A Simple and Efficient Two-Dimensional High-Speed Counter-Current Chromatography Linear Gradient and Isocratic Elution Modes for the Preparative Separation of Coumarins from Roots of Toddalia asiatica (Linn.) Lam.
Abstract
1. Introduction
2. Results
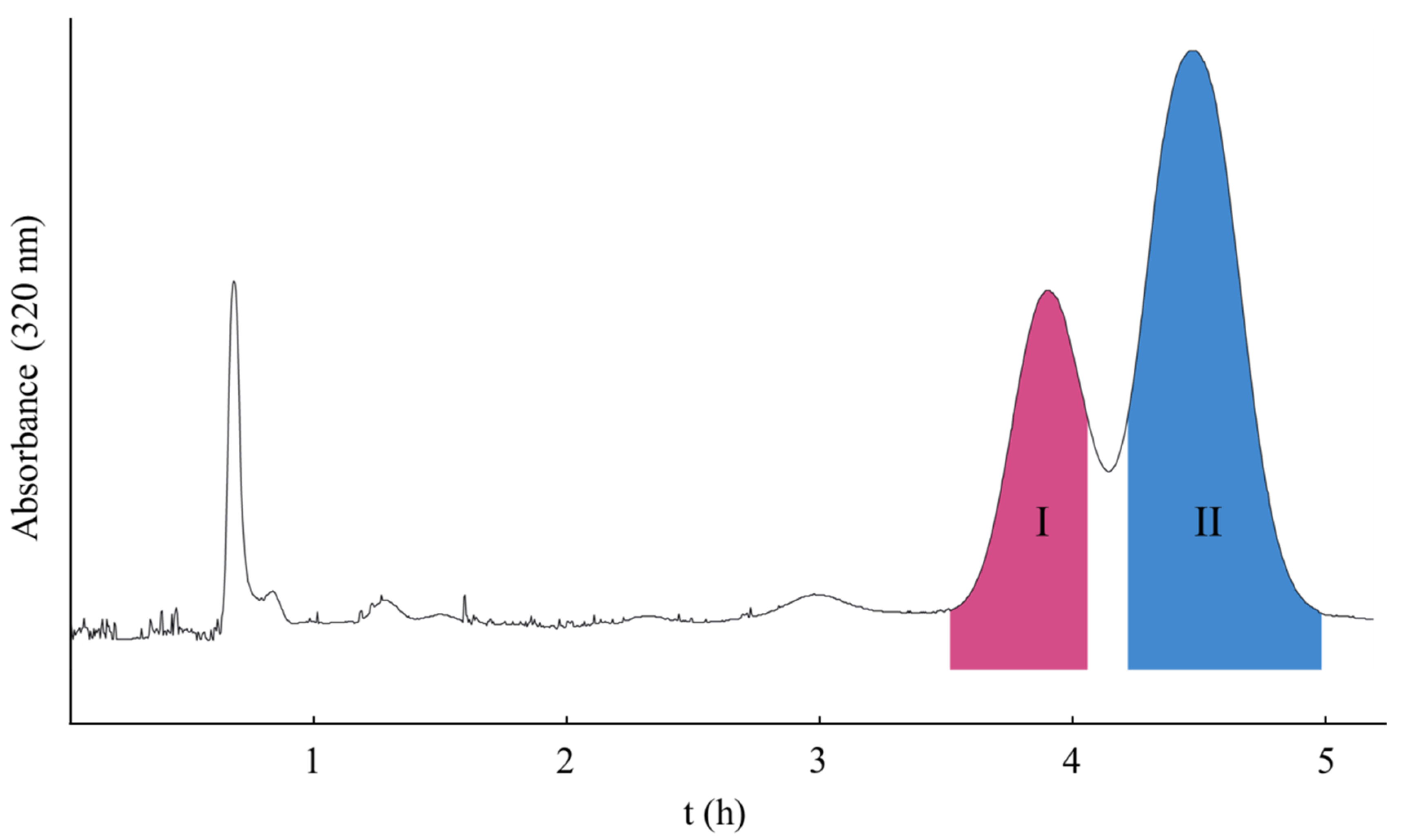
2.1. Selection of the HSCCC Solvent System
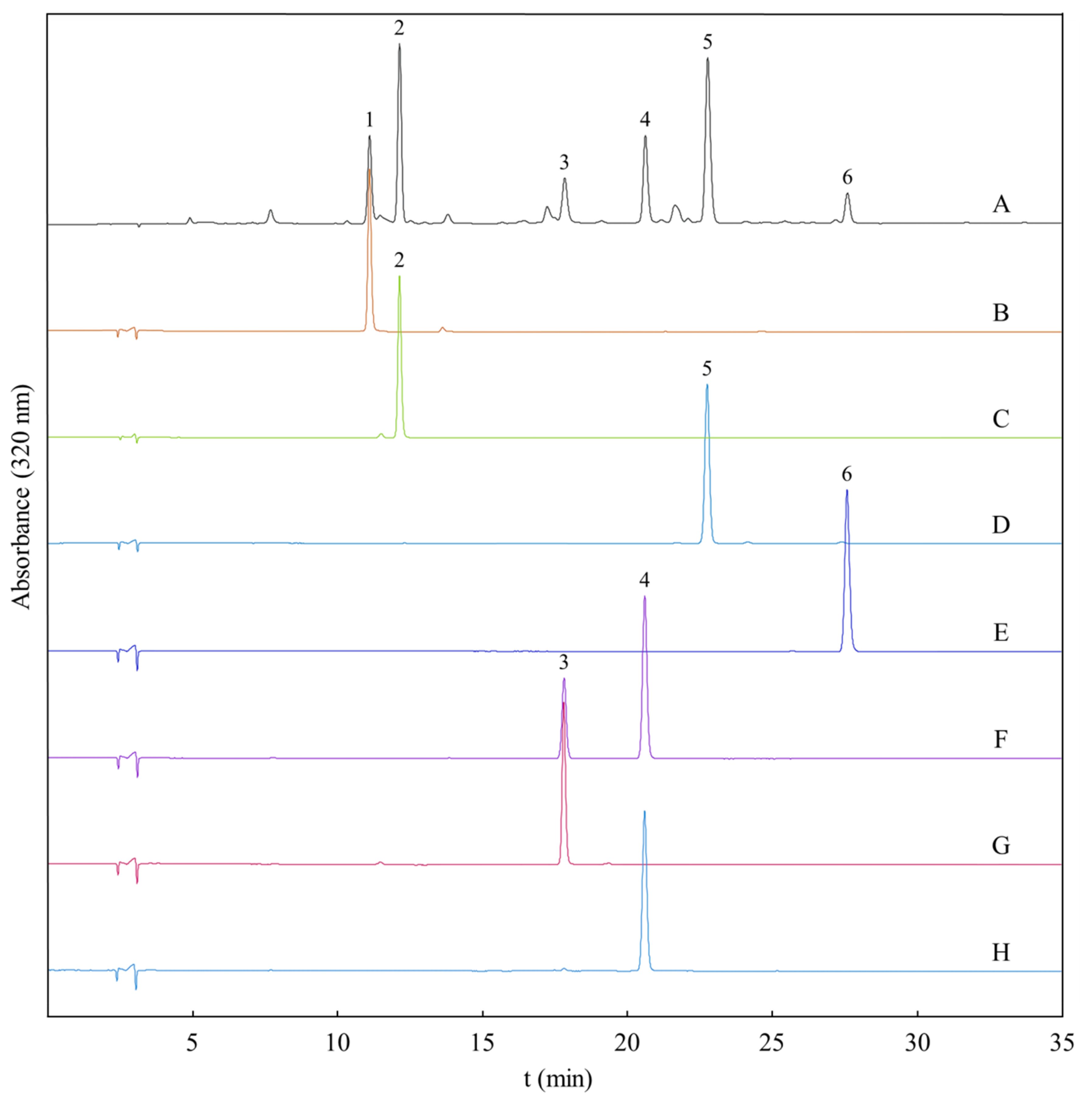
2.2. Optimization of HSCCC Conditions
2.3. Structure Identification
3. Materials and Methods
3.1. Reagents and Materials
3.2. Apparatus
3.3. Preparation of Crude Extract
3.4. Measurement of Partition Coefficient
3.5. Preparation of Solvent System and Sample Solution
3.6. HSCCC Separation Procedure
3.7. HPLC Analysis
3.8. Structural Identification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Schultz, F.; Osuji, O.F.; Wack, B.; Anywar, G.; Garbe, L.A. Antiinflammatory medicinal plants from the Ugandan greater Mpigi region act as potent inhibitors in the COX-2/PGH2 pathway. Plants 2021, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lei, K.; Ji, L. Characterization of the complete chloroplast genome of Toddalia asiatica (L.) Lam. Mitochondrial DNA B Resour. 2021, 6, 1650–1651. [Google Scholar] [CrossRef] [PubMed]
- Lobine, D.; Pairyanen, B.; Zengin, G.; Yilmaz, M.A.; Ouelbani, R.; Bensari, S.; Ak, G.; Abdallah, H.H.; Imran, M.; Mahomoodally, M.F. Chemical composition and pharmacological evaluation and of Toddalia asiatica (Rutaceae) extracts and essential oil by in vitro and in silico approaches. Chem. Biodivers. 2021, 18, e2000999. [Google Scholar] [CrossRef]
- Duraipandiyan, V.; Ignacimuthu, S. Antibacterial and antifungal activity of Flindersine isolated from the traditional medicinal plant, Toddalia asiatica (L.) Lam. J. Ethnopharmacol. 2009, 123, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Gumisiriza, H.; Sesaazi, C.D.; Olet, E.A.; Kembabazi, O.; Birungi, G. Medicinal plants used to treat ’African’ diseases by the local communities of Bwambara sub-county in Rukungiri District, Western Uganda. J. Ethnopharmacol. 2021, 268, 113578. [Google Scholar] [CrossRef]
