Biochemical Evaluation of the Effects of Hydroxyurea in Vitro on Red Blood Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Samples
2.3. Preparation of Hemolysate
2.4. Determination of the Hemoglobin (Hb) Concentration
2.5. Oxidative Stress Indicators
2.5.1. Lipid Peroxidation
2.5.2. Determination of Fe3+ in the Residual Plasma Samples
2.5.3. Quantification of Hydrogen Peroxide (H2O2)
2.5.4. Determination of the Superoxide Dismutase (SOD) Activity
2.5.5. Determination of the Catalase Activity
2.5.6. Determination of the Enzymatic Activity of Glutathione Peroxidase (GPx)
2.5.7. Determination of the Content of Reduced Glutathione (GSH)
2.5.8. Determination of the Glutathione Reductase (GR) Activity
2.6. Energy Metabolism of the RBC
2.6.1. Determination of the Glucose-6-Phosphate Dehydrogenase (G6PD) Activity
2.6.2. Determination of the Hexokinase Activity (HEX)
2.6.3. Determination of the Pyruvate Kinase (PK) Activity
2.7. Statistical Analysis
3. Results
3.1. Determination of the Oxidative Stress Indicators
3.2. Antioxidant Defense of the RBC
3.3. The Energy Metabolism of the RBC
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovacic, P. Hydroxyurea (therapeutics and mechanism): Metabolism, carbamoyl nitroso, nitroxyl, radicals, cell signaling, and clinical applications. Med. Hypotheses 2011, 76, 24–31. [Google Scholar] [CrossRef]
- Agrawal, R.K.; Patel, R.K.; Shah, V.; Nainiwal, L.; Trivedi, B. Hydroxyurea in sickle cell disease: Drug review. Indian J. Hematol. Blood Transfus. 2014, 30, 91–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrand, T.; Rakotoson, M.G.; Galacteros, F.; Bartolucci, P.; Hulin, A. Determination of hydroxyurea in human plasma by HPLC-UV using derivatization with xanthydrol. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1064, 85–91. [Google Scholar] [CrossRef]
