Mohajerani, A.; Suter, D.; Jeffrey-Bailey, T.; Song, T.; Arulrajah, A.; Horpibulsuk, S.; Law, D. Recycling waste materials in geopolymer concrete. Clean Technol. Environ. Policy 2019, 21, 493–515. [Google Scholar] [CrossRef]
- Bernardo, E.; Dattoli, A.; Bonomo, E.; Esposito, L.; Rambaldi, E.; Tucci, A. Application of an Industrial Waste Glass in “Glass–Ceramic Stoneware”. Int. J. Appl. Ceram. Technol. 2011, 8, 1153–1162. [Google Scholar] [CrossRef]
- Skenderovic, I.; Kalac, B.; Becirovic, S. Environmental pollution and waste management. Balk. J. Health Sci. 2015, 3, 2–10. [Google Scholar]
- Kowalski, Z.; Krupa-Żuczek, K. A model of the meat waste management. Pol. J. Chem. Technol. 2007, 9, 91–97. [Google Scholar] [CrossRef]
- Mladenov, M.; Pelovski, Y. Utilization of wastes from pulp and paper industry. J. Univ. Chem. Technol. Metall. 2010, 45, 33–38. [Google Scholar]
- Simão, L.; Hotza, D.; Raupp-Pereira, F.; Labrincha, J.A.; Montedo, O. Wastes from pulp and paper mills—A review of generation and recycling alternatives. Cerâmica 2018, 64, 443–453. [Google Scholar] [CrossRef]
- Fela, K.; Wieczorek-Ciurowa, K.; Konopka, M.; Woźny, Z. Present and prospective leather industry waste disposal. Pol. J. Chem. Technol. 2011, 13, 53–55. [Google Scholar] [CrossRef]
- Paramanandham, J.; Ronald Ross, P. Lignin and cellulose content in coir waste on subject to sequential washing. J. Chem. Chem. Res. Vol. 2011, 1, 10–13. [Google Scholar]
- Noguera, P.; Abad, M.; Noguera, V.; Puchades, R.; Maquieira, A. Coconut coir waste, a new and viable ecologically- Friendly peat substitute. In XXV International Horticultural Congress, Part 7: Quality of Horticultural Products 517; International Society for Horticultural Science: Brussels, Belgium, 1998; pp. 279–286. [Google Scholar]
- Baz, W.A.; Nawar, L.S.; Aly, M.M. Production of biofuel from sugarcane baggase wastes using Saccharomyces cerevisiae. J. Exp. Biol. 2017, 5, 6. [Google Scholar]
- Mzimela, Z.; Mochane, M.; Tshwafo, M. Sugarcane bagasse waste management. In “Waste-to-Profit”? (W-t-P): Value Added Products to Generate Wealth for a Sustainable Economy; Nova Science Publishers: Hauppauge, NY, USA, 2018; Volume 1, pp. 293–302. [Google Scholar]
- Xu, G.; Shi, X. Characteristics and applications of fly ash as a sustainable construction material: A state-of-the-art review. Resour. Conserv. Recycl. 2018, 136, 95–109. [Google Scholar] [CrossRef]
- Lieberman, R.N.; Knop, Y.; Izquierdo, M.; Palmerola, N.M.; de la Rosa, J.; Cohen, H.; Querol, X. Potential of hazardous waste encapsulation in concrete with coal fly ash and bivalve shells. J. Clean. Prod. 2018, 185, 870–881. [Google Scholar] [CrossRef]
- Sahu, G.; Bag, A.; Chatterjee, N.; Mukherjee, A. Potential use of flyash in agriculture: A way to improve soil health. J. Pharmacogn. Phytochem. 2017, 6, 873–880. [Google Scholar]
- Sheoran, H.S.; Duhan, B.S.; Kumar, A. Effect of Fly Ash Application on Soil Properties: A Review. J. Agroecol. Nat. Resour. Manag. 2014, 1, 98–103. [Google Scholar]
- Kalra, N.; Jain, M.C.; Joshi, H.C.; Choudhary, R.; Harit, R.C.; Vatsa, B.K.; Sharma, S.K.; Kumar, V. Flyash as a soil conditioner and fertilizer. Bioresour. Technol. 1998, 64, 163–167. [Google Scholar] [CrossRef]
- Yadav, V.K.; Fulekar, M.H. Advances in Methods for Recovery of Ferrous, Alumina, and Silica Nanoparticles from Fly Ash Waste. Ceramics 2020, 3, 34. [Google Scholar] [CrossRef]
- Yadav, V.K.; Pandita, R. Fly Ash Properties and Their Applications as a Soil Ameliorant. In Amelioration Technology for Soil Sustainability; IGI Global: Hershey, PA, USA, 2019; pp. 59–89. [Google Scholar]
- Ahmaruzzaman, M. Role of fly ash in the removal of organic pollutants from wastewater. Energy Fuels 2019, 23, 1494–1511. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, R.S.; Tiwari, N.; Singh, J.K.; Gode, F.; Sharma, Y.C. Removal of malathion from aqueous solutions and waste water using fly ash. J. Water Resour. Prot. 2010, 2, 322. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.S.; Alzboon, K.; Al-Makhadmeh, L.; Hararah, M.; Mahasneh, M. Fly ash based geopolymer for heavy metal removal: A case study on copper removal. J. Environ. Chem. Eng. 2011, 3, 1669–1677. [Google Scholar] [CrossRef]
- Ge, C.J.; Yoon, K.S.; Choi, J.N. Application of Fly Ash as an Adsorbent for Removal of Air and Water Pollutants. Appl. Sci. 2018, 8, 1116. [Google Scholar] [CrossRef]
- Yeole, K.; Kadam, P.; Mhaske, S. Synthesis and characterization of fly ash-zinc oxide nanocomposite. J. Mater. Res. Technol. 2014, 3, 186–190. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Yu, J.; Li, X.; Fleming, D.; Meng, Z.; Wang, D.; Tahmasebi, A. Analysis on Characteristics of Fly Ash from Coal Fired Power Stations. Energy Procedia 2012, 17, 3–9. [Google Scholar] [CrossRef]
