Experimental Animal Studies Support the Role of Dietary Advanced Glycation End Products in Health and Disease
Abstract
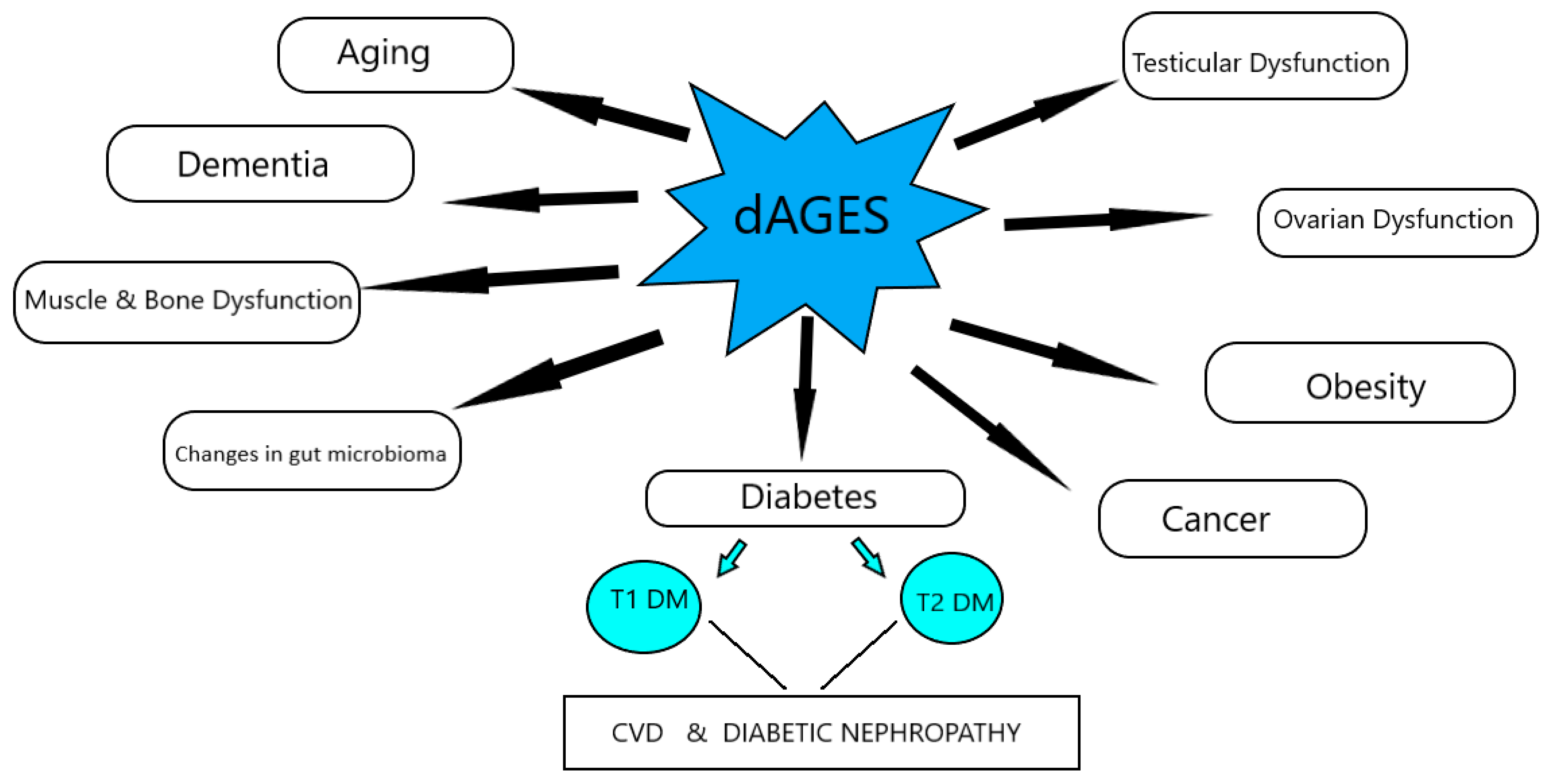
:1. Introduction
2. Dietary AGEs’ Homeostasis—Animal Studies
3. Dietary AGEs’ Effects on Health
4. Dietary AGEs’ Effects in Diabetes Mellitus
4.1. Type 1 Diabetes Mellitus (T1D)
4.2. Type 2 Diabetes Mellitus (T2D)
4.3. Diabetic Complications
4.3.1. Cardiovascular Disease
4.3.2. Diabetic Nephropathy
5. Dietary AGEs’ Effects on Obesity
6. Dietary AGEs Effects on Aging
7. Dietary AGEs’ Effects on Dementia
8. Dietary AGEs’ Effects on Muscle and Bone
9. Dietary AGEs’ Effects on the Gut
10. Dietary AGEs’ Effects on Reproductive Function
11. Dietary AGEs Effects on Cancer
12. Limitations of Animal Studies in dAGEs
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peppa, M.; Raptis, S.A. Advanced glycation end products and cardiovascular disease. Curr. Diabetes Rev. 2008, 4, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M.; Uribarri, J.; Vlassara, H. Aging and glycoxidant stress. Hormones 2008, 7, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Peppa, M.; Vlassara, H. Advanced glycation end products and diabetic complications: A General overview. Hormones 2005, 4, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Striker, G.E. AGE restriction in diabetes mellitus: A paradigm shift. Nat. Rev. Endocrinol. 2011, 7, 526–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, J.; Morrissey, P.A.; Ames, J.M. Nutritional and toxicological aspects of the Maillard browning reaction in foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 211–248. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Coordinate Contribution of Lipid Oxidation and Maillard Reaction to the Nonenzymatic Food Browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef]
- Tessier, F.J.; Niquet, C. The metabolic, nutritional and toxicological consequences of ingested dietary Maillard reaction products: A literature review. J. Soc. Biol. 2007, 201, 199–207. [Google Scholar] [CrossRef]
- van Boekel, M.A.J.S. Formation of flavour compounds in the Maillard reaction. Biotechnol. Adv. 2006, 24, 230–233. [Google Scholar] [CrossRef]
- He, C.; Sabol, J.; Mitsuhashi, T.; Vlassara, H. Dietary glycotoxins: Inhibition of reactive products by aminoguanidine facilitates renal clearance and reduces tissue sequestration. Diabetes 1999, 48, 1308–1315. [Google Scholar] [CrossRef]
- Koschinsky, T.; He, C.J.; Mitsuhashi, T.; Bucala, R.; Liu, C.; Buenting, C.; Heitmann, K.; Vlassara, H. Orally absorbed reactive glycation products (glyco-toxins): An environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1997, 94, 6474–6479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uribarri, J.; Cai, W.; Sandu, O.; Peppa, M.; Goldberg, T.; Vlassara, H. Diet-derived advanced glycation end products are major con-tributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann. N. Y. Acad. Sci. 2005, 1043, 461–466. [Google Scholar] [CrossRef]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.U.; Pyzik, R.; Striker, G.E. AGE’s in Foods and practical ways to reduce them. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Thornton, K.; Merhi, Z.; Jindal, S.; Goldsammler, M.; Charron, M.J.; Buyuk, E. Dietary Advanced Glycation End Products (AGEs) could alter ovarian function in mice. Mol. Cell. Endocrinol. 2020, 510, 110826. [Google Scholar] [CrossRef]