- Pereira, A.S.P.; den Haan, H.; Pena-Garcia, J.; Moreno, M.M.; Perez-Sanchez, H.; Apostolides, Z. Exploring African medicinal plants for potential anti-diabetic compounds with the DIA-DB inverse virtual screening web server. Molecules 2019, 24, 2002. [Google Scholar] [CrossRef]
- Rashid, M.A.; Gustafson, K.R.; Kashman, Y.; Cardellina, J.H.; McMahon, J.B.; Boyd, M.R. Anti-HIV alkaloids from Toddalia asiatica. Nat. Prod. Lett. 1995, 6, 153–156. [Google Scholar] [CrossRef]
- Zhou, J.W.; Li, Z.Q.; Zhang, J.J.; Wang, H.S.; Yin, S.; Du, J. 8-Acetonyldihydronitidine inhibits the proliferation of human colorectal cancer cells via activation of p53. Eur. J. Pharm. 2019, 854, 256–264. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Wang, P.; Wang, P.; Ma, C.; Kang, W. Antibacterial mechanism of chelerythrine isolated from root of Toddalia asiatica (Linn) Lam. Bmc Complement. Altern. Med. 2018, 18, 261. [Google Scholar] [CrossRef]
- Nyahanga, T.; Jondiko, J.I.; Manguro, L.O.A.; Orwa, J.A. Antiplasmodial and larvicidal compounds of Toddalia asiatica root bark. J. Chem. Sci. 2013, 125, 1115–1121. [Google Scholar] [CrossRef]
- Kavimani, S.; Vetrichel Van, T.; Ilango, R.; Jaykar, B. Antiinflammatory activity of the volatile oil of Toddalia asiatica. Indian J. Pharm. Sci. 1996, 58, 67–70. [Google Scholar]
- Tang, Z.H.; Liu, Y.B.; Ma, S.G.; Li, L.; Li, Y.; Jiang, J.D.; Qu, J.; Yu, S.S. Antiviral spirotriscoumarins A and B: Two pairs of oligomeric coumarin enantiomers with a spirodienone-sesquiterpene skeleton from Toddalia asiatica. Org. Lett. 2016, 18, 5146–5149. [Google Scholar] [CrossRef]
- Zhou, W.; Luo, C.; Liu, G.; Dong, W.; Zhang, X.; Shen, X. GC-MS analysis of chemical constituents of root bark oil of Toddalia asiatica. Guizhou Yike Daxue Xuebao 2019, 44, 147–152. [Google Scholar]
- Liang, J.-l.; Lin, Y.; Liu, L.-y.; Zhou, W.; He, M.-q.; Hao, X.-y. Rapid determination of four categories of pesticide residues in Toddalia asiatica (L.) Lam. by Carb /NH2-SPE-GC-MS. Zhongguo Yaoxue Zazhi (BeijingChina) 2015, 50, 435–441. [Google Scholar]