- Abbas, S.; Saleem, M.A.; Kazmi, S.M.S.; Munir, M.J. Production of sustainable clay bricks using waste fly ash: Mechanical and durability properties. J. Build. Eng. 2017, 14, 7–14. [Google Scholar] [CrossRef]
- Pawlik, T.; Michalik, D.; Sopicka-Lizer, M.; Godzierz, M. Manufacturing of Light Weight Aggregates from the Local Waste Materials for Application in the Building Concrete. In Materials Science Forum; Trans Tech Publications Ltd: Bäch, Switzerland, 2017; Volume 904, pp. 174–178. [Google Scholar] [CrossRef]
- Bouzoubaâ, N.; Zhang, M.H.; Zakaria, M.; Malhotra, V.M.; Golden, D.M. Blended fly ash cements—A review. ACI Mater. J. 1999, 96, 641–650. [Google Scholar]
- Nataatmadja, A.; Haigh, R.; Amaratunga, D. (Eds.) Development of low-cost fly ash bricks. In Proceedings of the CIB International Conference on Building Education and Research, Heritance Kandalama, Sri Lanka, 11–15 February 2008; pp. 831–843. [Google Scholar]
- Bohara, N. Study of the Influence of Fly Ash and Its Content in Marshall Properties of Asphalt Concrete. J. Sustain. Constr. Mater. Technol. 2018, 3, 261–270. [Google Scholar] [CrossRef]
- Mishra, M.K.; Karanam, U. Geotechnical characterization of fly ash composites for backfilling mine voids. Geotech. Geol. Eng. 2006, 24, 1749–1765. [Google Scholar] [CrossRef]
- Yadav, V.K.; Fulekar, M.H. Isolation and charcterization of iron nanoparticles from coal fly ash from Gandhinagar Gujarat thermal power plant a mechanical-method-of-isolation. Int. J. Eng. Res. Technol. 2014, 3, 471–477. [Google Scholar]
- Kumar, S.; Das, S.K.; Daspoddar, K. Synthesis of mullite aggregates from fly ash: Effect on thermomechanical behaviour of low cement castables. Br. Ceram. Trans. 2004, 103, 176–180. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, J.; Li, K.; Liu, N.; Chen, X.; Gao, Y.; Tian, S. Green Synthesis of Nanosilica from Coal Fly Ash and Its Stabilizing Effect on CaO Sorbents for CO2 Capture. Environ. Sci. Technol. 2017, 51, 7606–7615. [Google Scholar] [CrossRef] [PubMed]
- Ojha, K.; Pradhan, N.C.; Samanta, A.N. Zeolite from fly ash: Synthesis and characterization. Bull. Mater. Sci. 2004, 27, 555–564. [Google Scholar] [CrossRef]
- Kumar, S.; Srivastava, V.; Agarwal, V.C. Utilization of fly ash and lime in PPC concrete. Int. J. Eng. Tech. Res. (IJETR) 2015, 3, 121–124. [Google Scholar]
- Al-Shether, B.; Shamsa, M.; Al-Attar, T. Relationship between amorphous silica in source materials and compressive strength of geopolymer concrete. In MATEC Web of Conferences; EDP Sciences: Bhagdad, Iraq, 2018; Volume 162, p. 02019. [Google Scholar]
- Havlíček, D.; Přibil, R.; Kratochvíl, B. Content of quartz and mullite in some selected power-plant fly ash in Czechoslovakia. Atmos. Environ. 1989, 23, 701–706. [Google Scholar] [CrossRef]
- Ryu, G.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Fuller, A.; Maier, J.; Karampinis, E.; Kalivodova, J.; Grammelis, P.; Kakaras, E.; Scheffknecht, G. Fly Ash Formation and Characteristics from (co-)Combustion of an Herbaceous Biomass and a Greek Lignite (Low-Rank Coal) in a Pulverized Fuel Pilot-Scale Test Facility. Energies 2018, 11, 1581. [Google Scholar] [CrossRef]
- Inada, M.; Eguchi, Y.; Enomoto, N.; Hojo, J. Synthesis of zeolite from coal fly ashes with different silica–alumina composition. Fuel 2015, 84, 299–304. [Google Scholar] [CrossRef]
- Yadav, V.K.; Suriyaprabha, R.; Khan, S.H.; Singh, B.; Gnanamoorthy, G.; Choudhary, N.; Kalasariya, H. A novel and efficient method for the synthesis of amorphous nanosilica from fly ash tiles. Mater. Today Proc. 2020, 26, 701–705. [Google Scholar] [CrossRef]
- Colilla, M.; Baeza, A.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery and Controlled Release Applications. Sol-Gel Handb. 2015, 1309–1344. [Google Scholar] [CrossRef]
- Devi, L.R.; Singh, M.O. Application of silica based heterogeneous catalyst for the synthesis of bioactive heterocycles. In Heterogeneous Catalysis; Penoni, A., Penoni, A., Eds.; CRC Press: Boca Raton, FL, USA, 2014; Chapter 6; pp. 163–190. [Google Scholar]
- Sharma, P.; Han, M.H.; Cho, C.H. Synthesis of Zeolite Nanomolecular Sieves of Different Si/Al Ratios. J. Nanomater. 2015, 16, 9. [Google Scholar] [CrossRef]
- Singh, G.B.; Subramaniam, K. Characterization of Indian fly ashes using different Experimental Techniques. Indian Concr. J. 2018, 92, 10–23. [Google Scholar]
- Zhao, L.; Xiao, H.; Wang, B.; Sun, Q. Characterization of Glasses in One Type of Alumina Rich Fly Ash by Chemical Digestion Methods: Implications for Alumina Extraction. J. Chem. 2016, 2016, 10. [Google Scholar] [CrossRef]