- Sowndhar Rajan, B.; Manivasagam, S.; Dhanusu, S.; Chandrasekar, N.; Krishna, K.; Kalaiarasu, L.P.; Babu, A.A.; Vellaichamy, E. Diet with high content of advanced glycation end products induces systemic inflammation and weight gain in experimental mice: Protective role of curcumin and gallic acid. Food Chem. Toxicol. 2018, 114, 237–245. [Google Scholar] [CrossRef]
- Deng, G.; Zhang, L.; Wang, C.; Wang, S.; Xu, J.; Dong, J.; Kang, Q.; Zhai, X.; Zhao, Y.; Shan, Z. AGEs-RAGE axis causes endothelial-to-mesenchymal transition in early calcific aortic valve disease via TGF-β1 and BMPR2 signaling. Exp. Gerontol. 2020, 141, 111088. [Google Scholar] [CrossRef]
- Mastrocola, R.; Bello, F.D.; Cento, A.S.; Gaens, K.; Collotta, D.; Aragno, M.; Medana, C.; Collino, M.; Wouters, K.; Schalkwijk, C.G. Altered hepatic sphingolipid metabolism in insulin resistant mice: Role of advanced glycation endproducts. Free Radic. Biol. Med. 2021, 169, 425–435. [Google Scholar] [CrossRef]
- Verboven, M.; Deluyker, D.; Ferferieva, V.; Lambrichts, I.; Hansen, D.; Eijnde, B.O.; Bito, V. Western diet given to healthy rats mimics the human phenotype of diabetic cardiomyopathy. J. Nutr. Biochem. 2018, 61, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Lin, J.-A.; Lin, H.-T.; Chen, S.-Y.; Yen, G.-C. Potential effect of advanced glycation end products (AGEs) on spermatogenesis and sperm quality in rodents. Food Funct. 2019, 10, 3324–3333. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Ren, Y.; Zhang, Q.; Dong, S.; Han, K.; Feng, G.; Wu, H.; Zhao, Y. Glycated fish protein supplementation modulated gut microbiota composition and reduced inflammation but increased accumulation of advanced glycation end products in high-fat diet fed rats. Food Funct. 2019, 10, 3439–3451. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, M.T.; Yap, F.Y.; Tong, D.C.; Andrikopoulos, S.; Gasser, A.; Thallas-Bonke, V.; Webster, D.E.; Miyazaki, J.I.; Kay, T.W.; Slattery, R.M.; et al. Advanced Glycation End Products Are Direct Modulators of β-Cell Function. Diabetes 2011, 60, 2523–2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Sun, H.; Sun, Z. Advanced glycation end products (AGEs) increase renal lipid accumulation: A pathogenic factor of diabetic nephropathy (DN). Lipids Health Dis. 2017, 16, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borg, D.J.; Yap, F.Y.T.; Keshvari, S.; Simmons, D.; Gallo, L.A.; Fotheringham, A.; Zhuang, A.; Slattery, R.M.; Hasnain, S.; Coughlan, M.; et al. Perinatal exposure to high dietary advanced glycation end products in transgenic NOD8.3 mice leads to pancreatic beta cell dysfunction. Islets 2017, 10, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Yu, S.C.; Lo, Y.C.; Lin, I.H.; Tung, T.H.; Huang, S.Y. A high linoleic acid diet exacerbates metabolic responses and gut mi-crobiota dysbiosis in obese rats with diabetes mellitus. Food Funct. 2019, 10, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Żebrowska, E.; Zalewska, A.; Chabowski, A. Redox Balance, Antioxidant Defense, and Oxidative Damage in the Hypothalamus and Cerebral Cortex of Rats with High Fat Diet-Induced Insulin Resistance. Oxidative Med. Cell. Longev. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Qu, W.; Yuan, X.; Zhao, J.; Zhang, Y.; Hu, J.; Wang, J.; Li, J. Dietary advanced glycation end products modify gut microbial composition and partially increase colon permeability in rats. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Illien-Jünger, S.; Palacio-Mancheno, P.; Kindschuh, W.F.; Chen, X.; Sroga, G.E.; Vashishth, D.; Iatridis, J. Dietary Advanced Glycation End Products Have Sex- and Age-Dependent Effects on Vertebral Bone Microstructure and Mechanical Function in Mice. J. Bone Miner. Res. 2017, 33, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Peppa, M.; He, C.; Hattori, M.; McEvoy, R.; Zheng, F.; Vlassara, H. Fetal or Neonatal Low-Glycotoxin Environment Prevents Au-toimmune Diabetes in NOD Mice. Diabetes 2003, 52, 1441–1448. [Google Scholar] [CrossRef] [Green Version]
- Peppa, M.; Brem, H.; Ehrlich, P.; Zhang, J.G.; Cai, W.; Li, Z.; Croitoru, A.; Thung, S.; Vlassara, H. Adverse effects of dietary glycotoxins on wound healing in ge-netically diabetic mice. Diabetes 2003, 52, 2805–2813. [Google Scholar] [CrossRef] [Green Version]
- Vlassara, H.; Fuh, H.; Makita, Z.; Krungkrai, S.; Cerami, A.; Bucala, R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: A model for diabetic and aging complications. Proc. Natl. Acad. Sci. USA 1992, 89, 12043–12047. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; He, J.C.; Zhu, L.; Chen, X.; Wallenstein, S.; Striker, G.E.; Vlassara, H. Reduced Oxidant Stress and Extended Lifespan in Mice Exposed to a Low Glycotoxin Diet: Association with Increased AGER1 Expression. Am. J. Pathol. 2007, 170, 1893–1902. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, S.; Dong, H.H.; Li, Z.; Cai, W.; Altomonte, J.; Thung, S.N.; Zeng, F.; Fisher, E.; Vlassara, H. Improved Insulin Sensitivity Is Associated With Restricted Intake of Dietary Glycoxidation Products in the db/db Mouse. Diabetes 2002, 51, 2082–2089. [Google Scholar] [CrossRef] [Green Version]
- Sandu, O.; Song, K.; Cai, W.; Zheng, F.; Uribarri, J.; Vlassara, H. Insulin Resistance and Type 2 Diabetes in High-Fat-Fed Mice Are Linked to High Glycotoxin Intake. Diabetes 2005, 54, 2314–2319. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Ramdas, M.; Zhu, L.; Chen, X.; Striker, G.E.; Vlassara, H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc. Natl. Acad. Sci. USA 2012, 109, 15888–15893. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; He, J.C.; Zhu, L.; Chen, X.; Zheng, F.; Striker, G.E.; Vlassara, H. Oral Glycotoxins Determine the Effects of Calorie Restriction on Oxidant Stress, Age-Related Diseases, and Lifespan. Am. J. Pathol. 2008, 173, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandaraki, E.; Chatzigeorgiou, A.; Piperi, C.; Palioura, E.; Palimeri, S.; Korkolopoulou, P.; Koutsilieris, M.; Papavassiliou, A.G. Reduced Ovarian Glyoxalase-I Activity by Dietary Glycotoxins and Androgen Excess: A Causative Link to Polycystic Ovarian Syndrome. Mol. Med. 2012, 18, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; He, C.; Cai, W.; Hattori, M.; Steffes, M.; Vlassara, H. Prevention of diabetic nephropathy in mice by a diet low in glycoxidation products. Diabetes/Metab. Res. Rev. 2002, 18, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Kanatsuna, T.; Nakamura, N.; Kitagawa, Y.; Mori, H.; Kajiyama, S.; Nakano, K.; Kondo, M. Defect of the first-phase insulin secretion to glucose stimulation in the perfused pancreas of the nonobese diabetic (NOD) mouse. Diabetes 1986, 35, 486–490. [Google Scholar] [CrossRef]