- Liu, Z.-G.; Li, Y.; Zhu, F.-F.; He, Y.; Yuan, M.-M.; Xu, M.-J. Study on chemical composition of the essential oil from Toddalia asiatica Lam. by gas chromatography/mass spectrum. Liaoning Zhongyiyao Daxue Xuebao 2011, 13, 150–151. [Google Scholar]
- Liu, Z.; Liu, X.; Qin, J. HPLC fingerprint of Toddalia asiatica Lam. Zhongyaocai 2010, 33, 1240–1243. [Google Scholar]
- Zhu, M.; Wei, P.; Peng, Q.; Zhou, Y.; Zhang, L.; Qin, S.; Zhang, R.; Zhu, C. Simultaneous qualitative and quantitative evaluation of Toddalia asiatica root by using HPLC-DAD and UPLC-QTOF-MS/MS. Phytochem. Anal. 2019, 30, 164–181. [Google Scholar] [CrossRef]
- Liu, Z.-G.; Liu, X.-Y. Determination of pimpinelli and of isopimpinell in root of Toddalia asiatica by HPLC. Liaoning Zhongyiyao Daxue Xuebao 2009, 11, 224–225. [Google Scholar]
- Liu, Z.G.; Jiang, M.Y.; Lu, X.M.; Qin, F.; Song, Y.; Wen, J.; Li, F.M. Simultaneous determination of pimpinellin, isopimpinellin and phellopterin in rat plasma by a validated UPLC-MS/MS and its application to a pharmacokinetic study after administration of Toddalia asiatica extract. J. Chromatogr. B 2012, 891, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Praveena, C.; Veeresham, C. Quantitative determination of nitidine from roots and plant tissue culture extracts of Toddalia asiatica (Linn.) using HPTLC. Am. J. Anal. Chem. 2014, 5, 65–69. [Google Scholar] [CrossRef]
- Qiu, H.Y.; Xiao, X.H.; Li, G.K. Separation and purification of furanocoumarins from Toddalia asiatica (L.) Lam. using microwave-assisted extraction coupled with high-speed counter-current chromatography. J. Sep. Sci. 2012, 35, 901–906. [Google Scholar] [CrossRef]
- Cao, C.; Du, P.; Zhu, X.; Yan, H.; Song, X.; Zhu, H.; Geng, Y.; Wang, D. Rapid screening and purification of potential alkaloid neuraminidase inhibitors from Toddalia asiatica (Linn.) Lam. roots via ultrafiltration liquid chromatography combined with stepwise flow rate counter-current chromatography. J. Sep. Sci. 2019, 42, 2621–2627. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, L.; Yu, J.Q.; Cui, L.; Ali, I.; Song, X.Y.; Park, J.H.; Wang, D.J.; Wang, X. Flavonoid epimers from custard apple leaves, a rapid screening and separation by HSCCC and their antioxidant and hypoglycaemic activities evaluation. Sci. Rep. 2020, 10, 8819. [Google Scholar] [CrossRef]
