Salivary Oxidative Stress Markers’ Relation to Oral Diseases in Children and Adolescents
Abstract
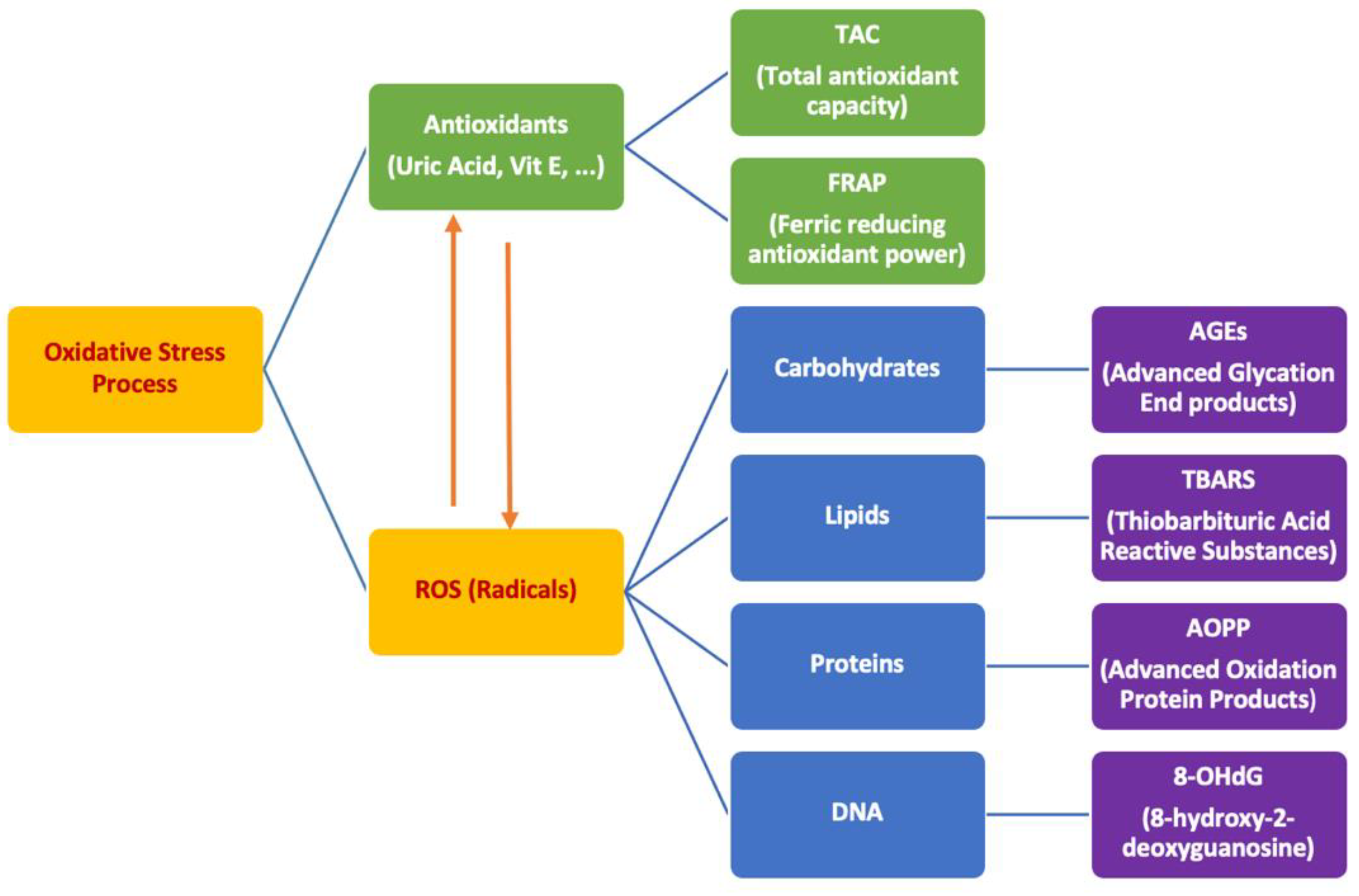
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Lab Procedures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Guo, L.; Shi, W. Salivary biomarkers for caries risk assessment. J. Calif. Dent. Assoc. 2013, 41, 107. [Google Scholar] [PubMed]
- Marcenes, W.; Kassebaum, N.J.; Bernabe, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C.J. Global burden of oral conditions in 1990–2010: A systematic analysis. J. Dent. Res. 2013, 92, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Luchian, I.; Moscalu, M.; Goriuc, A.; Nucci, L.; Tatarciuc, M.; Martu, I.; Covasa, M. Using Salivary MMP-9 to Successfully Quantify Periodontal Inflammation during Orthodontic Treatment. J. Clin. Med. 2021, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Nazemisalman, B.; Sajedinejad, N.; Darvish, S.; Vahabi, S.; Gudarzi, H. Evaluation of inductive effects of different concentrations of cyclosporine A on MMP-1, MMP-2, MMP-3, TIMP-1, and TIMP-2 in fetal and adult human gingival fibroblasts. J. Basic Clin. Physiol. Pharmacol. 2019, 30. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Amer. Oil. Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Tothova, L.; Kamodyova, N.; Cervenka, T.; Celec, P. Salivary markers of oxidative stress in oral diseases. Front. Cell. Infect. Microbiol. 2015, 5, 73. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Iannitti, T.; Rottigni, V.; Palmieri, B. Role of free radicals and antioxidant defences in oral cavity-related pathologies. J. Oral Pathol. Med. 2012, 41, 649–661. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Stephens, J.W.; Khanolkar, M.P.; Bain, S.C. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis 2009, 202, 321–329. [Google Scholar] [CrossRef]