- Navarra, P.; Preziosi, P. Hydroxyurea: New insights on an old drug. Crit. Rev. Oncol. Hematol. 1999, 29, 249–255. [Google Scholar] [CrossRef]
- Tracewell, W.G.; Trump, D.L.; Vaughan, W.P.; Smith, D.C.; Gwilt, P.R. Population pharmacokinetics of hydroxyurea in cancer patients. Cancer Chemother. Pharmacol. 1995, 35, 417–422. [Google Scholar] [CrossRef]
- Walker, A.L.; Franke, R.M.; Sparreboom, A.; Ware, R.E. Transcellular movement of hydroxyurea is mediated by specific solute carrier transporters. Exp. Hematol. 2011, 39, 446–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agil, A.; Sadrzadeh, S.M.H. Hydroxy-urea protects erythrocytes against oxidative damage. Redox Rep. 2000, 5, 29–34. [Google Scholar] [CrossRef]
- Malec, J.; Przybyszewski, W.M.; Grabarczyk, M.; Sitarska, E. Hydroxyurea has the capacity to induce damage to human erythrocytes, which can be modified by radical scavengers. Biochem. Biophys. Res. Commun. 1984, 120, 566–573. [Google Scholar] [CrossRef]
- Iyamu, E.W.; Fasold, H.; Roa, D.; del Pilar Aguinaga, M.; Asakura, T.; Turner, E.A. Hydroxyurea-induced oxidative damage of normal and sickle cell hemoglobins in vitro: Amelioration by radical scavengers. J. Clin. Lab. Anal. 2001, 15, 1–7. [Google Scholar] [CrossRef]
- Lorenzi, T.F. Manual of Propaedeutic and Clinical Hematology, 4th ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2006. [Google Scholar]
- Thom, C.S.; Dickson, C.F.; Gell, D.A.; Weiss, M.J. Hemoglobin variants: Biochemical properties and clinical correlates. Cold Spring Harb. Perspect. Med. 2013, 3, a011858. [Google Scholar] [CrossRef] [Green Version]
- Luzzatto, L.; Nannelli, C.; Notaro, R. Glucose-6-Phosphate Dehydrogenase Deficiency. Hematol. Oncol. Clin. N. Am. 2016, 30, 373–393. [Google Scholar] [CrossRef]