- Ali, I.; Mu, Y.; Atif, M.; Hussain, H.; Li, J.; Li, D.; Shabbir, M.; Bankeu, J.J.K.; Cui, L.; Sajjad, S.; et al. Separation and anti-inflammatory evaluation of phytochemical constituents from Pleurospermum candollei (Apiaceae) by high-speed countercurrent chromatography with continuous sample load. J. Sep. Sci. 2021, 44, 2663–2673. [Google Scholar] [CrossRef]
- Ali, I.; Li, J.; Cui, L.; Zhao, H.; He, Q.; Wang, D. Efficient extraction and purification of benzo[c]phenanthridine alkaloids from Macleaya cordata (Willd) R. Br. by combination of ultrahigh pressure extraction and pH-zone-refining counter-current chromatography with anti-breast cancer activity in vitro. Phytochem. Anal. 2021, 32, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Huang, S.H.; Wang, Y.; Shao, M.; Ye, W.C. Studies on the chemical constituents from Lobelia chinensis. J. Chin. Med. Mater. 2010, 33, 1721–1724. [Google Scholar]
- Liu, Z.; Wang, X.; Mao, B.; Xie, X. Study on chemical constituents of Toddalia asiatica. J. Chin. Med. Mater. 2014, 37, 1600–1603. [Google Scholar]
- Sun, W.B.; Yang, Z.; Liang, Y.; Li, L.I.; Hao, X.Y.; Zuo, G.Y.; Zhou, W.; Pharmacy, S.O.; University, G.M. Chemical constituents from n-butanol part in Toddalia asiatica root bark. Chin. Pharm. J. 2018, 53, 1052–1056. [Google Scholar]
- Garcia, G.R.M.; Hennig, L.; Rodríguez, E.F.R.; Bussmann, R.W. Coumarins of Loricaria ferruginea. Rev. Bras. Farm. 2016, 26, 471–473. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Simayi, R.; Long, S. Studies on chemical constituents of Toddalia asiatica stems. Northwest. Pharm. J. 2013, 28, 337–339. [Google Scholar]
- Yoo, S.W.; Kim, J.S.; Kang, S.S.; Son, K.H.; Chang, H.W.; Kim, H.P.; Bae, K.; Lee, C.-O. Constituents of the fruits and leaves of Euodia daniellii. Arch. Pharmacal Res. 2002, 25, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-L.; Yang, X.-W.; Wu, B.-F.; Shang, J.-H.; Liu, Y.-P.; Zhi, D.; Luo, X.-D. Anti-inflammatory effect of pomelo peel and its bioactive coumarins. J. Agric. Food Chem. 2019, 67, 8810–8818. [Google Scholar] [CrossRef] [PubMed]