- Ribeiro, M.J.; Abrantes, J.C.; Ferreira, J.M.; Labrincha, J.A. Recycling of Alrich industrial sludge in refractory ceramic pressed bodies. Ceram. Int. 2002, 28, 319–326. [Google Scholar] [CrossRef]
- Paglia, G.; Buckley, C.E.; Rohl, A.L.; Hart, R.D.; Winter, K.; Studer, A.J.; Hunter, B.A.; Hanna, J.V. Boehmite derived γ-alumina system. 1. Structural evolution with temperature, with the identification and structural determination of a new transition phase, γ ‘-alumina. Chem. Mater. 2004, 16, 220–236. [Google Scholar]
- Piconi, C. Alumina Ceramics for Biomedical Applications. In Proceedings of the 3rd International Conference on “High-Tech Aluminas and Unfolding their Business Prospects” (Aluminas-2013), Kolkata, West Bengal, India, 20 March 2013. [Google Scholar]
- Fomenko, E.; Anshits, N.; Solovyov, A.L.; Mikhaylova, O.A.; Anshits, A.G. Composition and Morphology Of Fly Ash Cenospheres Produced from the Combustion of Kuznetsk Coal. Energy Fuels 2013, 27, 5440–5448. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Vasil’Eva, N.G.; Rogovenko, E.S.; Mikhaylova, O.A.; Mazurova, E.V.; Solovyev, L.A.; Anshits, A.G. Composition and structure of the shells of aluminosilicate microspheres in fly ash formed on the combustion of Ekibastuz coal. Solid Fuel Chem. 2016, 50, 238–247. [Google Scholar] [CrossRef]
- Kruger, R.A. The Use of Cenospheres in Refractories. Energeia 1996, 7, 1–5. [Google Scholar]
- Ghosal, S.; Ebert, J.L.; Self, S.A. Chemical composition and size distributions for fly ashes. Fuel Process. Technol. 1995, 44, 81–94. [Google Scholar] [CrossRef]
- Alcala, J.C.; Davila, R.M.; Quintero, R.L. Recovery of cenospheres and magnetite from coal burning power plant fly ash. Trans. Iron Steel Inst. Jpn. 1987, 27, 531–538. [Google Scholar] [CrossRef]
- Yadav, V.K.; Yadav, K.K.; Tirth, V.; Jangid, A.; Gnanamoorthy, G.; Choudhary, N.; Islam, S.; Gupta, N.; Son, C.T.; Jeon, B.-H. Recent Advances in Methods for Recovery of Cenospheres from Fly Ash and Their Emerging Applications in Ceramics, Composites, Polymers and Environmental Cleanup. Crystals 2021, 11, 1067. [Google Scholar] [CrossRef]
- Rodrigo, D.D.; Boch, P. High purity mullite ceramics by reaction sintering. Int. J. High Technol. Ceram. 1985, 1, 3–30. [Google Scholar] [CrossRef]
- Schneider, H.; Fischer, R.X.; Schreuer, J. Mullite: Crystal Structure and Related Properties. J. Am. Ceram. Soc. 2015, 98, 2948–2967. [Google Scholar] [CrossRef]
- Schmucker, M.; Hildmann, B.; Schneider, H. Mechanism of 2/1-to 3/2-mullite transformation at 1650 C. Am. Mineral. 2002, 87, 1190–1193. [Google Scholar] [CrossRef]
- Jiangfeng, C.H.E.N.; Longyi, S.H.A.O.; Jing, L.U. Synthesis of Mullite from High-alumina Fly Ash: A Case from the Jungar Power Plant in Inner Mongolia, Northern China. Acta Geol. Sin. Engl. Ed. 2008, 82, 99–104. [Google Scholar] [CrossRef]
- Yadav, V.K.; Saxena, P.; Lal, C.; Gnanamoorthy, G.; Choudhary, N.; Singh, B.; Tavker, N.; Kalasariya, H.; Kumar, P. Synthesis and Characterization of Mullites From Silicoaluminous Fly Ash Waste. Int. J. Appl. Nanotechnol. Res. (IJANR) 2020, 5, 10–25. [Google Scholar] [CrossRef]
- Virendra, K.Y.; Suriyaprabha, R.; Inwati, G.K.; Gupta, N.; Singh, B.; Lal, C.; Kumar, P.; Godha, M.; Kalasariya, H. A Noble and Economical Method for the Synthesis of Low Cost Zeolites From Coal Fly Ash Waste. Adv. Mater. Process. Technol. 2021, 1–19. [Google Scholar] [CrossRef]
- Wahyudi, A.; Kurniawan, W.; Husaini, A.A.M.; Hinode, H. Potential Application of Red Mud (Bauxite Residue) in Indonesia. In Proceedings of the Seminar-Workshop on Utilization of Waste Materials, Manila, Philippines, 11–15 February 2015; pp. 57–62. [Google Scholar]
- Balomenos, E.; Giannopoulou, I.; Panias, D.; Paspaliaris, I.; Perry, K.; Boufounos, D. Efficient and complete exploitation of the bauxite residue (red mud) produced in Bayer Process. In Proceedings of the European Metallurgical Conference (EMC, 2011), Duesseldorf, Germany, 26–29 June 2011; Volume 3, pp. 745–757. [Google Scholar]
- Evans, K.; Nordheim, E.; Tsesmelis, K. Bauxite Residue Management. In Light Metals; Springer: Cham, Switzerland, 2012; pp. 63–66. [Google Scholar]
- Paramguru, R.K.; Rath, C.; Misra, V.N. Trends in red mud utilization—A review Mineral Processing and Extractive Metall. Miner. Process. Extr. Metall. Rev. 2005, 26, 1–29. [Google Scholar]
- Tan, R.B.; Khoo, H.H. An LCA study of a primary aluminum supply chain. J. Clean. Prod. 2005, 13, 607–618. [Google Scholar] [CrossRef]
- Pogue, A.I.; Lukiw, W.J. The mobilization of aluminum into the biosphere. Front. Neurol. 2014, 5, 262. [Google Scholar] [CrossRef]