- Vlkova, B.; Stanko, P.; Minarik, G.; Tothova, L.; Szemes, T.; Banasova, L.; Novotnakova, D.; Hodosy, J.; Celec, P. Salivary markers of oxidative stress in patients with oral premalignant lesions. Arch. Oral Biol. 2012, 57, 1651–1656. [Google Scholar] [CrossRef]
- Chapple, I.L.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol. 2000 2007, 43, 160–232. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Chen, H.S.; Chen, S.L.; Ho, Y.P.; Ho, K.Y.; Wu, Y.M.; Hung, C.C. Lipid peroxidation: A possible role in the induction and progression of chronic periodontitis. J. Periodontal. Res. 2005, 40, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Tothova, L.; Celecova, V.; Celec, P. Salivary markers of oxidative stress and their relation to periodontal and dental status in children. Dis. Markers 2013, 34, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Friedlander, M.; Capeillere-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Wang, J.; Schipper, H.M.; Velly, A.M.; Mohit, S.; Gornitsky, M. Salivary biomarkers of oxidative stress: A critical review. Free Radic. Biol. Med. 2015, 85, 95–104. [Google Scholar] [CrossRef]
- Vahabi, S.; Moslemi, M.; Nazemisalman, B.; Yadegari, Z. Phenytoin Effects on Proliferation and Induction of IL1β and PGE2 in Pediatric and Adults’ Gingival Fibroblasts. Open J. Stom. 2014, 4, 452. [Google Scholar] [CrossRef][Green Version]
- Celecova, V.; Kamodyova, N.; Tothova, L.; Kudela, M.; Celec, P. Salivary markers of oxidative stress are related to age and oral health in adult non-smokers. J. Oral Pathol. Med. 2013, 42, 263–266. [Google Scholar] [CrossRef]