| Compound No. | KD Values (n-Hex/EtOAc/MeOH/H2O, v/v) | ||||||
|---|---|---|---|---|---|---|---|
| 5:5:1:9 | 5:5:2:8 | 5:5:3:7 | 5:5:4:6 | 5:5:4.5:5.5 | 5:5:5:5 | 5:5:6:4 | |
| 1 | 1.74 | 0.90 | 0.66 | 0.33 | 0.24 | <0.20 | <0.20 |
| 2 | 0.94 | 0.53 | 0.40 | 0.21 | <0.20 | <0.20 | <0.20 |
| 3 | >5.00 | 4.27 | 2.94 | 1.17 | 0.94 | 0.54 | 0.25 |
| 4 | >5.00 | >5.00 | 3.23 | 1.28 | 1.05 | 0.62 | 0.30 |
| 5 | >5.00 | >5.00 | >5.00 | 2.89 | 2.35 | 1.25 | 0.52 |
| 6 | >5.00 | >5.00 | >5.00 | 3.20 | 2.68 | 1.49 | 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Ali, I.; Li, Y.; Hussain, H.; Zhao, H.; Sun, X.; Xie, L.; Cui, L.; Wang, D. A Simple and Efficient Two-Dimensional High-Speed Counter-Current Chromatography Linear Gradient and Isocratic Elution Modes for the Preparative Separation of Coumarins from Roots of Toddalia asiatica (Linn.) Lam. Molecules 2021, 26, 5986. https://doi.org/10.3390/molecules26195986
Ma W, Ali I, Li Y, Hussain H, Zhao H, Sun X, Xie L, Cui L, Wang D. A Simple and Efficient Two-Dimensional High-Speed Counter-Current Chromatography Linear Gradient and Isocratic Elution Modes for the Preparative Separation of Coumarins from Roots of Toddalia asiatica (Linn.) Lam. Molecules. 2021; 26(19):5986. https://doi.org/10.3390/molecules26195986
Chicago/Turabian StyleMa, Wenya, Iftikhar Ali, Yali Li, Hidayat Hussain, Huanzhu Zhao, Xuan Sun, Lei Xie, Li Cui, and Daijie Wang. 2021. "A Simple and Efficient Two-Dimensional High-Speed Counter-Current Chromatography Linear Gradient and Isocratic Elution Modes for the Preparative Separation of Coumarins from Roots of Toddalia asiatica (Linn.) Lam." Molecules 26, no. 19: 5986. https://doi.org/10.3390/molecules26195986
APA StyleMa, W., Ali, I., Li, Y., Hussain, H., Zhao, H., Sun, X., Xie, L., Cui, L., & Wang, D. (2021). A Simple and Efficient Two-Dimensional High-Speed Counter-Current Chromatography Linear Gradient and Isocratic Elution Modes for the Preparative Separation of Coumarins from Roots of Toddalia asiatica (Linn.) Lam. Molecules, 26(19), 5986. https://doi.org/10.3390/molecules26195986