- Abhilash, S.S.; Meshram, P.; Pandey, B.D.; Behera, K.; Satapathy, B.K. Redmud: A secondary resource for rare earth elements. In International bauxite, alumina and aluminium symposium. IBAAS Bind. 2014, 3, 148–162. [Google Scholar]
- Moise, G.; Capota, P.; Enache, L.; Neagu, E.; Dragut, D.; Mihaescu, D.; Sarbu, A. Material composition and properties of red mud coming from domestic alumina processing plant. In Proceedings of the 20th International Symposium “Environment and Industry” SIMI 2017, Section Pollution Control and Monitoring, Bucharest, Romania, 28–29 September 2017; pp. 279–289. [Google Scholar]
- Zhang, Y.; Wang, Y.; Meng, X.; Zheng, L.; Gao, J. The Suppression Characteristics of NH4H2PO4/Red Mud Composite Powders on Methane Explosion. Appl. Sci. 2018, 8, 1433. [Google Scholar] [CrossRef]
- Dentoni, V.; Grosso, B.; Massacci, G. Environmental Sustainability of the Alumina Industry in Western Europe. Sustainability 2014, 6, 9477–9493. [Google Scholar] [CrossRef]
- Milacic, R.; Zuliani, T.; Ščančar, J. Environmental impact of toxic elements in red mud studied by fractionation and speciation procedures. Sci. Total. Environ. 2012, 426, 359–365. [Google Scholar] [CrossRef]
- Li, X.B.; Xiao, W.; Liu, W.; Liu, G.H.; Peng, Z.H.; Zhou, Q.S.; Qi, T.G. Recovery of alumina and ferric oxide from Bayer red mud rich in iron by reduction sintering. Trans. Nonferrous Met. Soc. China 2009, 19, 1342–1347. [Google Scholar] [CrossRef]
- Salman, A.D.; Juzsakova, T.; Rédey, Á.; Le, P.C.; Nguyen, X.C.; Domokos, E.; Abdullah, T.A.; Vagvolgyi, V.; Chang, S.W.; Nguyen, D.D. Enhancing the Recovery of Rare Earth Elements from Red Mud. Chem. Eng. Technol. 2021, 44, 1768–1774. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, S.; Ma, S.; Zhang, Y. Recovery of alumina and alkali in Bayer red mud by the formation of andradite-grossular hydrogarnet in hydrothermal process. J. Hazard. Mater. 2011, 189, 827–835. [Google Scholar] [CrossRef]
- Zhu, X.F.; Zhang, T.A.; Wang, Y.X.; Lü, G.Z.; Zhang, W.G. Recovery of alkali and alumina from Bayer red mud by the calcification–carbonation method. Int. J. Miner. Metall. Mater. 2016, 23, 257–268. [Google Scholar] [CrossRef]
- Meher, S.N. Alumina extraction from red mud by CaCO3 and Na2CO3 sinter process. Int. J. Chem. Stud. 2016, 4, 122–127. [Google Scholar]
- Wang, S.; Ang, H.M.; Tadé, M.O. Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 2008, 72, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, A.I.; Kydros, K.A. Use of red mud for toxic metals removal: The case of nickel. J. Chem. Technol. Biotechnol. 1993, 58, 95–101. [Google Scholar] [CrossRef]
- Eamsiri, A.; Jackson, W.R.; Pratt, K.C.; Christov, V.; Marshall, M. Activated red mud as a catalyst for the hydrogenation of coals and of aromatic compounds. Fuel 1992, 71, 449–453. [Google Scholar] [CrossRef]
- Hodge, W.W. Wastes Problems of the Iron and Steel Industries. Ind. Eng. Chem. 1939, 31, 1364–1380. [Google Scholar] [CrossRef]
- Sarkar, S. Solid waste management in steel industry-challanges and opportunity. Int. J. Nucl. Energy Sci. Technol. 2015, 9, 884–887. [Google Scholar]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M.A. Remediation of wastewater using various nanomaterials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef]
- Weidner, E.; Ciesielczyk, F. Removal of Hazardous Oxyanions from the Environment Using Metal-Oxide-Based Materials. Materials 2019, 12, 927. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparattion, physical properties and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef]
- Tang, Z.; Ding, X.; Yan, X.; Dong, Y.; Liu, C. Recovery of Iron, Chromium, and Nickel from Pickling Sludge Using Smelting Reduction. Metals 2018, 8, 936. [Google Scholar] [CrossRef]
- Tang, C.; Li, K.; Ni, W.; Fan, D. Recovering Iron from Iron Ore Tailings and Preparing Concrete Composite Admixtures. Minerals 2019, 9, 232. [Google Scholar] [CrossRef]
- Zhang, G.F.; Yang, Q.R.; Yang, Y.D.; Wu, P.; McLean, A. Recovery of iron from waste slag of pyrite processing using reduction roasting magnetic separation method. Can. Metall. Q. 2013, 52, 153–159. [Google Scholar] [CrossRef]
- Wang, Y.U.; Peng, Y.L.; Zheng, Y.J. Recovery of iron from waste ferrous sulphate by co-precipitation and magnetic separation. Trans. Nonferrous Met. Soc. China 2017, 27, 211–219. [Google Scholar]
- Yadav, V.K.; Yadav, K.K.; Cabral-Pinto, M.M.S.; Choudhary, N.; Gnanamoorthy, G.; Tirth, V.; Prasad, S.; Khan, A.H.; Islam, S.; Khan, N.A. The Processing of Calcium Rich Agricultural and Industrial Waste for Recovery of Calcium Carbonate and Calcium Oxide and Their Application for Environmental Cleanup: A Review. Appl. Sci. 2021, 11, 4212. [Google Scholar] [CrossRef]