- Selmeci, L. Advanced oxidation protein products (AOPP): Novel uremic toxins, or components of the non-enzymatic antioxidant system of the plasma proteome? Free Radic. Res. 2011, 45, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Behuliak, M.; Palffy, R.; Gardlik, R.; Hodosy, J.; Halcak, L.; Celec, P. Variability of thiobarbituric acid reacting substances in saliva. Dis. Markers 2009, 26, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Motamayel, F.; Goodarzi, M.T.; Hendi, S.S.; Kasraei, S.; Moghimbeigi, A. Total antioxidant capacity of saliva and dental caries. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e553. [Google Scholar] [CrossRef]
- Pyati, S.A.; Naveen Kumar, R.; Kumar, V.; Praveen Kumar, N.; Parveen Reddy, K. Salivary flow rate, pH, buffering capacity, total protein, oxidative stress and antioxidant capacity in children with and without dental caries. J. Clin. Ped. Dent. 2018, 42, 445–449. [Google Scholar] [CrossRef]
- Ahmadi-Motamayel, F.; Goodarzi, M.T.; Mahdavinezhad, A.; Jamshidi, Z.; Darvishi, M. Salivary and serum antioxidant and oxidative stress markers in dental caries. Caries Res. 2018, 52, 565–569. [Google Scholar] [CrossRef]
- Su, H.; Gornitsky, M.; Velly, A.M.; Yu, H.; Benarroch, M.; Schipper, H.M. Salivary DNA, lipid, and protein oxidation in nonsmokers with periodontal disease. Free Radic. Biol. Med. 2009, 46, 914–921. [Google Scholar] [CrossRef]
- Zhang, T.; Andrukhov, O.; Haririan, H.; Muller-Kern, M.; Liu, S.; Liu, Z.; Rausch-Fan, X. Total Antioxidant Capacity and Total Oxidant Status in Saliva of Periodontitis Patients in Relation to Bacterial Load. Front. Cell. Infect. Microbiol. 2015, 5, 97. [Google Scholar] [CrossRef]
- De Caro, V.; Murgia, D.; Seidita, F.; Bologna, E.; Alotta, G.; Zingales, M.; Campisi, G. Enhanced in situ availability of aphanizomenon flos-aquae constituents entrapped in buccal films for the treatment of oxidative stress-related oral diseases: Biomechanical characterization and in vitro/ex vivo evaluation. Pharmaceutics 2019, 11, 35. [Google Scholar] [CrossRef]