- Vlassara, H.; Striker, L.J.; Teichberg, S.; Fuh, H.; Li, Y.M.; Steffes, M. Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc. Natl. Acad. Sci. USA 1994, 91, 11704–11708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onorato, J.M.; Jenkins, A.; Thorpe, S.R.; Baynes, J.W. Pyridoxamine, an Inhibitor of Advanced Glycation Reactions, Also Inhibits Advanced Lipoxidation Reactions. J. Biol. Chem. 2000, 275, 21177–21184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metz, T.O.; Alderson, N.L.; Chachich, M.E.; Thorpe, S.R.; Baynes, J.W. Pyridoxamine traps intermediates in lipid peroxidation reactions in vivo: Evidence on the role of lipids in chemical modification of protein and development of diabetic complications. J. Biol. Chem. 2003, 278, 42012–42019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolffenbuttel, B.H.; Boulanger, C.M.; Crijns, F.R.L.; Huijberts, M.S.P.; Poitevin, P.; Swennen, G.N.M.; Vasan, S.; Egan, J.J.; Ulrich, P.; Cerami, A.; et al. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc. Natl. Acad. Sci. USA 1998, 95, 4630–4634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degenhardt, T.P.; Alderson, N.L.; Arrington, D.D.; Beattie, R.J.; Basgen, J.M.; Steffes, M.W.; Thorpe, S.R.; Baynes, J.W. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 2002, 61, 939–950. [Google Scholar] [CrossRef] [Green Version]
- Vlassara, H.; Cai, W.; Goodman, S.; Pyzik, R.; Yong, A.; Chen, X.; Zhu, L.; Neade, T.; Beeri, M.; Silverman, J.M.; et al. Protection against Loss of Innate Defenses in Adulthood by Low Advanced Glycation End Products (AGE) Intake: Role of the Antiinflammatory AGE Receptor-1. J. Clin. Endocrinol. Metab. 2009, 94, 4483–4491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneto, H.; Nakatani, Y.; Kawamori, D.; Miyatsuka, T.; Matsuoka, T.-A. Involvement of Oxidative Stress and the JNK Pathway in Glucose Toxicity. Rev. Diabet. Stud. 2004, 1, 165. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [Green Version]
- Helzlsouer, K.J.; Alberg, A.J.; Huang, H.Y.; Hoffman, S.C.; Strickland, P.T.; Brock, J.W.; Burse, V.W.; Needham, L.L.; Bell, D.A.; Lavigne, J.A.; et al. Serum concentrations of organochlorine compounds and the subsequent development of breast cancer. Cancer Epidemiol. Biomark. Prev. 1999, 8, 525–532. [Google Scholar]
- Lin, R.-Y.; Choudhury, R.P.; Cai, W.; Lu, M.; Fallon, J.; Fisher, E.; Vlassara, H. Dietary glycotoxins promote diabetic atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 2003, 168, 213–220. [Google Scholar] [CrossRef]
- Lin, R.-Y.; Reis, E.D.; Dore, A.T.; Lu, M.; Ghodsi, N.; Fallon, J.; Fisher, E.; Vlassara, H. Lowering of dietary advanced glycation endproducts (AGE) reduces neointimal formation after arterial injury in genetically hypercholesterolemic mice. Atherosclerosis 2002, 163, 303–311. [Google Scholar] [CrossRef]
- Haesen, S.; Cöl, Ü.; Schurgers, W.; Evens, L.; Verboven, M.; Driesen, R.B.; Bronckaers, A.; Lambrichts, I.; Deluyker, D.; Bito, V. Glycolaldehyde-modified proteins cause adverse functional and structural aortic remodeling leading to cardiac pressure overload. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M.; Stavroulakis, P.; Raptis, S.A. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen. 2009, 17, 461–472. [Google Scholar] [CrossRef] [PubMed]