- Arbos, K.A.; Claro, L.M.; Borges, L.; Santos, C.A.; Weffort-Santos, A.M. Human erythrocytes as a system for evaluating the antioxidant capacity of vegetable extracts. Nutr. Res. 2008, 28, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Petit, K.; Suwalsky, M.; Colina, J.R.; Contreras, D.; Aguilar, L.F.; Jemiola-Rzeminska, M.; Strzalka, K. Toxic effects of the anticancer drug epirubicin in vitro assayed in human erythrocytes. Toxicol. In Vitro 2020, 68, 104964. [Google Scholar] [CrossRef]
- Morabito, R.; Romano, O.; La Spada, G.; Marino, A. H2O2-Induced Oxidative Stress Affects SO4= Transport in Human Erythrocytes. PLoS ONE 2016, 11, e0146485. [Google Scholar] [CrossRef] [Green Version]
- Dacie, J.V.; Lewis, S.M. Pratical Hematology, 8th ed.; Churchill Livingstone: Edinburgh, UK, 1996. [Google Scholar]
- Atmaca, G. Antioxidant effects of sulfur-containing amino acids. Yonsei Med. J. 2004, 45, 776–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, C.M.; Santos, R.C.C.; Lima, E.S. A simple automated procedure for thiol measurement in human serum samples. J. Bras. Patol. Med. Lab. 2006, 42, 345–350. [Google Scholar] [CrossRef]
- Adams, P.E. Determining Iron Content in Foods by Spectrophotometry. J. Chem. Educ. 1995, 72, 649–651. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Woollard, A.C.; Wolff, S.P. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990, 268, 69–71. [Google Scholar] [CrossRef] [Green Version]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Flohe, L.; Gunzler, W.A.; Schock, H.H. Glutathione peroxidase: A selenoenzyme. FEBS Lett. 1973, 32, 132–134. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, W.; Hosoda, S.; KazukoHayashib, K. Purification, and the properties of rat liver glutathione peroxidase. Biochim. Biophys. Acta 1974, 358, 251–261. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. An improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Racker, E. Glutathione reductase from bakers’ yeast and beef liver. J. Biol. Chem. 1955, 217, 855–865. [Google Scholar] [CrossRef]