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Vlkova, B.; Celec, P. Does Enterococcus faecalis contribute to salivary thiobarbituric acid-reacting substances? In Vivo 2009, 23, 343–345. [Google Scholar]
- Akalιn, F.A.; Baltacιoğlu, E.; Alver, A.; Karabulut, E. Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis. J. Clin. Periodontol. 2007, 34, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Kamodyova, N.; Tothova, L.; Celec, P. Salivary markers of oxidative stress and antioxidant status: Influence of external factors. Dis. Markers. 2013, 34, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Celec, P. Oxidative stress and antioxidants in the diagnosis and therapy of periodontitis. Front. Physiol. 2017, 8, 1055. [Google Scholar]
- Kumar, J.; Teoh, S.L.; Das, S.; Mahakknaukrauh, P. Oxidative stress in oral diseases: Understanding its relation with other systemic diseases. Front. Physiol. 2017, 8, 693. [Google Scholar] [CrossRef] [PubMed]

| Males | Females | p-Value | |
|---|---|---|---|
| CI = 0 | 41 (48.8%) | 43 (51.2%) | 0.75 |
| CI = 2 | 40 (51.3%) | 38 (48.7%) | |
| PBI = 0 | 28 (49.1%) | 29 (50.9%) | 0.32 |
| PBI = 1 | 34 (51.5%) | 32 (48.5%) | |
| PBI = 2 | 11 (35.5%) | 20 (64.5%) |
| CI = 0 Average ± Standard Deviation | CI = 2 Average ± Standard Deviation | p-Value | |
|---|---|---|---|
| Age | 4.4 ± 9.8 | 9.4 ± 4.5 | 0.39 |
| Age (3–12) | 7.6 ± 2.6 | 7.1 ± 2.4 | 0.34 |
| Age (13–18) | 16 ± 1.1 | 16.3 ± 1.2 | 0.33 |
| Plaque index | 24.3 ± 11.3 | 30.1 ± 16.5 | 0.04 * |
| Plaque index (3–12) | 23.7 ± 11.4 | 27.4 ± 15.5 | 0.27 |
| Plaque index (13–18) | 25.5 ± 11.4 | 35.2 ± 17.5 | 0.05 |
| TBARS (µmol/L) | 3 ± 1.4 | 3.1 ± 1.4 | 0.75 |
| TBARS (3–12) (µmol/L) | 2.9 ± 1.4 | 3.2 ± 1.3 | 0.28 |
| TBARS (13–18) (µmol/L) | 3.4 ± 1.1 | 2.9 ± 1.5 | 0.21 |
| AOPP (µmol/L) | 75.9 ± 65.3 | 71.8 ± 78 | 0.48 |
| AOPP (3–12) (µmol/L) | 74.6 ± 67.6 | 74.6 ± 85.1 | 0.99 |
| AOPP (13–18) (µmol/L) | 79.8 ± 59.6 | 63.4 ± 53.3 | 0.36 |