- Ko, J.H.; Xu, Q.; Jang, Y.C. Emissions and Control of Hydrogen Sulfide at Landfills: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2043–2083. [Google Scholar] [CrossRef]
- Xu, Q.; Townsend, T.; Bitton, G. Inhibition of hydrogen sulfide generation from disposed gypsum drywall using chemical inhibitors. J. Hazard. Mater. 2011, 191, 204–211. [Google Scholar] [CrossRef]
- De Beer, M.; Doucet, F.J.; Maree, J.; Liebenberg, L. Synthesis of high-purity precipitated calcium carbonate during the process of recovery of elemental sulphur from gypsum waste. Waste Manag. 2015, 46, 619–627. [Google Scholar] [CrossRef]
- Cuesta Mayorga, I.; Astilleros, J.M.; Fernández-Díaz, L. Precipitation of CaCO3 Polymorphs from Aqueous Solutions: The Role of pH and Sulphate Groups. Minerals 2019, 9, 178. [Google Scholar] [CrossRef]
- Sufang, W.U.; Peiqiang, L.A.N. Method for Preparing a Nano-Calcium Carbonate Slurry from Waste Gypsum as Calciumsource, the Product and Use Thereof. United States Patent Application Publication. U.S. Patent 8,846,562, 30 September 2014. [Google Scholar]
- Okumura, S.; Mihara, N.; Kamiya, K.; Ozawa, S.; Onyango, M.S.; Kojima, Y.; Iwashita, T. Recovery of CaO by Reductive Decomposition of Spent Gypsum in a CO−CO2−N2 Atmosphere. Ind. Eng. Chem. Res. 2003, 42, 6046–6052. [Google Scholar] [CrossRef]
- Ramachandran, N.; Maniam, G. Regeneration of calcium oxide (CaO) from waste gypsum via two-step reaction. In Proceedings of the 8th Malaysian Technical Universities Conference on Engineering & Technology (MUCET 2014), Malacca, Malaysia, 10–11 November 2014. [Google Scholar]
- Mbhele, N.R.; Van Der Merwe, W.; Maree, J.; Theron, D. Recovery of sulphur from waste gypsum. In Proceedings of the Abstracts of the International Mine Water Conference, Mpumalanga, South Africa, 19–23 October 2009; pp. 622–630. [Google Scholar]
- Nagrale, S.D.; Hajare, H.; Modak, R. Utilization of rice husk ash. Int. J. Eng. Res. Appl. 2012, 2, 42. [Google Scholar]
- Yadav, V.K.; Choudhary, N.; Tirth, V.; Kalasariya, H.; Gnanamoorthy, G.; Algahtani, A.; Yadav, K.K.; Soni, S.; Islam, S.; Yadav, S.; et al. A Short Review on the Utilization of Incense Sticks Ash as an Emerging and Overlooked Material for the Synthesis of Zeolites. Crystals 2021, 11, 1255. [Google Scholar] [CrossRef]
- Tomar, R.; Kishore, K.; Singh Parihar, H.; Gupta, N. A comprehensive study of waste coconut shell aggregate as raw material in concrete. Mater. Today Proc. 2021, 44, 437–443. [Google Scholar] [CrossRef]
- Channoy, C.; Maneewan, S.; Punlek, C.; Chirarattananon, S. Preparation and Characterization of Silica Gel from Bagasse Ash. In Advanced Materials Research; Trans Tech Publications Ltd.: Zurich, Switzerland, 2018; Volume 1145, pp. 44–48. [Google Scholar] [CrossRef]
- Balasubramaniam, P.; Peera, S.P.G.; Mahendran, P.P.; Tajuddin, A. Release of silicon from soil applied with graded levels of fly ash with silicate solubilizing bacteria and farm yard manure. In Proceedings of the 5th International Conference on Silicon in Agriculture, Beijing, China, 13–18 September 2011. [Google Scholar]
- Yadav, V.K.; Malik, P.; Khan, A.H.; Pandit, R.; Hasan, M.A.; Cabral-Pinto, M.M.S.; Islam, S.; Suriyaprabha, R.; Yadav, K.K.; Dinis, A.; et al. Recent Advances on Properties and Utility of Nanomaterials Generated from Industrial and Biological Activities. Crystals 2021, 11, 634. [Google Scholar] [CrossRef]
- Nayak, P.P.; Datta, A. Synthesis of SiO2-Nanoparticles from Rice Husk Ash and its Comparison with Commercial Amorphous Silica through Material Characterization. Silicon 2021, 13, 1209–1214. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Mochane, M.J.; Motaung, T.E.; Linganiso, L.Z.; Thekisoe, O.M.; Songca, S. Sugarcane Bagasse and Cellulose Polymer Composites. In Sugarcane-Technology and Research; IntechOpen: London, UK, 2018. [Google Scholar]
- Schettino, M.A.S.; Holanda, J.N.F. Characterization of sugarcane bagasse ash waste for its use in ceramic floor tile. Procedia Mater. Sci. 2015, 8, 190–196. [Google Scholar] [CrossRef]
- Drummond, A.R.F.; Drummond, W.I. Pyrolysis of Sugar Cane Bagasse in a Wire-Mesh Reactor. Ind. Eng. Chem. Res. 1996, 35, 1263–1268. [Google Scholar] [CrossRef]
- Harish, R.; Aru, A.; Ponnusami, V. Recovery of silica from various low cost precursors for the synthesis of silica gel. Pharm. Lett. 2015, 7, 208–213. [Google Scholar]
- Norsuraya, S.; Hamzah, F.; Rahmat, N. Sugarcane Bagasse as a Renewable Source of Silica to Synthesize Santa Barbara Amorphous-15 (SBA-15). Procedia Eng. 2016, 148, 839–846. [Google Scholar] [CrossRef]