- Beutler, E. Red Cell Metabolism: A Manual of Biochemical Methods, 3rd ed.; Grune e Stratton: New York, NY, USA, 1984. [Google Scholar]
- Ahmad, M.F.; Ansari, M.O.; Jameel, S.; Wani, A.L.; Parveen, N.; Siddique, H.R.; Shadab, G. Protective role of nimbolide against chemotherapeutic drug hydroxyurea induced genetic and oxidative damage in an animal model. Environ. Toxicol. Pharmacol. 2018, 60, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.E.; Bonovas, S.; Travlou, A.; Sitaras, N.M. Redox imbalance, macrocytosis, and RBC homeostasis. Antioxid. Redox Signal. 2006, 8, 1205–1216. [Google Scholar] [CrossRef]
- Belcher, J.D.; Beckman, J.D.; Balla, G.; Balla, J.; Vercellotti, G. Heme degradation and vascular injury. Antioxid. Redox Signal. 2010, 12, 233–248. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F. Lipid peroxidation: A review of causes, consequences, measurement, and dietary influences. Int. J. Food Sci. Nutr. 1996, 47, 233–261. [Google Scholar] [CrossRef]
- Rocha, S.; Gomes, D.; Lima, M.; Bronze-da-Rocha, E.; Santos-Silva, A. Peroxiredoxin 2, glutathione peroxidase, and catalase in the cytosol and membrane of erythrocytes under H2O2-induced oxidative stress. Free Radic. Res. 2015, 49, 990–1003. [Google Scholar] [CrossRef]
- Huang, M.E.; Facca, C.; Fatmi, Z.; Baille, D.; Benakli, S.; Vernis, L. DNA replication inhibitor hydroxyurea alters Fe-S centers by producing reactive oxygen species in vivo. Sci. Rep. 2016, 6, 29361. [Google Scholar] [CrossRef] [Green Version]