- West, R.K.; Moshier, E.; Lubitz, I.; Schmeidler, J.; Godbold, J.; Cai, W.; Uribarri, J.; Vlassara, H.; Silverman, J.M.; Beeri, M.S. Dietary advanced glycation end products are associated with decline in memory in young elderly. Mech. Ageing Dev. 2014, 140, 10–12. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-H.; Xie, J.-Z.; Jiang, X.; Lv, B.-L.; Cheng, X.-S.; Du, L.-L.; Zhang, J.-Y.; Wang, J.-Z.; Zhou, X.-W. Methylglyoxal Induces Tau Hyperphosphorylation via Promoting AGEs Formation. NeuroMol. Med. 2012, 14, 338–348. [Google Scholar] [CrossRef]
- Li, J.J.; Dickson, D.; Hof, P.R.; Vlassara, H. Receptors for advanced glycosylation endproducts in human brain: Role in brain ho-meostasis. Mol. Med. 1998, 4, 46–60. [Google Scholar] [CrossRef]
- Egawa, T.; Tsuda, S.; Goto, A.; Ohno, Y.; Yokoyama, S.; Goto, K.; Hayashi, T. Potential involvement of dietary advanced glycation end products in impairment of skeletal muscle growth and muscle contractile function in mice. Br. J. Nutr. 2017, 117, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dongen, K.C.; Linkens, A.M.; Wetzels, S.M.; Wouters, K.; Vanmierlo, T.; van de Waarenburg, M.P.; Scheijen, J.L.; de Vos, W.M.; Belzer, C.; Schalkwijk, C.G. Dietary advanced glycation endproducts (AGEs) increase their concentration in plasma and tissues, result in inflammation and modulate gut microbial composition in mice; evidence for reversibility. Food Res. Int. 2021, 147, 110547. [Google Scholar] [CrossRef] [PubMed]
- Janšáková, K.; Lengyelová, E.; Pribulová, N.; Somoza, V.; Celec, P.; Šebeková, K.; Ostatníková, D.; Tóthová, Ľ. Metabolic and Renal Effects of Dietary Advanced Glycation end Products in Pregnant Rats—A Pilot Study. Physiol. Res. 2019, 68, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, F.; Rahmani, M.; Tavalaee, M. Effect of Different High-Fat and Advanced Glycation End-Products Diets in Obesity and Diabetes-Prone C57BL / 6 Mice on Sperm Function. Int. J. Fertil. Steril. 2021, 15, 226–233. [Google Scholar]
- Van Heijst, J.W.J.; Niessen, H.W.M.; Hoekman, K.; Schalkwijk, C.G. Advanced glycation end products in human cancer tissues: Detection of Nε-(carboxymethyl)lysine and argpyrimidine. Ann. N. Y. Acad. Sci. 2005, 1043, 725–733. [Google Scholar] [CrossRef]
- Jiao, L.; Stolzenberg-Solomon, R.; Zimmerman, T.P.; Duan, Z.; Chen, L.; Kahle, L.; Risch, A.; Subar, A.F.; Cross, A.J.; Hollenbeck, A.; et al. Dietary consumption of advanced glycation end products and pancreatic cancer in the prospective NIH-AARP Diet and Health Study. Am. J. Clin. Nutr. 2014, 101, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Turner, D.P. Advanced Glycation End-Products: A Biological Consequence of Lifestyle Contributing to Cancer Disparity. Cancer Res. 2015, 75, 1925–1929. [Google Scholar] [CrossRef] [Green Version]
- Tessier, F.J.; Boulanger, E.; Howsam, M. Metabolic transit of dietary advanced glycation end-products—The case of N(Ɛ)-carboxymethyllysine. Glycoconj. J. 2021, 38, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud, A.; Niquet-Leridon, C.; Boulanger, E.; Tessier, F.J. How Can Diet Affect the Accumulation of Advanced Glycation End-Products in the Human Body? Foods 2016, 5, 84. Available online: https://pubmed.ncbi.nlm.nih.gov/28231179 (accessed on 6 December 2019). [CrossRef] [PubMed] [Green Version]