- Rovani, S.; Santos, J.J.; Corio, P.; Fungaro, D.A. Highly Pure Silica Nanoparticles with High Adsorption Capacity Obtained from Sugarcane Waste Ash. ACS Omega 2018, 3, 2618–2627. [Google Scholar] [CrossRef] [PubMed]
- Manjula, C.; Kukkamgai, S.; Rahman, S.; Rajesh, M.K. Characterization of Kuttiyadi ecotype of coconut (Cocos nucifera L.) using morphological and microsatellite markers. J. Plant. Crops 2014, 42, 301–315. [Google Scholar]
- Omer, A.M. Agricultural residues for future energy option in Sudan: An analysis. Ann. Adv. Chem. 2016, 2, 17–31. [Google Scholar] [CrossRef]
- Anuar, M.F.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Khaidir, R.E.M. Synthesis and structural properties of coconut husk as potential silica source. Results Phys. 2018, 11, 1–4. [Google Scholar] [CrossRef]
- Rodgers, K.; Hursthouse, A.; Cuthbert, S. The Potential of Sequential Extraction in the Characterisation and Management of Wastes from Steel Processing: A Prospective Review. Int. J. Environ. Res. Public Health 2015, 12, 11724–11755. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramanian, S.; Sravanthi, K. Synthesis and characterisation of silica nano particles from coconut shell. Int. J. Pharma Bio Sci. 2015, 6, 530–536. [Google Scholar]
- Melati, A.; Hidayati, E. Synthesis and characterization of carbon nanotube from coconut shells activated carbon. J. Phys. Conf. Ser. 2016, 694, 012073. [Google Scholar] [CrossRef]
- Adewumi, G.A.; Revaprasadu, N.; Eloka-Eboka, A.C.; Inambo, F.L.; Gervas, C. A facile low-cost synthesis of carbon nanosphere from coconut fibre. In Proceedings of the World Congress on Engineering and Computer Science, San Francisco, CA, USA, 25–27 October 2017; pp. 577–582. [Google Scholar]
- Hakim, Y.Z.; Yulizar, Y.; Nurcahyo, A.; Surya, M. Green Synthesis of Carbon Nanotubes from Coconut Shell Waste for the Adsorption of Pb(II) Ions. Acta Chim. Asiana 2018, 1, 6–10. [Google Scholar] [CrossRef]
- Kumar, S.; Bhatt, B. Status and production technology of maize. In Status of Agricultural Development in Eastern India; Bhatt, B.P., Sikka, A., Mukharjee, J., Islam, A., Dey, A., Eds.; ICAR RCER: Patna, India, 2012; pp. 151–167. [Google Scholar]
- Berber-Villamar, N.K.; Netzahuatl-Muñoz, A.R.; Morales-Barrera, L.; Chávez-Camarillo, G.M.; Flores-Ortiz, C.M.; Cristiani-Urbina, E. Corncob as an effective, eco-friendly, and economic biosorbent for removing the azo dye Direct Yellow 27 from aqueous solutions. PLoS ONE 2018, 13, e0196428. [Google Scholar] [CrossRef]
- Sun, Y.; Webley, P.A. Preparation of activated carbons from corncob with large specific surface area by a variety of chemical activators and their application in gas storage. Chem. Eng. J. 2010, 162, 883–892. [Google Scholar] [CrossRef]
- Sabir, M.; Zia-ur-Rehman, M.; Hakeem, K.R.; Saifullah, U. Phytoremediation of Metal-Contaminated Soils Using Organic Amendments: Prospects and Challenges. In Soil Remediation and Plants; Hakeem, K.R., Sabir, M., Öztürk, M., Mermut, A.R., Eds.; Academic Press: London, UK, 2015; pp. 503–523. [Google Scholar]
- Dias, J.M.; Alvim-Ferraz, M.C.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste Materials for Activated Carbon Preparation and Its Use in Aqueous-phase Treatment: A Review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Rashed, M.N. Adsorption Technique for the Removal of Organic Pollutants from Water and Wastewater. Org. Pollut. Monit. Risk Treat. 2013, 7, 167–194. [Google Scholar]
- Tsai, W.T.; Chang, C.Y.; Wang, S.Y.; Chang, C.F.; Chien, S.F.; Sun, H.F. Utilization of agricultural waste corn cob for the preparation of carbon adsorbent. J. Environ. Sci. Health 2011, 36, 677–686. [Google Scholar] [CrossRef]
- Tsai, W.T.; Chang, C.Y.; Wang, S.Y.; Chang, C.F.; Chien, S.F.; Sun, H.F. Preparation of activated carbons from corn cob catalyzed by potassium salts and subsequent gasification with CO2. Bioresour. Technol. 2001, 78, 203–208. [Google Scholar] [CrossRef]
- Kaźmierczak-Raźna, J.; Nowicki, P.; Pietrzak, R. Sorption properties of activated carbons obtained from corn cobs by chemical and physical activation. Adsorption 2013, 19, 273–281. [Google Scholar] [CrossRef]
- Hashemian, S.; Salari, K.; Yazdi, Z.A. Preparation of activated carbon from agricultural wastes (almond shell and orange peel) for adsorption of 2-pic from aqueous solution. J. Ind. Eng. Chem. 2014, 20, 1892–1900. [Google Scholar] [CrossRef]
- Ratan, J.K.; Kaur, M.; Adiraju, B. Synthesis of activated carbon from agricultural waste using a simple method: Characterization, parametric and isotherms study. Mater. Today Proc. 2018, 5, 3334–3345. [Google Scholar] [CrossRef]
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef]