- Torres, L.S.; Silva, D.G.H.; Belini Junior, E.; Almeida, E.A.; Lobo, C.L.C.; Cancado, R.D.; Ruiz, M.A.; Bonini-Domingos, C.R. The influence of hydroxyurea on oxidative stress in sickle cell anemia. Rev. Bras. Hematol. Hemoter. 2012, 34, 421–425. [Google Scholar] [CrossRef] [Green Version]
- Brose, R.D.; Lehrmann, E.; Zhang, Y.; Reeves, R.H.; Smith, K.D.; Mattson, M.P. Hydroxyurea attenuates oxidative, metabolic, and excitotoxic stress in rat hippocampal neurons and improves spatial memory in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2018, 72, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Nader, E.; Grau, M.; Fort, R.; Collins, B.; Cannas, G.; Gauthier, A.; Walpurgis, K.; Martin, C.; Bloch, W.; Poutrel, S.; et al. Hydroxyurea therapy modulates sickle cell anemia red blood cell physiology: Impact on RBC deformability, oxidative stress, nitrite levels, and nitric oxide synthase signaling pathway. Nitric Oxide 2018, 81, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Rhee, S.G.; Chae, H.Z.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005, 38, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.S.; Kato, G.J.; Yang, S.H.; Bae, S.W.; Lee, J.S.; Gladwin, M.T.; Rhee, S.G. Hydroxyurea-induced expression of glutathione peroxidase 1 in red blood cells of individuals with sickle cell anemia. Antioxid. Redox Signal. 2010, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurata, M.; Suzuki, M.; Agar, N.S. Antioxidant systems and erythrocyte life span in mammals. Comp. Biochem. Physiol. B 1993, 106, 477–487. [Google Scholar] [CrossRef]
- Teixeira Neto, P.F.; Gonçalves, R.P.; Elias, D.B.D.; Araújo, C.P.; Magalhães, H.I.F. Analysis of oxidative status and biochemical parameters in adult patients with sickle cell anemia treated with hydroxyurea, Ceará, Brazil. Rev. Bras. Hematol. Hemoter. 2011, 33, 207–210. [Google Scholar] [CrossRef]