- Semba, R.D.; Gebauer, S.K.; Baer, D.J.; Sun, K.; Turner, R.; Silber, H.A.; Talegawkar, S.; Ferrucci, L.; Novotny, J.A. Dietary Intake of Advanced Glycation End Products Did Not Affect Endothelial Function and Inflammation in Healthy Adults in a Randomized Controlled Trial. J. Nutr. 2014, 144, 1037–1042. [Google Scholar] [CrossRef] [Green Version]
- Kellow, N.J.; Savige, G.S. Dietary advanced glycation end-product restriction for the attenuation of insulin resistance, oxidative stress and endothelial dysfunction: A systematic review. Eur. J. Clin. Nutr. 2013, 67, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Author | Animal Model | Diet | Results |
|---|---|---|---|
| Thornton et al. 2020 [15] | C57BL/6 J mice | H-AGEs diet: 16.6% fat, 18.8% protein, 64.6% carbohydrate, 3.73 kcal/g, subjected to heating at 125 °C for 30 min | H-AGEs mice showed a lower number of corpora lutea |
| Rajan et al. 2018 [16] | Swiss albino mice | H-AGEs diet (18% of protein, 64% of carbohydrate, 5.2% of fat) subjected to heating (90 °C for 10 min) to generate diet-derived AGEs | Increased body weight, pro-inflammatory cytokines, chemokines, CML, CRP, HbA1c, BUN, creatinine |
| Deng et al. 2020 [17] | apoE−/− mice | High fat diet (42% fat and 0.2% cholesterol) and normal diet (13% fat, no cholesterol) and aminoguanidine | Elevation in the aortic valve transvalvular velocity, alleviation of LDL, TC reduced HFD-induced CML accumulation and RAGE expression in the aortic valve, and restricted EndMT in the aortic valve calcification |
| Mastrocola et al. 2021 [18] | C57BLKS-Db/Db mice | Standard diet and high fat diet (HFD) and pyridoxamine | Accumulation of AGEs in the liver impaired hepatic sphingolipids metabolism and hepatic insulin signaling, prevented by pyridoxamine |
| Verboven et al. 2018 [19] | Sprague–Dawley rats | High-sugar and high-fat diet (Western diet) | Altered glucose tolerance, early signs of cardiac alteration |
| Chen et al. 2019 [20] | Sprague–Dawley | L-AGEs and H-AGEs diets | H-AGEs diet decreases the total number of epididymal sperm and increases the abnormal sperm ratio |
| Mao et al. 2019 [21] | Sprague–Dawley rats | Low-fat control diet and high-fat diet | High fat diet increased cecal contents and decreased proteobacteria |
| Coughlan et al. 2011 [22] | Sprague–Dawley rats | L-AGEs diet and H-AGEs diet | H-AGEs diet increased cellular pathways linked to b-cell damage and b-cell apoptosis and the incidence of autoimmune diabetes in NOD mice |
| Coughlan et al. 2011 [22] | Sprague–Dawley rats | Daily intraperitoneal injections of either AGEs-modified rat serum albumin (AGE-RSA), RSA at 20 mg/kg/day, or saline (sham) | H-AGEs diet increased cellular pathways linked to b-cell damage and b-cell apoptosis and the incidence of autoimmune diabetes in NOD mice |
| Yuan et al. 2017 [23] | Sprague–Dawley rats | Intraperitoneal injection of high-fat/high-sucrose diet and low-dose streptozocin, untreated diabetic and treated with aminoguanidine hydrochloride (100 mg/Kg/day, i.g., for 8 weeks) | AG reduced serum and renal CML deposition, and improved urine protein and uNGAL in type 2 diabetic rats |