| TAC (µmol/L) | 541.4 ± 228 | 522.5 ± 204.1 | 0.57 |
| TAC (3–12) (µmol/L) | 494.1 ± 229.7 | 520 ± 216 | 0.52 |
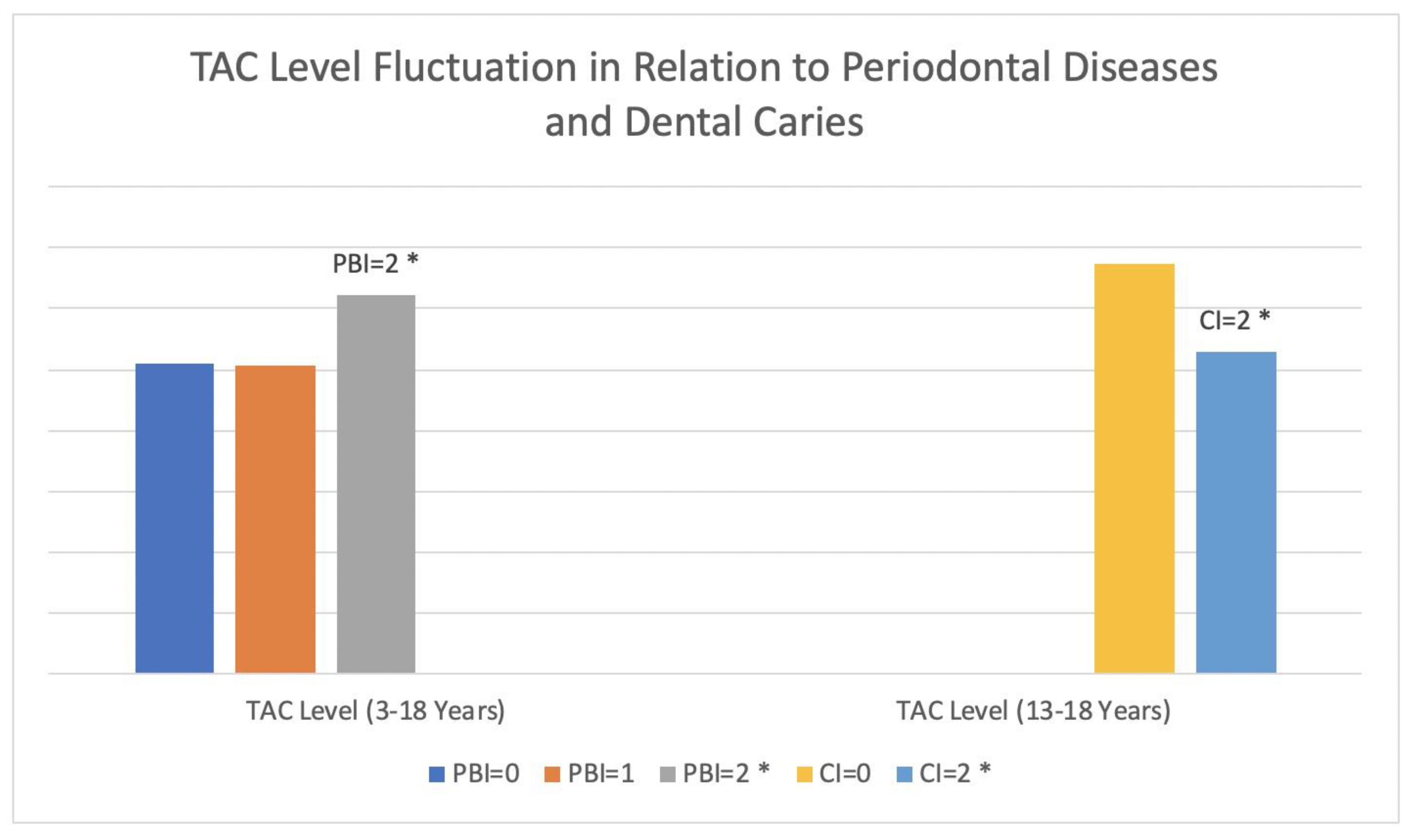
| TAC (13–18) (µmol/L) | 675 ± 163.9 | 530.1 ± 166.8 | 0.008 * |
| PBI = 2 Average ± Standard Deviation | PBI = 1 Average ± Standard Deviation | PBI = 0 Average ± Standard Deviation | p-Value | |
|---|---|---|---|---|
| Age | 14.1 ± 2.9 | 10.8 ± 3.2 | 5.25 ± 1.9 | 0.0001 * |
| Plaque index | 32.1 ± 16.7 | 25.4 ± 12.7 | 19.1 ± 7.8 | 0.03 * |
| TBARS (µmol/L) | 3.1 ± 1.1 | 3.1 ± 1.5 | 3 ± 1.2 | 0.97 |
| AOPP (µmol/L) | 84.5 ± 90.9 | 68.4 ± 75.3 | 73.7 ± 54.8 | 0.31 |
| TAC (µmol/L) | 623.4 ± 176.3 | 507.3 ± 210.4 | 509.2 ± 233.2 | 0.02 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salman, B.N.; Darvish, S.; Goriuc, A.; Mazloomzadeh, S.; Hossein Poor Tehrani, M.; Luchian, I. Salivary Oxidative Stress Markers’ Relation to Oral Diseases in Children and Adolescents. Antioxidants 2021, 10, 1540. https://doi.org/10.3390/antiox10101540
Salman BN, Darvish S, Goriuc A, Mazloomzadeh S, Hossein Poor Tehrani M, Luchian I. Salivary Oxidative Stress Markers’ Relation to Oral Diseases in Children and Adolescents. Antioxidants. 2021; 10(10):1540. https://doi.org/10.3390/antiox10101540
Chicago/Turabian StyleSalman, Bahareh Nazemi, Shayan Darvish, Ancuta Goriuc, Saeideh Mazloomzadeh, Maryam Hossein Poor Tehrani, and Ionut Luchian. 2021. "Salivary Oxidative Stress Markers’ Relation to Oral Diseases in Children and Adolescents" Antioxidants 10, no. 10: 1540. https://doi.org/10.3390/antiox10101540
APA StyleSalman, B. N., Darvish, S., Goriuc, A., Mazloomzadeh, S., Hossein Poor Tehrani, M., & Luchian, I. (2021). Salivary Oxidative Stress Markers’ Relation to Oral Diseases in Children and Adolescents. Antioxidants, 10(10), 1540. https://doi.org/10.3390/antiox10101540