- Al Omari, M.M.H.; Rashid, I.S.; Qinna, N.A.; Jaber, A.M.; Badwan, A.A. Calcium Carbonate. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: London, UK, 2016; Volume 41, pp. 31–132. [Google Scholar]
- Cho, S.H.; Park, J.K.; Lee, S.K.; Joo, S.M.; Kim, I.H.; Ahn, J.-W.; Kim, H. Synthesis of Precipitated Calcium Carbonate Using a Limestone and its Application in Paper Filler and Coating Color. In Materials Science Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2007; pp. 881–884. [Google Scholar]
- Huwald, E. Calcium carbonate-pigment and filler. In Calcium Carbonate: From the Cretaceous Period into the 21st Century; Tegethoff, F.W., Ed.; Birkhäuser Basel: Basel, Switzerland, 2001; pp. 160–170. [Google Scholar]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Thomas, S.; Sharma, H.; Mishra, P.; Talegaonkar, S. Ceramic Nanoparticles: Fabrication Methods and Applications in Drug Delivery. Curr. Pharm. Des. 2015, 21, 6165–6188. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K.; Lotfipour, F. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin. Drug Deliv. 2015, 12, 1649–1660. [Google Scholar] [CrossRef]
- Hua, K.H.; Wang, H.C.; Chung, R.S.; Hsu, J.C. Calcium carbonate nanoparticles can enhance plant nutrition and insect pest tolerance. J. Pestic. Sci. 2015, 40, 208–213. [Google Scholar] [CrossRef]
- Aframehr, W.M.; Molki, B.; Heidarian, P.; Behzad, T.; Sadeghi, M.; Bagheri, R. Effect of calcium carbonate nanoparticles on barrier properties and biodegradability of polylactic acid. Fibers Polym. 2017, 18, 2041–2048. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Kovalchuk, M.V.; Antipina, M.N. CaCO3 vaterite microparticles for biomedical and personal care applications. Mater. Sci. Eng. C 2014, 45, 644–658. [Google Scholar] [CrossRef]
- Grasby, S.E. Naturally precipitating vaterite (μ-CaCO3) spheres: Unusual carbonates formed in an extreme environment. Geochim. Et Cosmochim. Acta 2013, 67, 1659–1666. [Google Scholar] [CrossRef]
- De Leeuw, N.H.; Parker, S.C. Surface Structure and Morphology of Calcium Carbonate Polymorphs Calcite, Aragonite, and Vaterite: An Atomistic Approach. J. Phys. Chem. B 1998, 102, 2914–2922. [Google Scholar] [CrossRef]
- Buasri, A.; Chaiyut, N.; Loryuenyong, V.; Worawanitchaphong, P.; Trongyong, S. Calcium Oxide Derived from Waste Shells of Mussel, Cockle, and Scallop as the Heterogeneous Catalyst for Biodiesel Production. Sci. World J. 2013, 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Dewangan, S.; Chakrabarty, R.; Zielinska, B.; Pervez, S. Emission of volatile organic compounds from religious and ritual activities in India. Environ. Monit. Assess. 2013, 185, 9279–9286. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, P.; Dutta, N.B.; Biswas, S.C.; Dutta, R.C.; Jayaraj, R.S.C. Status of agarbatti industry in India with special reference to Northeast. Int. J. Adv. Res. Biol. Sci. 2018, 5, 173–186. [Google Scholar]
- Settimo, G.; Tirler, W. Incense, sparklers and cigarettes are significant contributors to indoor benzene and particle levels. Ann. Ist. Super. Sanita. 2015, 51, 28–33. [Google Scholar]
- Abdulrahman, I.; Tijani, H.I.; Mohammed, B.A.; Saidu, H.; Yusuf, H.; Jibrin, M.N.; Mohammed, S. From garbage to biomaterials:an overview on eggshell based hydroxyapatite. J. Mater. 2014, 2014, 802467. [Google Scholar]
- Asri, N.P.; Podjojono, B.; Fujiani, R. Utilization of eggshell waste as low-cost solid base catalyst for biodiesel production from used cooking oil. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; Volume 67, p. 012021. [Google Scholar]
- Ummartyotin, S.; Tangnorawich, B. Utilization of eggshell waste as raw material for synthesis of hydroxyapatite. Colloid Polym. Sci. 2015, 293, 2477–2483. [Google Scholar] [CrossRef]
- Hassan, T.; Rangari, V.; Rana, R.; Jeelani, S. Sonochemical effect on size reduction of CaCO3 nanoparticles derived from waste eggshells. Ultrason. Sonochem. 2013, 20, 1308–1315. [Google Scholar] [CrossRef]
- Hassan, T.; Rangari, V.; Jeelani, S. Sonochemical synthesis and characterisation of bio-based hydroxyapatite nanoparticles. Int. J. Nano Biomater. 2014, 5, 103–112. [Google Scholar] [CrossRef]
- Hariharan, M.; Varghese, N.; Cherian, A.B.; Paul, J. Synthesis and characterisation of CaCO3 (Calcite) nano particles from cockle shells using chitosan as precursor. Int. J. Sci. Res. Publ. 2014, 4, 5. [Google Scholar]
- Channappa, B.; Kambalagere, Y.; Mahadevan, K.M.; Narayanappa, M. Synthesis of Calcium Oxide Nanoparticles and Its Mortality Study on Fresh Water Fish Cyprinus Carpio. IOSR J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 55–60. [Google Scholar]