| Borg et al. 2018 [24] | NOD8.3 + NOD/ShiLt +NOD8.3C | H-AGEs diet and L-AGEs diet | Exposure to low dietary AGEs from conception to early postnatal life increased islet hormone secretion ex vivo; reduced insulitis; increased the variance of CD4C and CD8C T cells and cDCs in local lymphoid tissues and proportions of pDCs in the spleen; and altered islet expression of the AGEs, CML, and AGE receptors |
| Lee et al. 2019 [25] | Sprague–Dawley rats | Normal diet and regular, low, or high high-fat high-sucrose diet | L-AGEs showed lower fasting glucose, insulin, HOMA-IR, TC, TG, HDLc levels, higher blood urea nitrogen, n-3 PUFAs, decreased proliferation of the mesangial cells, glomerular capillaries, basement membranes around the glomeruli, lower Firmicutes/Bacteroidetes ratio, and increased Allobaculum |
| Maciejczyk et al. 2018 [26] | male Wistar rats | High fat diet (59.8 kcal% fat, 20.1 kcal% protein, and 20.1 kcal% carbs) and normal diet (g 13.5 kcal% fat, 24 kcal% protein, and 62.5 kcal% carbohydrates) | High fat fed rats showed increased body weight, free fatty acids, glucose, insulin, HOMA-IR index, enzymatic antioxidant activity, total antioxidant/oxidant status and oxidative damage products (TAC, TOS, OSI, and FRAP), activity of NOX and XO) in the cerebral cortex and hypothalamus, and enzymatic antioxidants (GPx, CAT, and SOD-1) in the cerebral cortex |
| Qu et al. 2017 [27] | Sprague–Dawley rats | L-AGEs diet or high-AGEs diet | H-AGEs fed rats showed markedly decreased diversity of cecal microbiota, after 18 weeks of feeding, increased proportion of proteobacteria, and decreased proportion of Bacteroidetes; in the short-term feeding period of 6 weeks, significantly higher relative abundance of five genera, including Prevotella, Oscillibacter, Phascolarctobacterium, Akkermansia, and Gastranaerophilales; higher concentration of ammonia in cecal contents and significantly lower concentration of two other genera, Lachnospiraceae and Mucispirillum at 12 and 18 weeks of feeding; decreased acetate and propionate from 6 to 18 weeks; and modestly increased butyric acid and histological score of colonic tissue |
| Illien-Juünger et al. 2018 [28] | C57BL/6J mice | L-AGEs; containing 7.6 μg/mg AGE and H-AGEs: 40.9 μg/m | H-AGEs fed mice showed elevated serum AGEs levels in female mice, sex- and age-dependent effects on vertebral AGEs accumulation and on vertebral bone microstructure, and decreased vertebral mechanical properties |
| Peppa et al. 2003 [29] | Prediabetic NOD mice | H-AGEs diet produced vy exposure to heating (100 °C for 20–60 s and at 125 °C for 20–30 min) and L-AGEs = identical chow mix without heating | L-AGEs fed mice showed a striking reduction in fasting blood glucose, increased plasma insulin levels, decreased affected (20%) pancreatic islets, low levels of IFN-γ and IL-4, high IL-10-to-actin mRNA ratio, and prevention of Type 1 diabetes transmitted to next generations |
| Peppa et al. 2003 [30] | db/db +/+ and db/db −/+ | H-AGEs diet produced by heating (100 °C for 20–60 min and at 125 °C for 20–30 min), L-AGEs identical chow mix without heating | H-AGEs decreased the albumin/creatinine ratio, increased protein-linked tissue deposition of MG- and CML-like AGEs, delayed closure, lead to less re-epithelialization, and delayed wound healing |