- Van Zwieten, R.; Verhoeven, A.J.; Roos, D. Inborn defects in the antioxidant systems of human red blood cells. Free Radic. Biol. Med. 2014, 67, 377–386. [Google Scholar] [CrossRef]
- Kalpakcioglu, B.; Senel, K. The interrelation of glutathione reductase, catalase, glutathione peroxidase, superoxide dismutase, and glucose-6-phosphate in the pathogenesis of rheumatoid arthritis. Clin. Rheumatol. 2008, 27, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.C.; Ross, J.G.; d’Avignon, A.; Gibbons, L.B.; Gazit, V.; Hassan, M.N.; McLaughlin, D.; Griffin, S.; Neumayr, T.; Debaun, M.; et al. Sickle hemoglobin disturbs normal coupling among erythrocyte O2 content, glycolysis, and antioxidant capacity. Blood 2013, 121, 1651–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reno, C.O.; Barbosa, A.R.; de Carvalho, S.S.; Pinheiro, M.B.; Rios, D.R.; Cortes, V.F.; Barbosa, L.A.; Santos, H.L. Oxidative stress assessment in sickle cell anemia patients treated with hydroxyurea. Ann. Hematol. 2020, 99, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Raththagala, M.; Karunarathne, W.; Kryziniak, M.; McCracken, J.; Spence, D.M. Hydroxyurea stimulates the release of ATP from rabbit erythrocytes through an increase in calcium and nitric oxide production. Eur. J. Pharmacol. 2010, 645, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Naoum, P.C.; Radispiel, J.; Moraes, M.S. Methaemoglobin spectrometric dosage without chemical or enzymatic interferers. Rev. Bras. Hematol. Hemoter. 2004, 26, 9–22. [Google Scholar]
- Cimen, M.Y. Free radical metabolism in human erythrocytes. Clin. Chim. Acta 2008, 390, 1–11. [Google Scholar] [CrossRef] [PubMed]
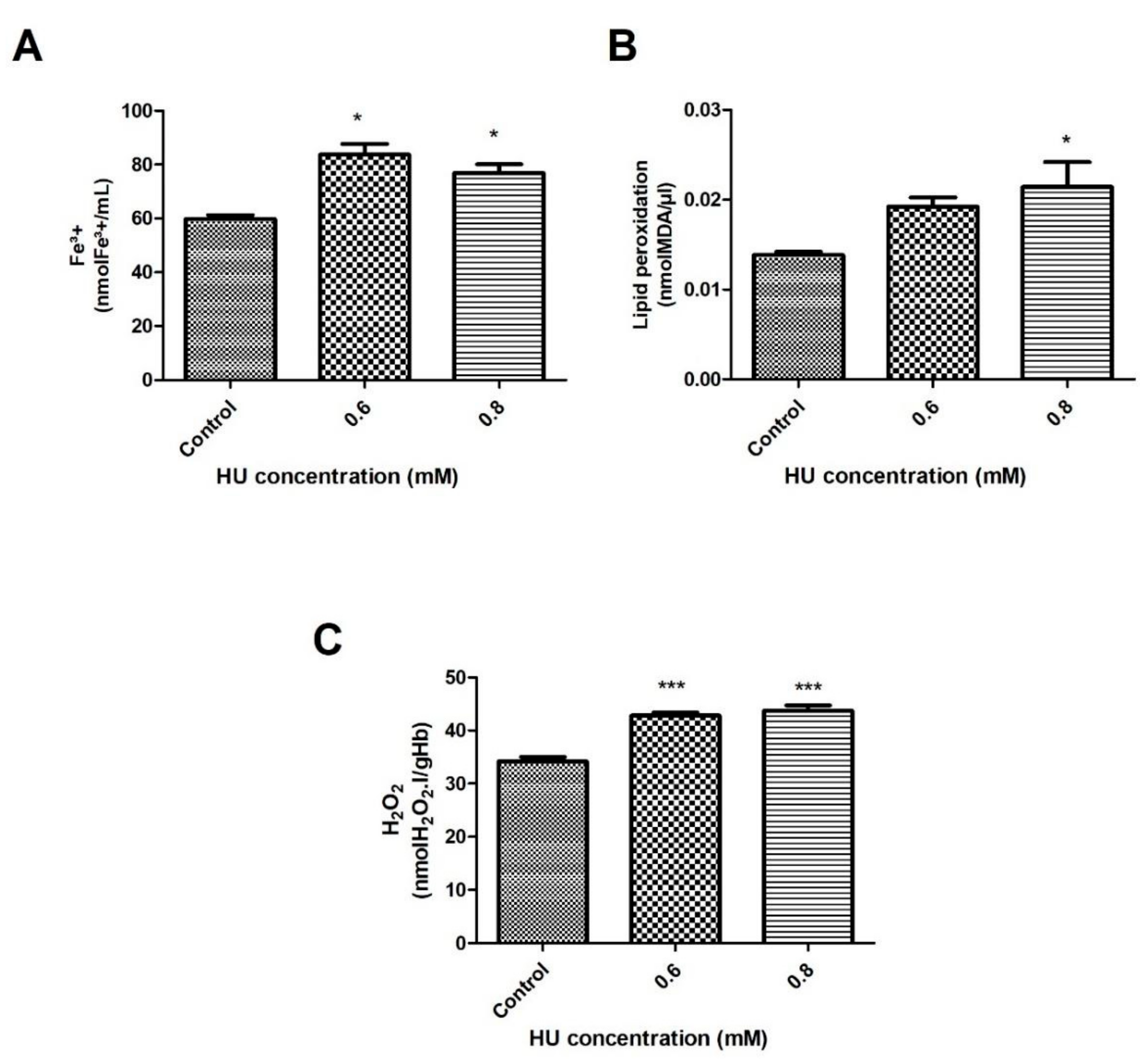

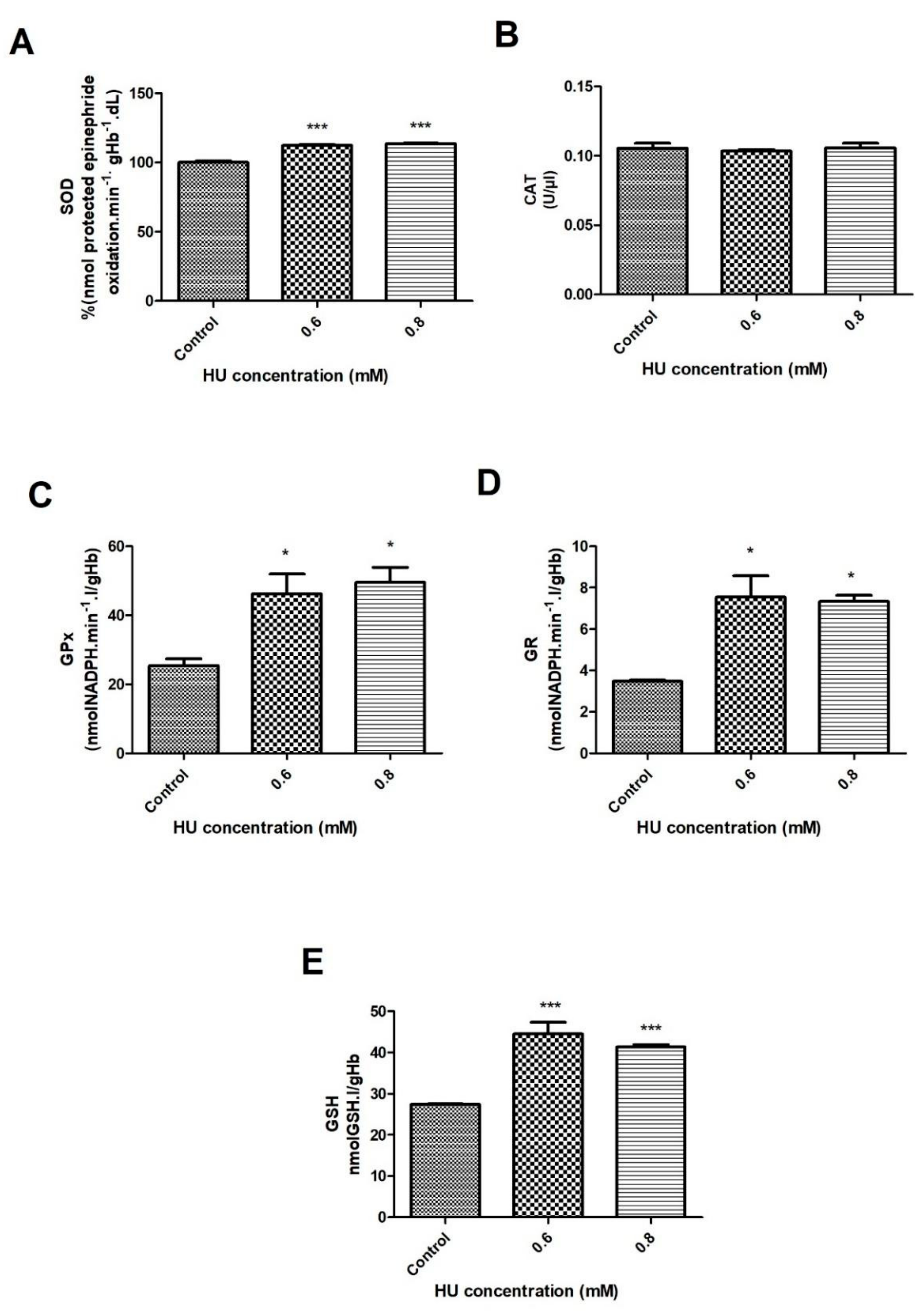

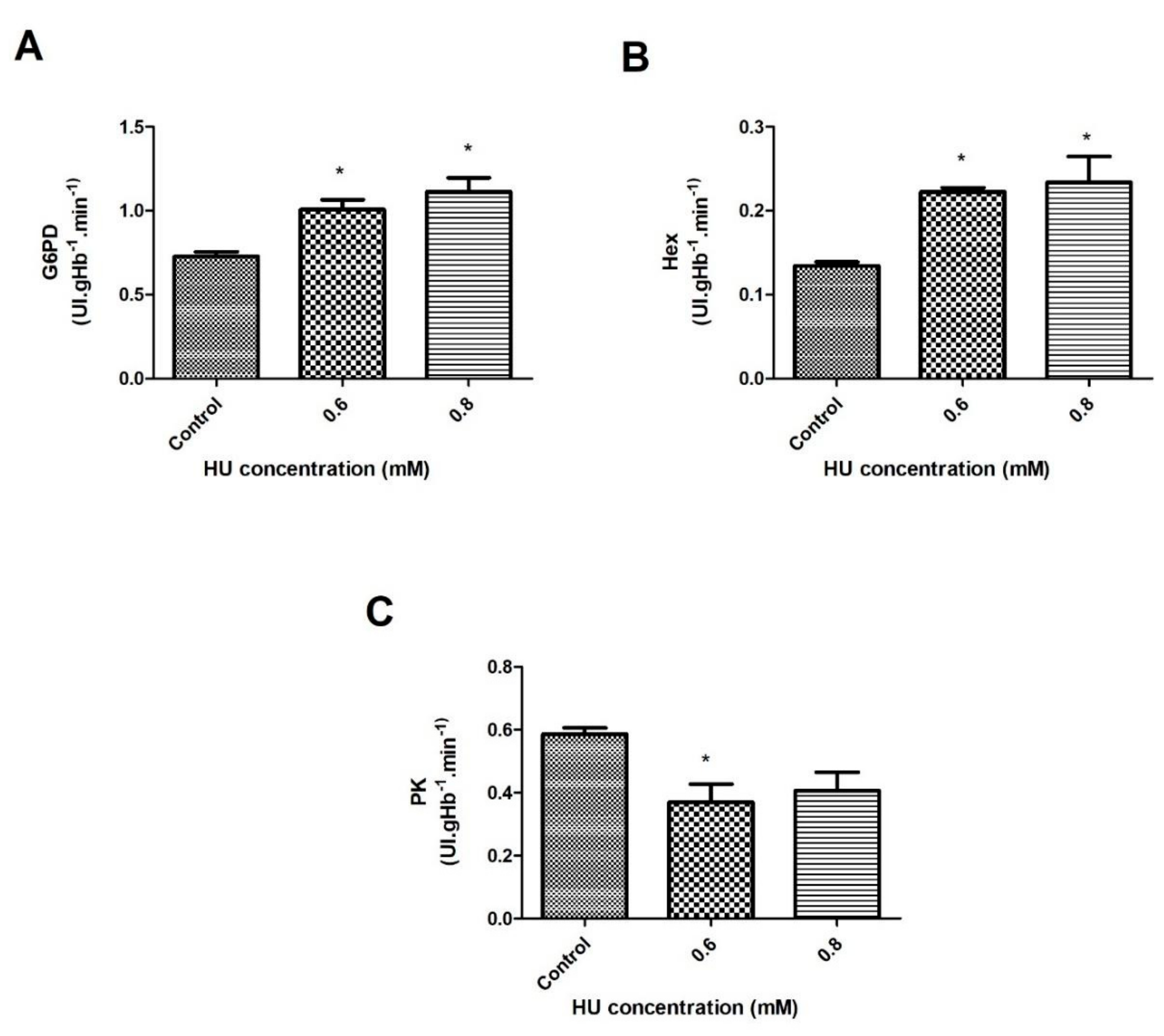

| Time of Treatment | 30 min | 1 h | ||
|---|---|---|---|---|
| HU Concentration | 0.6 mM | 0.8 mM | 0.6 mM | 0.8 mM |