- Mirghiasi, Z.; Bakhtiari, F.; Darezereshki, E.; Esmaeilzadeh, E. Preparation and characterization of CaO nanoparticles from Ca(OH)2 by direct thermal decomposition method. J. Ind. Eng. Chem. 2014, 20, 113–117. [Google Scholar] [CrossRef]
- Roy, A.; Bhattacharya, J. Microwave-assisted synthesis and characterization of CaO nanoparticles. Int. J. Nanosci. 2011, 10, 413–418. [Google Scholar] [CrossRef]
- Darcanova, O.; Beganskienė, A.; Kareiva, A. Sol-gel synthesis of calcium nanomaterial for paper conservation. Chemija 2015, 26, 25–31. [Google Scholar]
- Ali, S.; Butt, A.; Ejaz, S.; Baron, C.J.; Ikram, D.M. CaO nanoparticles as a potential drug delivery agent for biomedical applications. Dig. J. Nanomater. Biostructures 2015, 10, 799. [Google Scholar]
- Sadeghi, M.; Husseini, M.H. A Novel Method for the Synthesis of CaO Nanoparticle for the Decomposition of Sulfurous Pollutant. J. Appl. Chem. Res. 2013, 7, 10. [Google Scholar]
- Liu, T.; Zhu, Y.; Zhang, X.; Zhang, T.; Zhang, T.; Li, X. Synthesis and characterization of calcium hydroxide nanoparticles by hydrogen plasma-metal reaction method. Mater. Lett. 2010, 64, 2575–2577. [Google Scholar] [CrossRef]
- Yorug, A.H.; Ipek, Y. Sonochemical Synthesis of Hydroxyapatite Nanoparticles with Different Precursor Reagents. Acta Phys. Pol.-Ser. A Gen. Phys. 2012, 121, 230. [Google Scholar]
- Mohd Abd Ghafar, S.L.; Hussein, M.Z.; Abu Bakar Zakaria, Z. Synthesis and Characterization of Cockle Shell-Based Calcium Carbonate Aragonite Polymorph Nanoparticles with Surface Functionalization. J. Nanoparticles 2017, 2017, 12. [Google Scholar] [CrossRef]
- Mohadi, R.; Anggraini, K.; Riyanti, F.; Lesbani, A. Preparation Calcium Oxide from Chicken Eggshells. Sriwijaya J. Environ. 2016, 1, 32–35. [Google Scholar] [CrossRef]
- Mulopo, L.; Radebe, V. Recovery of calcium carbonate from waste gypsum and utilization for remediation of acid mine drainage from coal mines. Water Sci. Technol. 2012, 66, 1296–1300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Render, D.; Samuel, T.; King, H.; Vig, M.; Jeelani, S.; Babu, R.J.; Rangari, V. Biomaterial-Derived Calcium Carbonate Nanoparticles for Enteric Drug Delivery. J. Nanomater. 2016, 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Tangboriboon, N.; Kunanuruksapong, R.; Sirivat, A. Preparation and properties of calcium oxide from eggshells via calcination. Mater. Sci.-Pol. 2012, 30, 313–322. [Google Scholar] [CrossRef]
- Jirimali, H.D.; Chaudhari, B.C.; Khanderay, J.C.; Joshi, S.A.; Singh, V.; Patil, A.M.; Gite, V.V. Waste Eggshell-Derived Calcium Oxide and Nanohydroxyapatite Biomaterials for the Preparation of LLDPE Polymer Nanocomposite and Their Thermomechanical Study. Polym. Plast. Technol. Eng. 2018, 57, 804–811. [Google Scholar] [CrossRef]





| Composition | Percentage |
|---|---|
| Fe2O3 | 30–60% |
| Al2O3 | 10–20% |
| SiO2 | 3–50% |
| Na2O | 2–10% |
| CaO | 2–8% |
| TiO2 | 2–5% |
| Elements | Raw Sample | Sample after Acid Treatment |
|---|---|---|
| SiO2 | 53.10 | 88.13 |
| MgO | 20.72 | 3.04 |
| CaO | 3.77 | 0.57 |
| SO3 | 11.20 | 4.69 |
| P2O5 | 7.36 | 1.15 |
| K2O | 1.26 | 0.50 |
| Elements | Composition (%) |
|---|---|
| SiO2 | 8–12 |
| CaO | 27–31.5 |
| K2O | 17–20 |
| Al2O3 | 0.3–0.8 |
| SO3 | 2–3.5 |
| Fe2O3 | 0.3–1.0 |
| P2O5 | 0.05–0.3 |
| Cl | 35–38 |
| Chemical Elements | Concentration (mg/L) |
|---|---|
| Ca | 2296–2304 |
| Mg | 849–852 |
| Na | 33–35 |
| K | 16–19 |
| Fe | 1.01–1.43 |
| Zn | 0.95–1.03 |
| Cu | 0.062–0.064 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, V.K.; Yadav, K.K.; Tirth, V.; Gnanamoorthy, G.; Gupta, N.; Algahtani, A.; Islam, S.; Choudhary, N.; Modi, S.; Jeon, B.-H. Extraction of Value-Added Minerals from Various Agricultural, Industrial and Domestic Wastes. Materials 2021, 14, 6333. https://doi.org/10.3390/ma14216333
Yadav VK, Yadav KK, Tirth V, Gnanamoorthy G, Gupta N, Algahtani A, Islam S, Choudhary N, Modi S, Jeon B-H. Extraction of Value-Added Minerals from Various Agricultural, Industrial and Domestic Wastes. Materials. 2021; 14(21):6333. https://doi.org/10.3390/ma14216333
Chicago/Turabian StyleYadav, Virendra Kumar, Krishna Kumar Yadav, Vineet Tirth, Govindhan Gnanamoorthy, Nitin Gupta, Ali Algahtani, Saiful Islam, Nisha Choudhary, Shreya Modi, and Byong-Hun Jeon. 2021. "Extraction of Value-Added Minerals from Various Agricultural, Industrial and Domestic Wastes" Materials 14, no. 21: 6333. https://doi.org/10.3390/ma14216333
APA StyleYadav, V. K., Yadav, K. K., Tirth, V., Gnanamoorthy, G., Gupta, N., Algahtani, A., Islam, S., Choudhary, N., Modi, S., & Jeon, B.-H. (2021). Extraction of Value-Added Minerals from Various Agricultural, Industrial and Domestic Wastes. Materials, 14(21), 6333. https://doi.org/10.3390/ma14216333