| Vlassara et al. 1992 [31] | Lewis rats and New Zealand White rabbits | Tail vein injections with either AGEs-modified or native RSA (100 mg/kg per day) or AGEs-RSA, followed immediately by i.v. injection of aminoguanidine hydrochloride (100 mg/kg per day) | AGEs’ administration resulted in significantly increased vascular permeability, mononuclear cell migratory activity in subendothelial and periarteriolar spaces in various tissues, markedly defective vasodilatory responses to acetylcholine and nitroglycerin, promoted glomerulosclerosis, and normal characteristics in treated with aminoguanidine |
| Cai et al. 2007 [32] | C57BL/6 mice | H-AGEs diet and L-AGEs diet | L-AGEs fed mice showed amelioration of insulin resistance, albuminuria, and glomerulosclerosis, as well as extended lifespan |
| Hofmann et al. 2002 [33] | db/db (+/+) | H-AGEs diet and L-AGEs diet | L-AGEs fed mice showed reduced body weight, improved responses to both glucose and insulin tolerance tests, increased HDL and lowered CML and MG concentrations, and better preservation of the islets |
| Sandu et al. 2005 [34] | C57/BL6 mice | Regular and high-fat diets | H-AGEs fed mice showed higher body weight, fasting glucose, insulin, serum AGEs, altered pancreatic islet structure and function, plasma 8-isoprostanes, and lower adiponectin |
| Cai et al. 2012 [35] | wild-type C57BL6 mice | Nonheated, isocaloric diet, where the content of AGEs was increased by a single synthetic MG-AGE (MG+) | High fat fed mice showed increased adiposity and premature insulin resistance; severe deficiency of AGER1 and SIRT1 in white adipose tissue, skeletal muscle, and liver; impaired 2-deoxy-glucose uptake; marked changes in insulin receptor IRS-1, IRS-2; Akt activation; and a macrophage and adipocyte shift to a pro-oxidant/inflammatory phenotype |
| Cai et al. 2008 [36] | C57BL/6 mice | Caloric restriction (40% reduction in calories), caloric restriction exposed to heating, the same CR diet, in which the content of (by 15 min at 120 °C, and standard formula) | CR-high AGEs fed mice showed high levels of 8-isoprostanes, AGEs, RAGE, p66shc, low AGER1 and GSH/GSSG levels, insulin resistance, marked myocardial and renal fibrosis, and shortened lifespan |
| Kandaraki et al. 2012 [37] | Wistar rats | L-AGEs diet and H-AGEs diet | H-AGEs fed rats showed reduced GLO-I activity, positively correlated with body weight gain and progesterone levels |
| Zheng et al. 2002 [38] | db/db mice | L-AGEs diet and H-AGEs diet | L-AGEs fed mice showed minimal glomerular pathology; modest increase in urinary albumin/creatinine ratio; extended survival; lower serum; and kidney AGEs low levels of renal cortex TGFb-1, laminin B1 mRNA, a1 IV collagen mRNA, and protein |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peppa, M.; Mavroeidi, I. Experimental Animal Studies Support the Role of Dietary Advanced Glycation End Products in Health and Disease. Nutrients 2021, 13, 3467. https://doi.org/10.3390/nu13103467
Peppa M, Mavroeidi I. Experimental Animal Studies Support the Role of Dietary Advanced Glycation End Products in Health and Disease. Nutrients. 2021; 13(10):3467. https://doi.org/10.3390/nu13103467
Chicago/Turabian StylePeppa, Melpomeni, and Ioanna Mavroeidi. 2021. "Experimental Animal Studies Support the Role of Dietary Advanced Glycation End Products in Health and Disease" Nutrients 13, no. 10: 3467. https://doi.org/10.3390/nu13103467