| Variables | ||||
| Fe3+ | ↑ | ↑ | - | ↓ |
| TBARS | - | ↑ | - | ↓ |
| H2O2 | ↑ | ↑ | ↑ | ↑ |
| SOD | ↑ | ↑ | ↑ | ↑ |
| CAT | - | - | - | - |
| GPx | ↑ | ↑ | ↑ | ↑ |
| GR | ↑ | ↑ | ↓ | ↓ |
| GSH | ↑ | ↑ | ↑ | ↓ |
| G6PD | ↑ | ↑ | ↑ | ↓ |
| HEX | ↑ | ↑ | - | - |
| PK | ↓ | - | - | ↓ |
| Concentration | 0.8 mM |
|---|---|
| Fe3+ | - |
| TBARS | ↓ |
| H2O2 | ↓ |
| SOD | ↓ |
| CAT | - |
| GPx | - |
| GR | - |
| GSH | ↓ |
| G6PD | ↓ |
| HEX | ↓ |
| PK | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renó, C.O.; Maia, G.A.S.; Nogueira, L.S.; de Barros Pinheiro, M.; Rios, D.R.A.; Cortes, V.F.; de Oliveira Barbosa, L.A.; de Lima Santos, H. Biochemical Evaluation of the Effects of Hydroxyurea in Vitro on Red Blood Cells. Antioxidants 2021, 10, 1599. https://doi.org/10.3390/antiox10101599
Renó CO, Maia GAS, Nogueira LS, de Barros Pinheiro M, Rios DRA, Cortes VF, de Oliveira Barbosa LA, de Lima Santos H. Biochemical Evaluation of the Effects of Hydroxyurea in Vitro on Red Blood Cells. Antioxidants. 2021; 10(10):1599. https://doi.org/10.3390/antiox10101599
Chicago/Turabian StyleRenó, Cristiane Oliveira, Grazielle Aparecida Silva Maia, Leilismara Sousa Nogueira, Melina de Barros Pinheiro, Danyelle Romana Alves Rios, Vanessa Faria Cortes, Leandro Augusto de Oliveira Barbosa, and Hérica de Lima Santos. 2021. "Biochemical Evaluation of the Effects of Hydroxyurea in Vitro on Red Blood Cells" Antioxidants 10, no. 10: 1599. https://doi.org/10.3390/antiox10101599
APA StyleRenó, C. O., Maia, G. A. S., Nogueira, L. S., de Barros Pinheiro, M., Rios, D. R. A., Cortes, V. F., de Oliveira Barbosa, L. A., & de Lima Santos, H. (2021). Biochemical Evaluation of the Effects of Hydroxyurea in Vitro on Red Blood Cells. Antioxidants, 10(10), 1599. https://doi.org/10.3390/antiox10101599






